Abstract
We examined the tear film proteome of patients with Sjögren's syndrome (SS) and dry eye syndrome (group A), patients with dry eye symptoms (group B) and normal volunteers (group C). Tear samples were pooled from 8 subjects from each group and were subjected to two-dimensional-nano-liquid chromatography coupled with tandem mass spectrometry (2D-nano-LC-MS/MS). The tear breakup time for group A was significantly reduced compared with group B and C (P < 0.001). Group A (Schirmer I test, 2.13 ± 2.38 mm/5 min) had markedly lower tear volume than group B (5.94 ± 4.75 mm/5 min) and C (14.44 ± 6.57 mm/5 min) (P < 0.001). Group A had significantly higher normalized tear protein content (1.8291 ± 0.2241 μg/mm) than group B (1.0839 ± 0.1120 μg/mm) (P = 0.001) and C (0.2028 ± 0.0177 μg/mm) (P = 0.001). The 2D-nano-LC-MS/MS analysis identified a total of 435 proteins, including 182 (54.8%), 247 (74.4%) and 278 (83.7%) in group A, B, and C, respectively, with 56 (16.7%) proteins including defensin α1, clusterin and lactotransferrin unique to group A. In conclusion, dry eye syndrome in SS patients is associated with an altered proteomic profile with dysregulated expression of proteins involved in a variety of important cellular process including inflammation, immunity, and oxidative stress.
Dry eye syndrome is a multifactorial condition characterized by eye irritation symptoms, blurred and fluctuating vision, tear film instability, increased tear osmolarity and ocular surface epithelial dysfunction1,2,3,4. Sjögren's syndrome (SS) is an autoimmune disorder in which the exocrine glands are the principal target organs, particularly the lachrymal and salivary glands5,6, and dry eyes are frequently are the presenting symptoms of SS.
Multiple proteomics-based studies have identified several proteins and peptides as candidate SS biomarkers. A proteomic analysis of the saliva of SS patients exhibited differences in the levels of a number of proteins such as α-amylase precursor, carbonic anhydrase VI, and β-2 microglobulin compared to healthy controls7. Other studies also reveal that lactoferrin is a tear-specific biomarker for SS while anti-transglutaminase, anti-histone, anti-SSA and anti-SSB antibodies are saliva biomarkers of SS8,9. The ability to probe the protein content of human tear fluid has enormous potential for deepening our understanding of the pathology of ocular and systemic diseases such as SS and diabetes and enabling novel noninvasive tear-based diagnostic technologies.
Recently, electrospray ionization (ESI) tandem mass spectroscopy (MS/MS) has been used to identify novel protein species in tears10,11,12,13. The number of proteins found in the tear film continues to grow; however, there is disagreement in the literature regarding the number of proteins in the tear film and the functions of individual proteins. Fung et al.14 reported that approximately 500 proteins were detected and unambiguously identified by liquid chromatography (LC)/MS/MS. Some of these functions are thought to be protective by aiding the ocular surface defense system, or maintain stability of the ocular surface by promoting interaction with other ligands (i.e., lipid binding proteins). The up- or downregulation of these proteins may be indicative of disease mechanisms. In addition, proteomic patterns in tear fluids may reflect histological and functional changes of the lachrymal gland15.
The aims of this study were to examine and compare the tear film proteome of SS, dry eye patients and normal volunteers using 2D-LC-nano-MS/MS-based proteomics.
Methods
The study population
Eight subjects with SS and dry eye syndrome (group A) were recruited for the study. SS was diagnosed according to the Fox criteria16. Dry eye syndrome was diagnosed by a modified Dry Eye Workshop classification17 and was considered present if Schirmer I test < 5 mm and tear breakup time (BUT) < 5 seconds, and absent if Schirmer I test > 10 mm and BUT > 10 seconds. Eight patients with dry eye symptoms (group B, Schirmer I test > 10 mm and BUT > 10 sec) but no systemic diseases were also recruited. A subject was excluded if he or she 1) had a history of ocular trauma or abnormality of the nasolacrimal drainage apparatus; 2) used eye drops within one month prior to the study, 3) had systemic immunologic syndromes other than SS; or 4) any other ocular syndromes than dry eye syndrome. Four acinae of the labial gland were taken from the patients and immediately preserved in formalin for pathological examination. In addition, 8 age and sex-matched healthy subjects who received regular physical checkup (group C) in the same interim were included as normal controls.
