Abstract
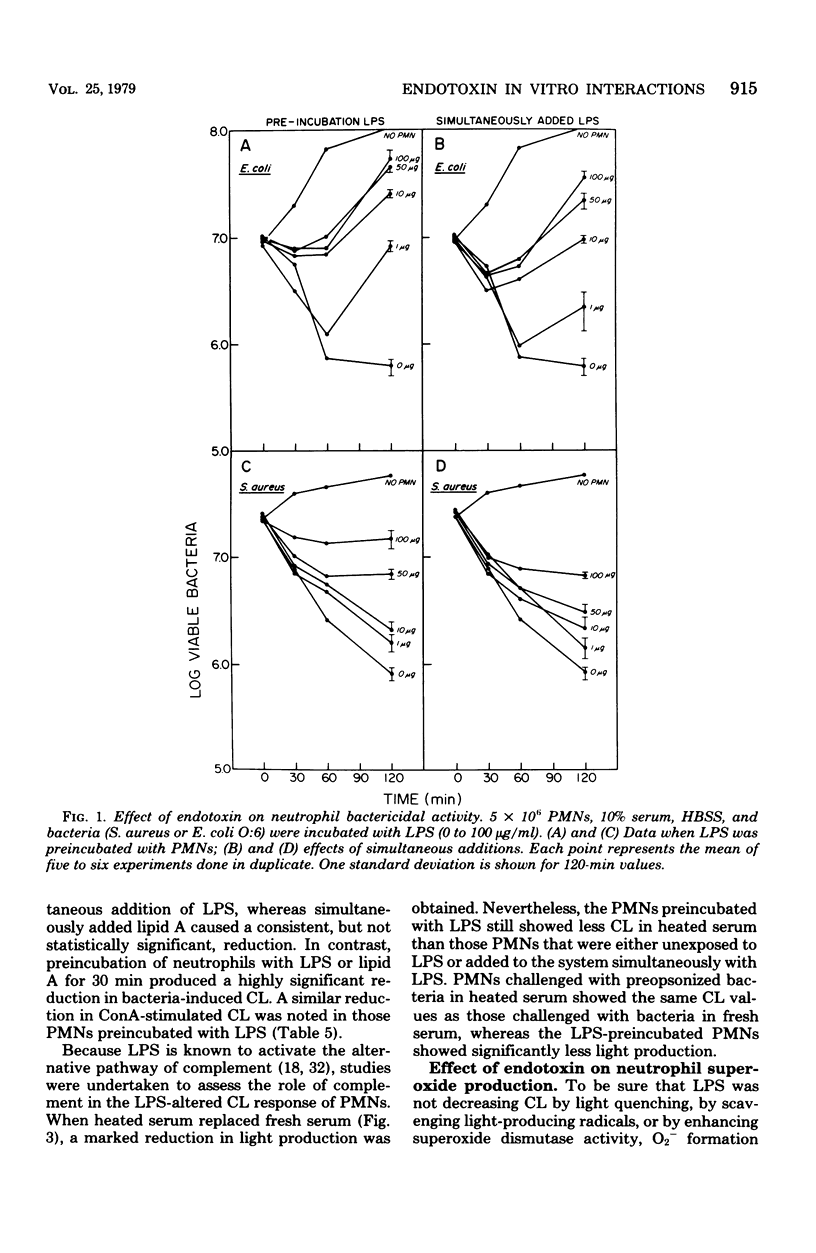
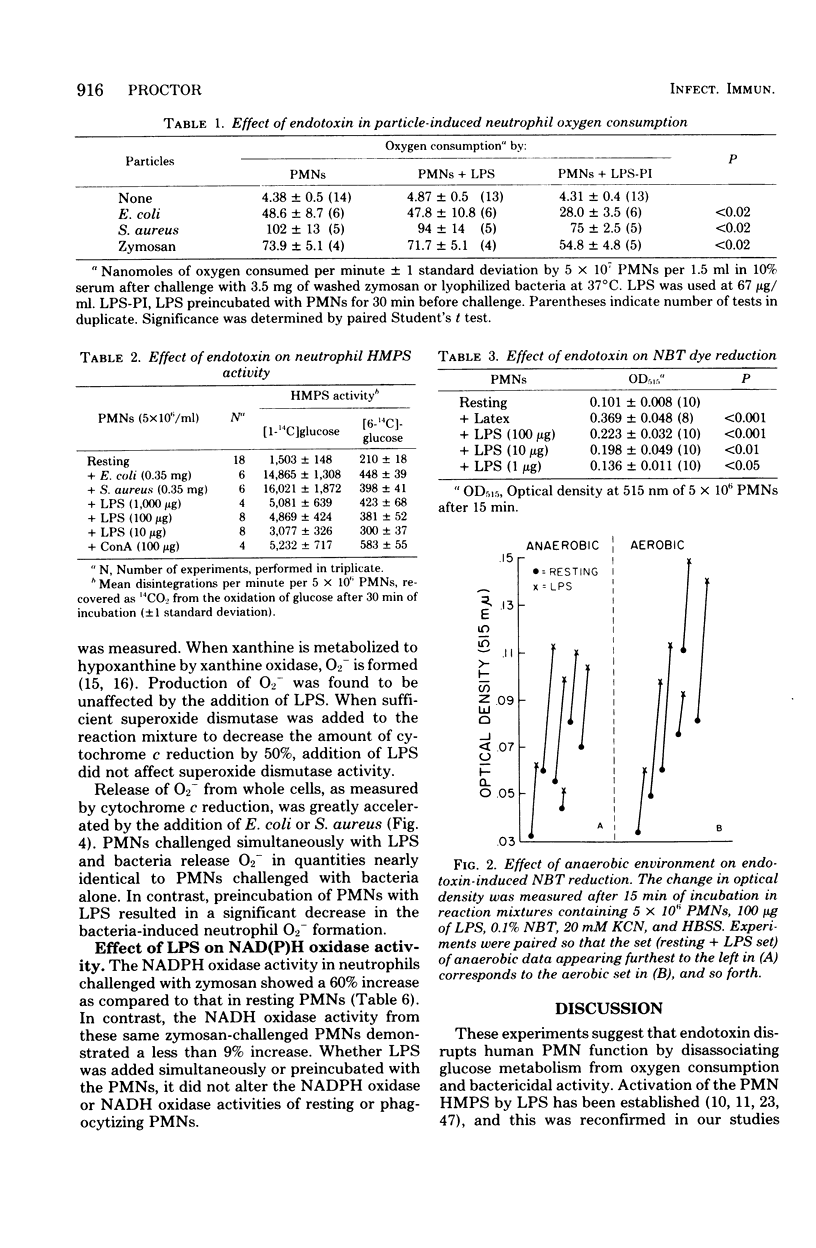
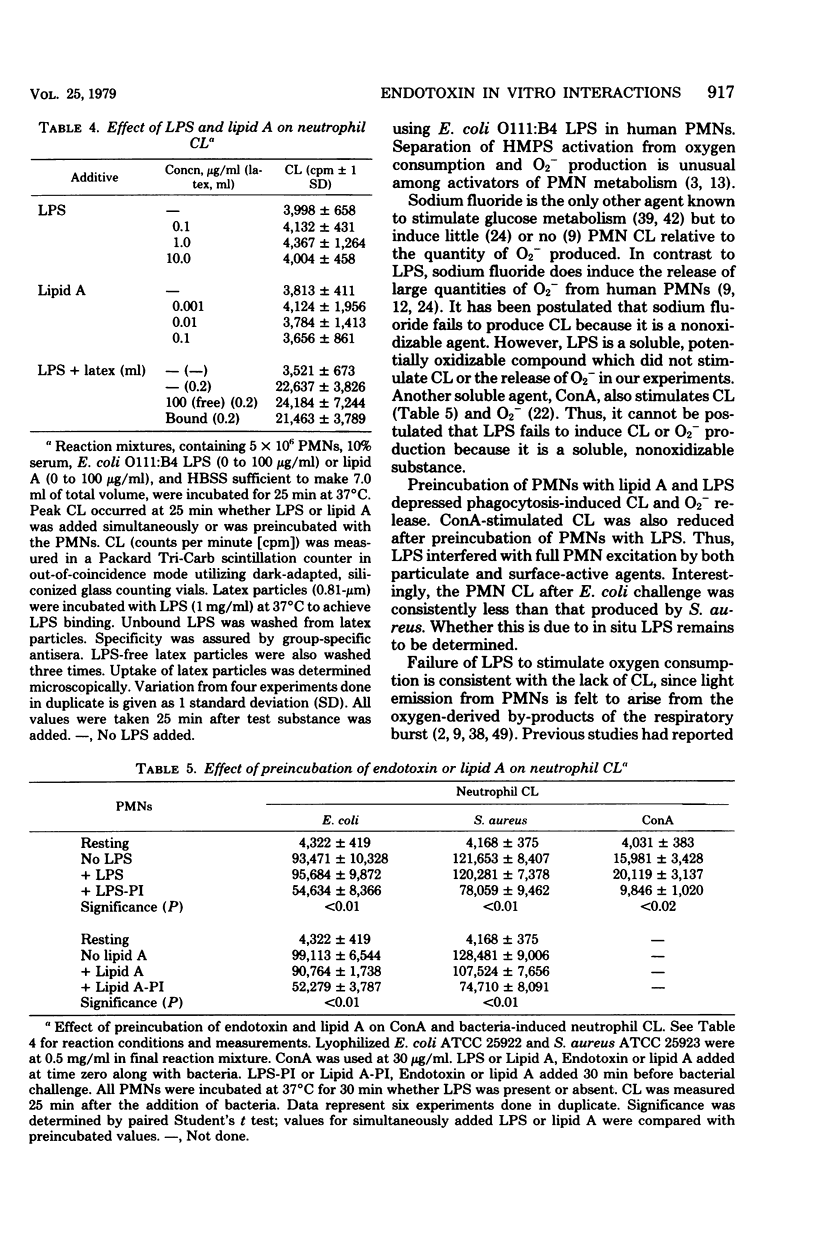
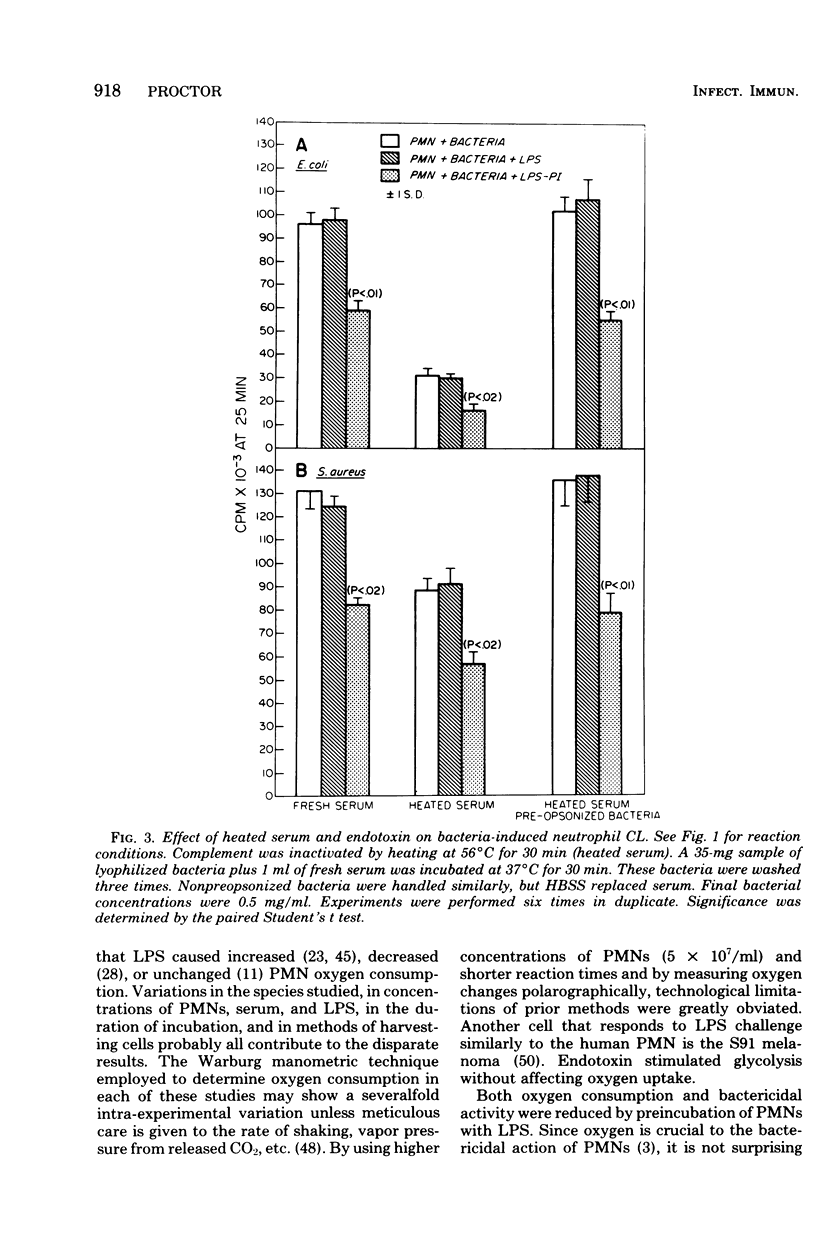
Endotoxin was shown to depress neutrophil bactericidal activity while enhancing Nitro Blue Tetrazolium reduction and hexose monophosphate shunt activity. Separation of bactericidal action from oxidative metabolism suggests that the effect of endotoxin might involve the formation of reactive oxygen radicals such as superoxide. Chemiluminescence often accompanies metabolic activation of polymorphonuclear neutrophils (PMNs). However, human PMNs did not show chemiluminescence when challenged with endotoxin (lipopolysaccharide; LPS) or lipid A. Superoxide formation was also unaffected by endotoxin. In contrast, preincubation of PMNs with LPS for 30 min produced significant depression of chemiluminescence, oxygen consumption, and superoxide formation. Decreased chemiluminescence was not the result of complement consumption. In a cell-free system, superoxide was not scavenged by LPS, nor did LPS stimulate superoxide dismutase. Oxidase enzymes for reduced nicotinamide adenine dinucleotide or reduced nicotinamide adenine dinucleotide phosphate harvested from broken cells were not affected by LPS. The toxicity of LPS may reside in its ability to activate the PMNs while simultaneously blocking bactericidal capacity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Andersen B. R., Brendzel A. M., Lint T. F. Chemiluminescence spectra of human myeloperoxidase and polymorphonuclear leukocytes. Infect Immun. 1977 Jul;17(1):62–66. doi: 10.1128/iai.17.1.62-66.