Abstract
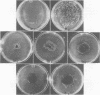
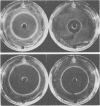
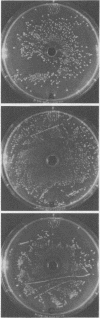
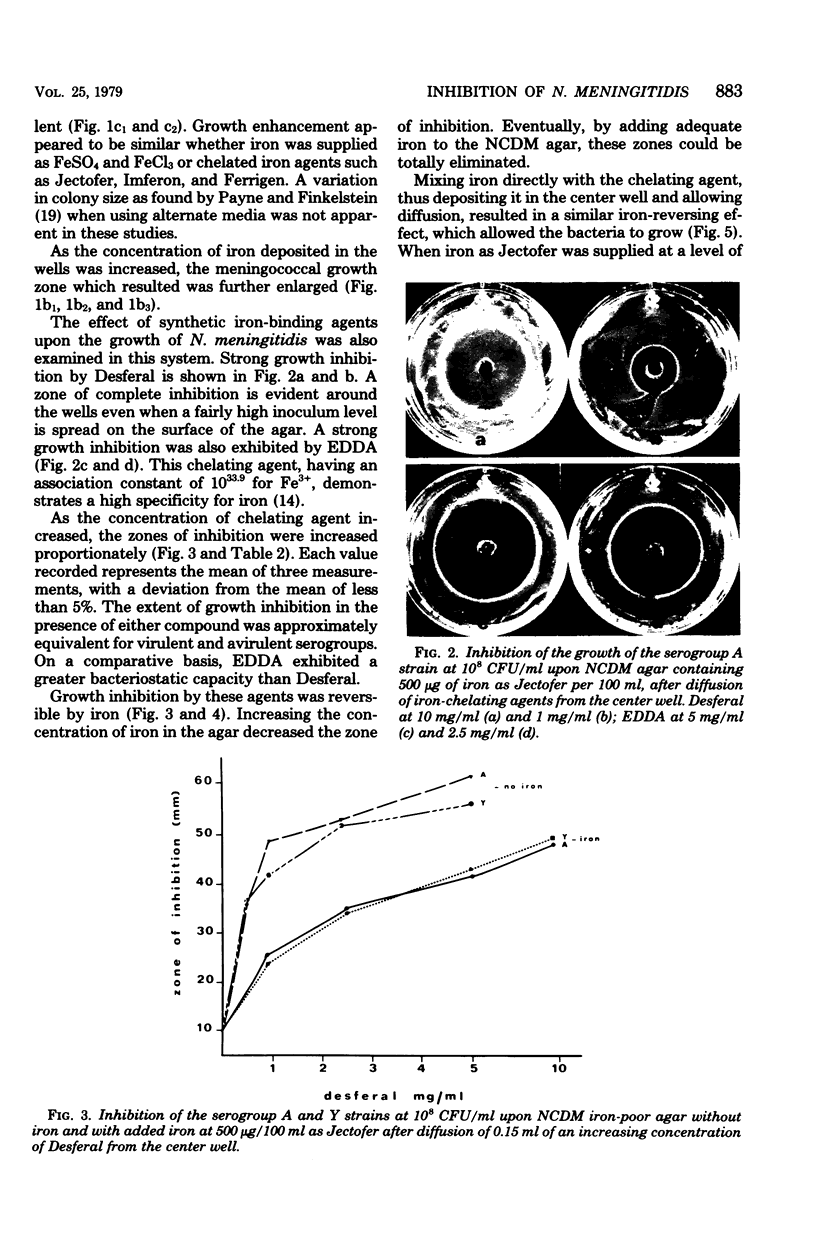
Serogroups of N. meningitidis were characterized as virulent or avirulent according to their capacity to establish meningococcal infection in mice. An agar plate diffusion technique demonstrated that iron had a definite growth-supporting role for both of these meningococcal types. The avirulent strains could use ionic or chelated iron as well as the virulent strains. Iron-reversible growth inhibition occurred to the same extent for both bacterial types in the presence of the synthetic iron-chelating agents Desferal and ethylenediamine-di-orthohydroxy phenylacetic acid. A difference in response was demonstrated for these bacterial types when grown in the presence of various iron-binding proteins from animal body fluids and tissues. The growth of the avirulent strain was inhibited to a greater degree by egg white conalbumin. The humoral iron-binding protein transferrin showed a significant inhibitory capacity only when used in conjunction with bicarbonate. Under conditions of increased iron saturation of this protein, the avirulent strain was inhibited to the furthest extent. In the presence of ferritin, the cellular iron-binding protein, which had been reduced, inhibition of the growth of either strain type did not occur on iron-poor media (less than 5 micrograms/100 ml). However, with the incorporation of iron into the media, the inhibitory effect of the protein became evident. As the concentration of iron increased, the inhibition increased to a certain level and subsequently declined. A substantial difference in the ability of the avirulent type to grow in the presence of reduced horse spleen ferritin was observed. For this microorganism, a correlation appears to exist between the capacity to grow by utilizing the available iron in the presence of reduced ferritin and the ability to establish infection. The host protein ferritin, in the reduced state, apart from simply being a storage protein for iron, can prevent the growth of a procaryotic organism. Our experiments suggest a role for ferritin in the prevention of emningococcal disease. A cehmotherapeutic potential for Desferal is also implied.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates G. W., Schlabach M. R. The reaction of ferric salts with transferrin. J Biol Chem. 1973 May 10;248(9):3228–3232. [PubMed] [Google Scholar]
- Bates G. W., Wernicke J. The kinetics and mechanism of iron(3) exchange between chelates and transferrin. IV. The reaction of transferrin with iron(3) nitrilotriacetate. J Biol Chem. 1971 Jun 10;246(11):3679–3685. [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Lewin J. E. The bacteriostatic effect of serum on Pasteurella septica and its abolition by iron compounds. Immunology. 1971 Mar;20(3):391–406. [PMC free article] [PubMed] [Google Scholar]
- Calver G. A., Kenny C. P., Lavergne G. Iron as a replacement for mucin in the establishment of meningococcal infection in mice. Can J Microbiol. 1976 Jun;22(6):832–838. doi: 10.1139/m76-120. [DOI] [PubMed] [Google Scholar]
- Drysdale J. W. Heterogeneity in tissue ferritins displayed by gel electrofocusing. Biochem J. 1974 Sep;141(3):627–632. doi: 10.1042/bj1410627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden C. A., Kochan I., Spriggs D. R. Role of mycobactin in the growth and virulence of tubercle bacilli. Infect Immun. 1974 Jan;9(1):34–40. doi: 10.1128/iai.9.1.34-40.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. L., Peterson C. M., Grady R. W., Kumbaraci T., Cerami A., Graziano J. H. Effects of iron chelators and iron overload on Salmonella infection. Nature. 1977 May 5;267(5606):63–65. doi: 10.1038/267063a0. [DOI] [PubMed] [Google Scholar]