The study protocol was approved by the Institutional Review Board of Shanghai Tenth People's Hospital and all study participants provided written informed consent. The study was carried out in accordance with the Declaration of Helsinki.
Western blot assays
Five mL of blood was collected via the antecubital vein of participants in group A, B, and C for measurement of anti-SSA/SSB antibodies using commercially available kits as instructed by the manufacturer (Emoimraw, Germany). The immunoblotting procedure was carried out as previously described18.
Sample collection and preparation
Tears were collected by placing a Schirmer strip in the inferior fornix approximately 6 mm from the lateral canthus. The conjunctiva was not anesthetized. The subjects were instructed to keep the eyes closed during the 5-min test. After the wet length was recorded, the strip was placed in a 1-mL amber Eppendorf tube. Gloves were worn by the examiner and by all investigators handling any tear film samples. All the samples were processed in a masked fashion. The samples were placed immediately in ice transport tanks and stored at −80°C until processed.
Proteins were extracted from the Schirmer strip by incubation in 1 mL Tris buffer (pH = 8.3) at 4°C for 10 h. After centrifugation at 12,000 g for 10 min, the supernatants from all patients within each group were pooled and precipitated by acetone as described previously19. Precipitated proteins were resuspended in 100 μL Tris buffer and quantitated using the BCA assay kit (Pierce, Rockford, IL). The normalized protein content in the tear samples pooled from all subjects from each group was calculated using the following formula:
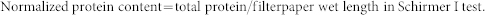 |
Then, 12.5 μg protein from each group was incubated with 25 mM ammonium bicarbonate and 10 mM dithiothreitol (DTT) for 60 min at 56°C followed by incubation with 25 mM iodoacetamide (IAA) for 30 min in the dark at room temperature. Trypsin digestion was done at 37°C for 12 h. The resultant peptides were dried and saved at −80°C.
Two-dimensional strong cation-exchange/reversed-phase nano-scale liquid chromatography mass spectrometry
Extracted peptides were desalted using a 1.3 mL C18 solid phase extraction column (Sep-Pak Cartridge, Waters, Milford, MA), dried using a vacuum centrifuge and resuspended with loading buffer (5 mM ammonium formate containing 5% acetonitrile, pH 3.0), which were then separated and analyzed by 2D-LC-nano-MS/MS. The experiments were performed on a Nano Aquity UPLC system (Waters) connected to an LTQ Orbitrap XL mass spectrometer (Thermo Electron, Bremen, Germany) equipped with an online nano-electrospray ion source (Michrom Bioresources, Auburn, CA). A 180 μm × 2.4 cm SCX column (Waters), which was packed with a 5-μm PolySULFOETHYL Aspartamide (PolyLC, Columbia, MD), was used for the first dimension for recovery of hydrophobic peptides after a conventional salt step gradient. Then, a RP step gradient from 15% to 50% acetonitrile was applied to the SCX column as previously described18. The RP analytical column (20 cm × 75 μm), which was packed with a 1.7-μm Bridged Ethyl Hybrid (BEH) C18 material (Waters), was used for the second dimension separation to elute peptides using a three-step linear gradient, starting from 5% B to 45% B in 40 min (A: water with 0.1% formic acid; B: acetonitrile with 0.1% formic acid), increased to 80% B in 3 min, and then to 5% B in 2 min. The electrospray voltage of 1.1 kV versus the inlet of the mass spectrometer was used.
LTQ Orbitrap XL mass spectrometer was operated in the data-dependent mode to switch automatically between MS and MS/MS acquisition. Survey full-scan MS spectra with two microscans (m/z 300–1800) were acquired in the Obitrap with a mass resolution of 60,000 at 400 m/z, followed by ten sequential LTQ-MS/MS scans. Dynamic exclusion was used with two repeat counts, 10 s repeat duration, and 60 s exclusion duration. For MS/MS, precursor ions were activated using 35% normalized collision energy at the default activation q of 0.25.