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECK W. S. Occurrence and control of the phosphogluconate oxidation pathway in normal and leukemic leukocytes. J Biol Chem. 1958 May;232(1):271–283. [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978 Mar 30;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Baehner R. L., Boxer L. A., Davis J. The biochemical basis of nitroblue tetrazolium reduction in normal human and chronic granulomatous disease polymorphonuclear leukocytes. Blood. 1976 Aug;48(2):309–313. [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G. Quantitative nitroblue tetrazolium test in chronic granulomatous disease. N Engl J Med. 1968 May 2;278(18):971–976. doi: 10.1056/NEJM196805022781801. [DOI] [PubMed] [Google Scholar]
- Baehner R. L. Subcellular distribution of nitroblue tetrazolium reductase (NBT-R) in human polymorphonuclear leukocytes (PMN). J Lab Clin Med. 1975 Nov;86(5):785–792. [PubMed] [Google Scholar]
- COHN Z. A., MORSE S. I. Functional and metabolic properties of polymorphonuclear leucocytes. II. The influence of a lipopolysaccharide endotoxin. J Exp Med. 1960 May 1;111:689–704. doi: 10.1084/jem.111.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheson B. D., Christensen R. L., Sperling R., Kohler B. E., Babior B. M. The origin of the chemiluminescence of phagocytosing granulocytes. J Clin Invest. 1976 Oct;58(4):789–796. doi: 10.1172/JCI108530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline M. J., Melmon K. L., Davis W. C., Williams H. E. Mechanism of endotoxin interaction with human leucocytes. Br J Haematol. 1968 Dec;15(6):539–547. doi: 10.1111/j.1365-2141.1968.tb01576.x. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Effects of anaerobiosis and inhibitors on O2-production by human granulocytes. Blood. 1975 Jun;45(6):851–861. [PubMed] [Google Scholar]