- Kenny C. P., Ashton F. E., Diena B. B., Greenberg L. A chemically defined protein-free liquid medium for the cultivation of some species of Neisseria. Bull World Health Organ. 1967;37(4):569–573. [PMC free article] [PubMed] [Google Scholar]
- Kingdon G. C., Sword C. P. Effects of Listeria monocytogenes Hemolysin on Phagocytic Cells and Lysosomes. Infect Immun. 1970 Apr;1(4):356–362. doi: 10.1128/iai.1.4.356-362.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan I., Kvach J. T., Wiles T. I. Virulence-associated acquisition of iron in mammalian serum by Escherichia coli. J Infect Dis. 1977 Apr;135(4):623–632. doi: 10.1093/infdis/135.4.623. [DOI] [PubMed] [Google Scholar]
- Kochan I. Mecahnism of tuberculostasis in mammalian serum. I. Role of transferrin in human serum tuberculostasis. J Infect Dis. 1969 Jan;119(1):11–18. doi: 10.1093/infdis/119.1.11. [DOI] [PubMed] [Google Scholar]
- Kochan I. The role of iron in bacterial infections, with special consideration of host-tubercle bacillus interaction. Curr Top Microbiol Immunol. 1973;60:1–30. doi: 10.1007/978-3-642-65502-9_1. [DOI] [PubMed] [Google Scholar]
- Macara I. G., Hoy T. G., Harrison P. M. The formation of ferritin from apoferritin. Kinetics and mechanism of iron uptake. Biochem J. 1972 Jan;126(1):151–162. doi: 10.1042/bj1260151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles A. A., Khimji P. L. Enterobacterial chelators of iron: their occurrence, detection, and relation to pathogenicity. J Med Microbiol. 1975 Nov;8(4):477–490. doi: 10.1099/00222615-8-4-477. [DOI] [PubMed] [Google Scholar]
- Pasieka A. E., Calver G., Kenny C. P., Perusse C. Antibody production in milk serum after antigen instillation of the goat mammary gland. IX. Sham infection studies with Neisseria meningitudis L.C.D.C. 608B. Can J Microbiol. 1975 May;21(5):662–667. doi: 10.1139/m75-095. [DOI] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. Detection and differentiation of iron-responsive avirulent mutants on Congo red agar. Infect Immun. 1977 Oct;18(1):94–98. doi: 10.1128/iai.18.1.94-98.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. Imferon agar: improved medium for isolation of pathogenic Neisseria. J Clin Microbiol. 1977 Sep;6(3):293–297. doi: 10.1128/jcm.6.3.293-297.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. Pathogenesis and immunology of experimental gonococcal infection: role of iron in virulence. Infect Immun. 1975 Dec;12(6):1313–1318. doi: 10.1128/iai.12.6.1313-1318.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price E. M., Gibson J. F. A re-interpretation of bicarbonate-free ferric transferrin E.P.R. spectra. Biochem Biophys Res Commun. 1972 Jan 31;46(2):646–651. doi: 10.1016/s0006-291x(72)80189-x. [DOI] [PubMed] [Google Scholar]
- Rogers H. J., Bullen J. J., Cushnie G. H. Iron compounds and resistance to infection. Further experiments with Clostridium welchii type A in vivo and in vitro. Immunology. 1970 Oct;19(4):521–538. [PMC free article] [PubMed] [Google Scholar]
- Stefanini S., Chiancone E., Vecchini P., Antonini E. Studies on iron uptake and micelle formation in ferritin and apoferritin. Mol Cell Biochem. 1976 Oct 30;13(1):55–61. doi: 10.1007/BF01732396. [DOI] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Role of iron in host-parasite interactions. J Infect Dis. 1971 Oct;124(4):401–410. doi: 10.1093/infdis/124.4.401. [DOI] [PubMed] [Google Scholar]