Data analyses
All MS/MS spectra were identified by using SEQUEST [v.28 (revision 12), Thermo Electron] against the human International Protein Index (IPI) database (IPI human v3.45 Fasta with 71983 entries). To reduce false positive identification results, a decoy database containing the reverse sequences was appended to the database. The searching parameters were set up as follows: partial trypsin (KR) cleavage with two missed cleavages was included for analysis, the variable modification was oxidation of methionine, peptide mass tolerance was 20 ppm, and fragment ion tolerance was 1 Da. The Trans Proteomic Pipeline software (revision 4.0) (Institute of Systems Biology, Seattle, WA) was then utilized to identify proteins based upon corresponding peptide sequences with ≥95% confidence. The peptide results were filtered by the Peptide Prophet18 with a P value over 0.95 and a Protein Prophet19 probability of 0.95 was used for protein identification results. Spectral counts (SC) correlate with protein abundance20. The relative abundance of individual proteins was assessed by spectral counting, in which we counted the number of times the unlabeled version of a protein was identified by the fragmentation spectra of its peptides. Additionally, we normalized SC using the following formula:
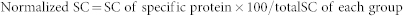 |
For analysis of the difference of abundance among the proteomes, proteins were divided into three groups according to SC: the high abundance group (SC ≥ 50), the medium abundance group (20 < SC < 49), and the low abundance group (SC < 20). Significant upregulation was defined as a SC ratio ≥ 3 and SC distance ≥ 5; significant downregulation was defined as a SC ratio ≤ 0.33 and SC distance ≤ −5. SC ratio was calculated using the equation:
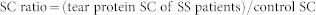 |
and SC distance was calculated using the following formula:
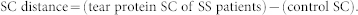 |
Statistical analysis
Data were expressed as mean ± standard deviation and analyzed using the SPSS version 16.0 (SPSS, Chicago, IL). Chi-square tests and Student's t tests were performed and a P value of 0.05 or less was considered significant in our study.
Results
Demographic and baseline characteristics of the study subjects
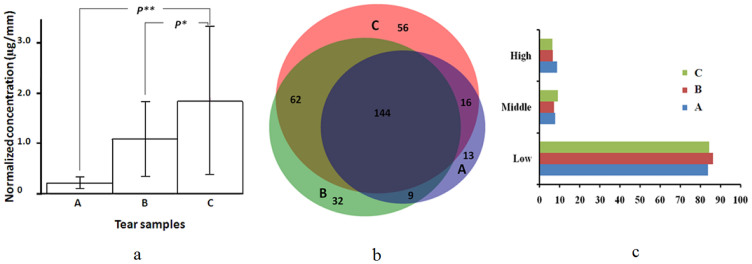
Demographic and baseline characteristics of the study subjects are summarized in Table 1. The subjects of the three groups were matched in age and sex. Group A patients had a median duration of symptoms for 10.5 (range, 8 to 14) years. The BUT for group A patients was 1.07 ± 1.022seconds, which was markedly reduced compared with that of group B (P < 0.001) and that of group C (P < 0.001). The BUT for group B patients was also markedly reduced compared to that of group C (P < 0.001). In addition, group A patients had a marked reduction in tear volumes (2.13 ± 2.38 mm/5 min) compared to group B and C (P < 0.001 in both). Furthermore, SSA/SSB antibodies were present in 75% (6/8) of the sera samples of group A patients with dry eye syndrome, which was not detected in group B and C.
Table 1. Demographic and baseline characteristics of the study subjects.
| Healthy subjects n = 8 | Patients with dry eye symptoms n = 8 | Patients with SS and dry eye syndrome n = 8 | |
|---|---|---|---|
| Age, years | |||
| Mean | 61.75 | 56.38 | 60.13 |
| Standard deviation | 6.61 | 4.63 | 5.89 |
| Gender | |||
| Male | 4 | 4 | 4 |
| Tear breakup time, sec | 12.38 ± 3.88 | 3.06 ± 1.76 | 1.07 ± 1.22 |
| Schirmer I test, mm/5 min | 14.44 ± 6.57 | 5.94 ± 4.75 | 2.13 ± 1.88 |
| Fluorescent test | 0.00 ± 0.00 | 8.44 ± 6.18 | 26.49 ± 0.99 |
| Best corrected visual acuity | 0.91 ± 0.23 | 0.91 ± 0.21 | 0.45 ± 0.35 |
| SSA/SSB antibody (+) | 0 | 0 | 6 |
| Lip gland biopsy (+) | 0 | 0 | 8 |
SS: Sjögren's syndrome.