- DeChatelet L. R. Initiation of the respiratory burst in human polymorphonuclear neutrophils: a critical review. J Reticuloendothel Soc. 1978 Jul;24(1):73–91. [PubMed] [Google Scholar]
- DeChatelet L. R., Shirley P. S., McPhail L. C., Iverson D. B., Doellgast G. J. Allosteric transformation of reduced nicotinamide adenine dinucleotide (phosphate) oxidase induced by phagocytosis in human polymorphonuclear leukocytes. Infect Immun. 1978 May;20(2):398–405. doi: 10.1128/iai.20.2.398-405.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIDOVICH I., HANDLER P. Xanthine oxidase. IV. Participation of iron in internal electron transport. J Biol Chem. 1958 Dec;233(6):1581–1585. [PubMed] [Google Scholar]
- Galanos C., Rietschel E. T., Lüderitz O., Westphal O. Interaction of lipopolysaccharides and lipid A with complement. Eur J Biochem. 1971 Mar 1;19(1):143–152. doi: 10.1111/j.1432-1033.1971.tb01298.x. [DOI] [PubMed] [Google Scholar]
- Goihman-Yahr M., Villalba-Pimentel L., Rodríguez-Ochoa G., Aranzazu N., Convit J., Ocanto A., Elena de Gómez M. Studies on the effect of serum and proteins on in vitro-induced neutrophil activation. J Reticuloendothel Soc. 1978 Jun;23(6):435–446. [PubMed] [Google Scholar]
- Goldstein I. M., Cerqueira M., Lind S., Kaplan H. B. Evidence that the superoxide-generating system of human leukocytes is associated with the cell surface. J Clin Invest. 1977 Feb;59(2):249–254. doi: 10.1172/JCI108635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Wünschmann B., Astrup T., Henderson E. S. Effects of bacterial endotoxin on the fibrinolytic activity of normal human leukocytes. Blood. 