Patients with SS and dry eye syndrome have increased normalized protein content in tears
The total protein contents in tear samples per Schirmer strip were 395.50 ± 78.45, 499.41 ± 145.59 and 423.58 ± 151.77 μg in group A, B and C, respectively, with no apparent difference statistically among the three groups (P = 0.274). We further determined the normalized protein content in tear samples. The normalized tear protein content in group A was 1.8291 ± 0.2241 μg/mm, which was markedly higher than that of group B (1.0839 ± 0.1120 μg/mm) (P = 0.001) and group C (0.2028 ± 0.0177 μg/mm) (P = 0.001) (Fig. 1a).
Figure 1.
(a) Normalized protein content in tear samples from healthy subjects (A), patients with dry eye symptoms (B) and Sjögren's syndrome (SS) patients with dry eye syndrome (C). Data are expressed as mean ± sd of at least three independent experiments. P** = 0.035, healthy subjects vs. SS patients with dry eye syndrome and P* = 0.213 patients with dry eye symptoms vs. SS patients with dry eye syndrome. (b) Protein distribution in healthy subjects (A), patients with dry eye symptoms (B) and SS patients with dry eye syndrome (C) identified by 2D-nano-LC-MS/MS. (c) Abundance of tear proteins in healthy subjects (A), patients with dry eye symptoms (B) and SS patients with dry eye syndrome (C) The relative abundance of individual proteins was assessed by spectral counting.
Tear proteomic characteristics of the study subjects
The 2D-nano-LC-MS/MS analysis identified a total of 435 unique proteins from the tear samples, including 278 (83.7%) proteins in group A, 247 (74.4%) proteins in group B and 182 (54.8%) proteins in group C. One hundred forty-four (43.4%) proteins were shared among group A, B and C. In addition, 62 (18.7%) proteins were present in group A and B, 9 (2.7%) proteins were found in both group B and C, and 16 (4.8%) proteins were shared by group A and C. Fifty-six (16.7%) proteins were unique to group A, 32 (9.6%) proteins to group B and thirteen (3.9%) to group C (Fig. 1b). In addition, the percentage of high and low abundance proteins was comparable among the three groups (P > 0.05) (Fig. 1c). Meanwhile, the percentage of medium abundance proteins in group A was markedly higher than that of group C (P < 0.05) (Fig. 1c) while the percentage of high abundance proteins was markedly reduced in group A.
Changes in tear proteins of SS patients with dry eye syndrome
The 12 most upregulated proteins in tear fluids of group A are shown in Table 2 and the 10 most downregulated proteins in tear proteins of group A are shown in Table 3. Tear proteins unique to group A included proteins in host defense such as defensin α1 and lactotransferrin, proteins involved in the immune response or inflammatory reaction such as neutrophil elastase 2 and C3, and apoptosis-related proteins like clusterin and annexin (Table 4).
Table 2. The 12 most upregulated tear proteins in Sjögren's syndrome patients with dry eye syndrome.
| Accession No. | Protein Name | Spectral counts |
|---|---|---|
| A8K008 | CDNA FLJ78387 | 115 |
| NP_001002858 | Annexin A2 isoform 1 | 94 |
| P02787 | Serotransferrin precursor | 92 |
| OTTHUMP00000167597 | Keratin 4 | 90 |
| P06702 | Protein S100-A9 | 77 |
| P98088 | Mucin-5AC precursor (fragment) | 59 |
| P04083 | Annexin A1 | 58 |
| P13645 | Keratin, type I cytoskeletal 10 | 50 |
| P04264 | Keratin, type II cytoskeletal 1 | 47 |
| P05109 | Protein S100-A8 | 41 |
| P01024 | Complement C3 precursor (fragment) | 41 |
| P60709 | Actin, cytoplasmic 1 | 41 |
Table 3. The 10 most downregulated tear proteins in Sjögren's syndrome patients with dry eye syndrome.
| Accession | Protein Name | SC |
|---|---|---|
| Q5DSM0 | Growth-inhibiting protein 12 | 516 |
| P31025 | Lipocalin-1 precursor | 507 |
| P12273 | Prolactin-inducible protein precursor | 191 |
| Q8N5K4 | Igha1 Protein | 152 |
| P01833 | Polymeric immunoglobulin receptor precursor | 85 |
| Q9GZZ8 | Extracellular glycoprotein lacritin precursor | 80 |
| P61626 | Lysozyme C precursor | 60 |
| Q16378 | Proline-rich protein 4 precursor | 56 |
| P01036 | Cystatin-S precursor | 29 |
| P13647 | Keratin, type II cytoskeletal 5 | 28 |
Table 4. Changes and functional classification of tear proteins in Sjögren's syndrome patients with dry eye syndrome.
| Accession | Protein Name | |
|---|---|---|
| Host defense proteins | ||
| P59665 | Defensin, α1 | upregulated |
| P81605 | Dermcidin | upregulated |
| P80511 | S100 calcium binding protein A12 | upregulated |
| P31151 | S100 calcium binding protein A7 | upregulated |
| Q71DI3 | Histone 1, H2Bd | upregulated |
| P20160 | Azurocidin 1 (cationic antimicrobial protein 37) | upregulated |
| Q5DSM0 | Lactotransferrin | downregulated |
| P61626 | Lysozyme (renal amyloidosis) | downregulated |
| Proteins involved in the immune response and inflammatory reaction | ||
| P02765 | α-2-Hs-glycoprotein | upregulated |
| P05155 | Serpin peptidase inhibitor, clade G (C1 inhibitor) | upregulated |
| P08246 | Elastase 2, neutrophil | upregulated |
| P04264 | Keratin 1 (epidermolytic hyperkeratosis) | upregulated |
| P00734 | Coagulation factor II (thrombin) | upregulated |
| P02787 | Transferrin | upregulated |
| P02763 | Orosomucoid 1 | upregulated |
| P01024 | Complement component 3 | upregulated |
| P19652 | Orosomucoid 2 | upregulated |
| P0C0L4 | Complement component 4A | upregulated |
| P02652 | Apolipoprotein A- II | ―― |
| Apoptosis-related proteins | ||
| P55072 | Valosin-containing protein | upregulated |
| O43707 | Actinin, α4 | upregulated |
| P00441 | Superoxide dismutase 1, soluble | upregulated |
| P27797 | Calreticulin | upregulated |
| P10909 | Clusterin | upregulated |
| P08107 | Heat shock 70 Kda protein 1A | upregulated |
| Q96BY2 | Modulator Of apoptosis 1 | upregulated |
| P04792 | Heat shock 27 Kda protein 1 | upregulated |
| P14174 | Macrophage migration inhibitory factor | upregulated |
| P04083 | Annexin A1 | upregulated |
| P30101 | Protein disulfide isomerase family A, member 3 | upregulated |
| P00734 | Coagulation factor II (thrombin) | upregulated |
| P23528 | Cofilin 1 (non-muscle) | upregulated |
| P47895 | Aldehyde dehydrogenase 1 family, member A3 | upregulated |
| Q15121 | Phosphoprotein enriched in astrocytes 15 | upregulated |
| P63104 | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein | upregulated |
| P12814 | Actinin, α1 | upregulated |
| P08758 | Annexin A5 | downregulated |
| P31944 | Caspase 14, apoptosis-related cysteine peptidase | downregulated |
| P32119 | Peroxiredoxin 2 | downregulated |
| P07858 | Cathepsin B | downregulated |
| P04179 | Superoxide dismutase 2, mitochondrial | ―― |
| P09211 | Glutathione S-transferase PI | ―― |
| Q04760 | Glyoxalase I | ―― |
| Q6NUS1 | Programmed cell death 6 interacting protein | ―― |
Discussion
Tear proteomic profile of SS patients with dry eye syndrome may offer important insight into the mechanisms of disease process at the target organs and may lead to identification of biomarkers for the conditions. In the current study, we examined the proteomic properties of tear fluids of SS patients with dry eye syndrome, patients with dry eye symptoms and healthy subjects using nano-flow 2D-LC-ESI-MS/MS. We identified a total of 435 proteins in the tear fluids from all three groups together, suggesting the presence of a wealth of proteins in the tear fluid. The findings indicate that the nano-flow 2D-LC-ESI-MS/MS approach may yield a promising proteomic profile of tear proteins in SS patients with dry eye syndrome from relatively small amounts of samples. We also identified 56 proteins that were uniquely present in the tear fluid of SS patients with dry eye syndrome, which include proteins in the host defense, immune response, inflammation and apoptosis. The findings suggest that the development of dry eye syndrome in SS is a complicated process involving proteins of multiple body systems with participation of novel proteins. These proteins are involved in the inflammatory response (α-2-HS-glycoprotein, coagulation factor II, transferrin, and orosomucoid 1 and 2), biosynthesis of IL-8 (apolipoprotein A-II and elastase 2) and activation of the host immune response and the inflammatory response (serpin peptidase inhibitor, clusterin, keratin 1, C3 and 4A). Gene co-expression modules related to primary SS and primary