1971 Apr;37(4):447–453. [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J., Shafer A. W., Glass E. A., Karnovsky M. L. Metabolic and morphological observations on the effect of surface-active agents of leukocytes. J Cell Biol. 1967 Mar;32(3):629–647. doi: 10.1083/jcb.32.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvath L., Amirault H. J., Andersen B. R. Chemiluminescence of human and canine polymorphonuclear leukocytes in the absence of phagocytosis. J Clin Invest. 1978 May;61(5):1145–1154. doi: 10.1172/JCI109029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson D., DeChatelet L. R., Spitznagel J. K., Wang P. Comparison of NADH and NADPH oxidase activities in granules isolated from human polymorphonuclear leukocytes with a fluorometric assay. J Clin Invest. 1977 Feb;59(2):282–290. doi: 10.1172/JCI108639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos H., Roos D., Weening R., Houwerzijl J. Familial deficiency of glutathione reductase in human blood cells. Blood. 1976 Jul;48(1):53–62. [PubMed] [Google Scholar]
- MARTIN S. P., MCKINNEY G. R., GREEN R. The metabolism of human polymorphonuclear leukocytes. Ann N Y Acad Sci. 1955 Mar 24;59(5):996–1002. doi: 10.1111/j.1749-6632.1955.tb45997.x. [DOI] [PubMed] [Google Scholar]
- Matula G., Paterson P. Y. Spontaneous in vitro reduction of nitroblue tetrazolium by neutrophils of adult patients with bacterial infection. N Engl J Med. 1971 Aug 5;285(6):311–317. doi: 10.1056/NEJM197108052850603. [DOI] [PubMed] [Google Scholar]