SS/MALT lymphoma were significantly enriched with genes known to be involved in the immune/defense response, apoptosis, cell signaling, gene regulation, and oxidative stress21. We further found in this study that tear proteins in SS patients with dry eye syndrome and patients with dry eye symptoms exhibited a marked increase in the contents of proteins involved in the immune response and stress response, development and differentiation. These proteins also interact with one another and they also show increased oxidative activities. The findings indicate that proteins in the tear fluid of SS patients with dry eye syndrome and patients with dry eye symptoms are more likely the result of the host stress response, which is consistent with the findings by Hu et al21.
Beta-2-microglobulin, lactoferrin, immunoglobulin (Ig) kappa light chain, polymeric Ig receptor, lysozyme C and cystatin C were found to be involved in all stages of SS22. Two presumed proline-rich proteins, amylase and carbonic anhydrase VI, were downregulated in SS patients. In our study, we found that polymeric immunoglobulin receptor precursor, cystatin-S precursor and proline-rich protein 4 precursor were downregulated in the tear fluids of group A patients. Lactotransferrin, which was downregulated in the tear fluids of group A patients in the current study, is a secreted protein and possesses antimicrobial activities against bacteria, fungi and viruses23. The protein acts downstream of the inflammatory and immune response cascade and could modulate the host response to bacteria and the immune response. Defensin belongs to the α-defensin family and is a secreted protein. Defensin 1α, which was upregulated in the tear fluid of SS patients with dry eye syndrome in this study, is capable of inhibiting the replication of viruses and synthesis of viral proteins24. Lysozyme, which has bacteriolytic activities, was downregulated in the tear fluid of SS patients with dry eye syndrome. Our findings that lactotransferrin and lysozymes were downregulated in the tear fluid of group A patients indicate that the ocular surface defense system becomes lessened in group A patients, which may render the patients more susceptible to microbial infections.
Consistent with the findings by Giusfi et al25, proteins related to acute and chronic inflammation or involved in oxidative stress injury in this study were upregulated. We found that S100 A 12 and S100 A7 were upregulated in the tear fluids of group A patients. S100 proteins are calcium binding proteins. The secretion of S100 A12 is induced by TNF and S100 A12 is highly expressed in the inflammatory response26. Other members of the S100 family including S100 A6, S100 A8, S100 A9, and S100 A11 were also upregulated in the tear fluids of group A patients (data not shown), indicating their involvement in the inflammatory response. In addition, proteins associated with apoptosis were markedly upregulated or downregulated. Superoxide dismutase 1 and heat shock proteins were markedly upregulated while peroxiredoxin 2 was downregulated.
Our results lend support to the concept that autoimmunity-mediated dry eye disease has an inflammatory component27. Overexpressed proteins were interferon-inducible or were related to lymphocyte filtration and antigen presentation known to be involved in the pathogenesis of primary SS28. In the current study, neutrophil elastase 2, α-2-HS–glycoprotein, C3, Orosomucoid 1 and 2 were significantly upregulated while APO A-II and clusterin showed no apparent changes from normal controls, suggesting the presence of enhanced immune and inflammatory response at the ocular surface. Complement C3 is a pivotal component of the complement system, interacts with C3d and CR2, and plays a critical role in the activation and proliferation of B cells. In C3 knockout SS mice, SS was diminished or abolished29. In addition, apoptosis of ductal cells in the exocrine glands was decreased and caspase-3 levels declined with apparent reduction in the infiltration of leukocytes into the submandibular gland and diminished production of autoantibodies. Cuida et al.30 reported that complement regulatory proteins in the saliva of SS patients including CD59, CD55, CD46 and clusterin could inhibit the activation of complements in tissues. We found that these proteins were reduced in levels or absent in the tear fluids of group A patients, indicating that complements may show unrestrained activities in the tissues. We found here that C3 was upregulated in the tear fluid of group A patients. Given its pivotal role in immune response and inflammation, C3 may be an important molecule for targeting and as a predictor of patient outcomes.