- Maxie M. G., Valli V. E. Studies with radioactive endotoxin. III. Localization of 3H-labelled endotoxin in the formed elements of the blood and detection of endotoxin in calf blood with the Limulus amebocyte lysate. Can J Comp Med. 1974 Oct;38(4):383–390. [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Nydegger U. E., Miescher A., Anner R. M., Greighton D. W., Lambert P. H., Miescher P. A. Serum and cellular factor involvement in nitroblue tetrazolium (NBT) reduction by human neutrophils. Klin Wochenschr. 1973 Apr 15;51(8):377–382. doi: 10.1007/BF01468085. [DOI] [PubMed] [Google Scholar]
- Park H. H., Good R. A. N.B.T. test stimulated. Lancet. 1970 Sep 19;2(7673):616–616. doi: 10.1016/s0140-6736(70)90207-2. [DOI] [PubMed] [Google Scholar]
- Proctor R. A., White J. D., Ayala E., Canonico P. G. Phagocytosis of Francisella tularensis by Rhesus monkey peripheral leukocytes. Infect Immun. 1975 Jan;11(1):146–151. doi: 10.1128/iai.11.1.146-151.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quie P. G., White J. G., Holmes B., Good R. A. In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J Clin Invest. 1967 Apr;46(4):668–679. doi: 10.1172/JCI105568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed P. W. Glutathione and the hexose monophosphate shunt in phagocytizing and hydrogen peroxide-treated rat leukocytes. J Biol Chem. 1969 May 10;244(9):2459–2464. [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Chemiluminescence and superoxide production by myeloperoxidase-deficient leukocytes. J Clin Invest. 1976 Jul;58(1):50–60. doi: 10.1172/JCI108458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- Segal A. W., Levi A. J. Cell damage and dye reduction in the quantitative nitroblue tetrazolium (NBT) test. Clin Exp Immunol. 1975 Feb;19(2):309–318. [PMC free article] [PubMed] [Google Scholar]