In conclusion, we have demonstrated that dry eye syndrome in SS patients is associated with an altered proteomic profile with dysregulated expression of proteins involved in a variety of important cellular process including inflammation, immunity, and oxidative stress. These findings suggest broad derangement in tear proteins in SS patients with dry eye syndrome. Furthermore, SS and dry eye have similar pathologies such as increased apoptosis, inflammation at the ocular surface, immune response and cytoskeletal remodeling. Further characterization of these proteins could provide potential diagnostic markers and therapeutic targets that may lead to better outcomes for these patients.
Author Contributions
B.L., W.W. and Y.C. designed the experiments; B.L., M.S., J.L. and G.Y. collected the date; B.L., M.S., W.W. and Y.C. wrote the main manuscript text; all authors reviewed the manuscript.
Acknowledgments
This work was supported by Shanghai Health Hospital Development Center Foundation Project (SHDC12007104); the Youth Research Project of Shanghai Municipal Health Bureau (No. 2010Y164); the Young Talent Training Plan of Tongji University (No. 2010KJ018); the Natural Science Foundation of Shanghai (No.11ZR1427900); the Young Talent Training Plan of Shanghai Tenth People's Hospital (No.11RQ108).
References
- Pflugfelder S. C. et al. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea 17, 38 (1998). [DOI] [PubMed] [Google Scholar]
- Musch D. C., Sugar A. & Meyer R. F. Demographic and predisposing factors in corneal ulceration. Arch ophthalmol 101, 1545 (1983). [DOI] [PubMed] [Google Scholar]
- Sade de Paiva C., Lindsey J. L. & Pflugfelder S. C. Assessing the severity of keratitis sicca with videokeratoscopic indices. Ophthalmology 110, 1102–1109 (2003). [DOI] [PubMed] [Google Scholar]
- Goto E., Yagi Y., Matsumoto Y. & Tsubota K. Impaired functional visual acuity of dry eye patients. Am J ophthalmol 133, 181–186 (2002). [DOI] [PubMed] [Google Scholar]
- De Franceschi L. et al. Proteome analysis of biological fluids from autoimmune -rheumatological disorders. Proteomics Clin Appls 5, 78–89 (2011). [DOI] [PubMed] [Google Scholar]
- Vissink A. et al. Current and Future Challenges in Primary Sjogrens Syndrome. Curr Pharm Biotechnol 13, 2026–2045 (2012). [DOI] [PubMed] [Google Scholar]
- Baldini C. et al. Proteomic analysis of saliva: a unique tool to distinguish primary SjögrenLs syndrome from secondary SjögrenLs syndrome and other sicca syndromes. (2011). [DOI] [PMC free article] [PubMed]
- Karns K. & Herr A. E. Human tear protein analysis enabled by an alkaline microfluidic homogeneous immunoassay. Anal chem 83, 8115–8122 (2011). [DOI] [PubMed] [Google Scholar]
- Hu S. et al. Identification of autoantibody biomarkers for primary Sjögren's syndrome using protein microarrays. Proteomics 11, 1499–1507 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Beuerman R. W., Barathi A. & Tan D. Analysis of rabbit tear proteins by high-pressure liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom 17, 401–412 (2003). [DOI] [PubMed] [Google Scholar]
- Fung K. Y., Morris C., Sathe S., Sack R. & Duncan M. W. Characterization of the in vivo forms of lacrimal - specific proline - rich proteins in human tear fluid. Proteomics 4, 3953–3959 (2004). [DOI] [PubMed] [Google Scholar]
- Zhou L. et al. Characterisation of human tear proteins using high-resolution mass spectrometry. Ann Acad Med Singapore 35, 400 (2006). [PubMed] [Google Scholar]