- Segal A. W., Levi A. J. The mechanism of the entry of dye into neutrophils in the nitroblue tetrazolium (NBT) test. Clin Sci Mol Med. 1973 Dec;45(6):817–826. doi: 10.1042/cs0450817. [DOI] [PubMed] [Google Scholar]
- Selvaraj R. J., Sbarra A. J. Relationship of glycolytic and oxidative metabolism to particle entry and destruction in phagocytosing cells. Nature. 1966 Sep 17;211(5055):1272–1276. doi: 10.1038/2111272a0. [DOI] [PubMed] [Google Scholar]
- Springer G. F., Adye J. C. Endotoxin-binding substances from human leukocytes and platelets. Infect Immun. 1975 Nov;12(5):978–986. doi: 10.1128/iai.12.5.978-986.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsan M. F., Newman B., Chusid M. J., Wolff S. M., McIntyre P. A. Surface sulphydryl groups and hexose monophosphate pathway activity in resting human polymorphonuclear leucocytes. Br J Haematol. 1976 Jun;33(2):205–211. doi: 10.1111/j.1365-2141.1976.tb03531.x. [DOI] [PubMed] [Google Scholar]
- Tsan M., McIntyre P. A. The requirement for membrane sialic acid in the stimulation of superoxide production during phagocytosis by human polymorphonuclear leukocytes. J Exp Med. 1976 Jun 1;143(6):1308–1316. doi: 10.1084/jem.143.6.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODS M. W., LANDY M., WHITBY J. L., BURK D. Symposium on bacterial endotoxins. III. Metabolic effects of endotoxins on mammalian cells. Bacteriol Rev. 1961 Dec;25:447–456. doi: 10.1128/br.25.4.447-456.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb L. S., Keele B. B., Jr, Johnston R. B., Jr Inhibition of phagocytosis-associated chemiluminescence by superoxide dismutase. Infect Immun. 1974 Jun;9(6):1051–1056. doi: 10.1128/iai.9.6.1051-1056.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