- Koo B.-S., Lee D.-Y., Ha H.-S., Kim J.-C. & Kim C.-W. Comparative analysis of the tear protein expression in blepharitis patients using two-dimensional electrophoresis. J Proteome Res 4, 719–724 (2005). [DOI] [PubMed] [Google Scholar]
- Fung K., Morris C. & Duncan M. Mass spectrometric techniques applied to the analysis of human tears: a focus on the peptide and protein constituents. Adv Exp Med Biol 506, 601 (2002). [DOI] [PubMed] [Google Scholar]
- Tomosugi N., Kitagawa K., Takahashi N., Sugai S. & Ishikawa I. Diagnostic potential of tear proteomic patterns in Sjögren's syndrome. J Proteome Res 4, 820–825 (2005). [DOI] [PubMed] [Google Scholar]
- Fox R. I., Robinson C. A., Curd J. G., Kozin F. & Howelly F. V. Sjögren's syndrome. Proposed criteria for classification. Arthritis Rheum 29, 577–585 (1986). [DOI] [PubMed] [Google Scholar]
- Asbell P. & Lemp M. Dry eye disease: the clinician's guide to diagnosis and treatment. (Thieme, 2006). [Google Scholar]
- Liu H., Finch J. W., Luongo J. A., Li G.-Z. & Gebler J. C. Development of an online two-dimensional nano-scale liquid chromatography/mass spectrometry method for improved chromatographic performance and hydrophobic peptide recovery. J Chromatogr A 1135, 43–51 (2006). [DOI] [PubMed] [Google Scholar]
- Nesvizhskii A. I., Keller A., Kolker E. & Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem 75, 4646–4658 (2003). [DOI] [PubMed] [Google Scholar]
- Dong M.-Q. et al. Quantitative mass spectrometry identifies insulin signaling targets in C. elegans. Science 317, 660–663 (2007). [DOI] [PubMed] [Google Scholar]
- Hu S. et al. Systems biology analysis of sjögren's syndrome and mucosa -associated lymphoid tissue lymphoma in parotid glands. Arthritis & Rheumatism 60, 81–92 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu O., Atkinson J., Hoehn G., Illei G. & Hart T. Identification of parotid salivary biomarkers in Sjögren's syndrome by surface-enhanced laser desorption/ionization time-of-flight mass spectrometry and two-dimensional difference gel electrophoresis. Rheumatology 45, 1077–1086 (2006). [DOI] [PubMed] [Google Scholar]
- Weinberg E. D. Human lactoferrin: a novel therapeutic with broad spectrum potential. J Pharm Pharmacol 53, 1303–1310 (2001). [DOI] [PubMed] [Google Scholar]
- Salvatore M. et al. β-Defensin Inhibits Influenza Virus Replication by Cell-Mediated Mechanism (s). J Infect Dis 196, 835–843 (2007). [DOI] [PubMed] [Google Scholar]
- Giusti L. et al. Proteome analysis of whole saliva: a new tool for rheumatic diseases–the example of Sjögren's syndrome. Proteomics 7, 1634–1643 (2007). [DOI] [PubMed] [Google Scholar]
- Foell D. et al. Neutrophil derived human S100A12 (EN-RAGE) is strongly expressed during chronic active inflammatory bowel disease. Gut 52, 847–853 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. et al. Antibody protein array analysis of the tear film cytokines. Optometry Vision Sci 85, E653–E660 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. et al. Salivary proteomic and genomic biomarkers for primary Sjögren's syndrome. Arthritis Rheum 56, 3588–3600 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen C. Q., Kim H., Cornelius J. G. & Peck A. B. Development of Sjögren's syndrome in nonobese diabetic-derived autoimmune-prone C57BL/6. NOD-Aec1Aec2 mice is dependent on complement component-3. J Immunol 179, 2318–2329 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuida M., Legler D., Eidsheim M. & Jonsson R. Complement regulatory proteins in the salivary glands and saliva of Sjogren's syndrome patients and healthy subjects. Clin Exp Rheumatol 15, 615–623 (1996). [PubMed] [Google Scholar]