Abstract
We have previously shown that microRNAs (miRNAs) miR-760, miR-186, miR-337-3p, and miR-216b stimulate premature senescence through protein kinase CK2 (CK2) down-regulation in human colon cancer cells. Here, we examined whether these four miRNAs are involved in the replicative senescence of human lung fibroblast IMR-90 cells. miR-760 and miR-186 were significantly upregulated in replicatively senescent IMR-90 cells, and their joint action with both miR-337-3p and miR-216b was necessary for efficient downregulation of the α subunit of CK2 (CK2α) in IMR-90 cells. A mutation in any of the four miRNA-binding sequences within the CK2α 3′-untranslated region (UTR) indicated that all four miRNAs should simultaneously bind to the target sites for CK2α downregulation. The four miRNAs increased senescence-associated β-galactosidase (SA-β-gal) staining, p53 and p21Cip1/WAF1 expression, and reactive oxygen species (ROS) production in proliferating IMR-90 cells. CK2α over-expression almost abolished this event. Taken together, the present results suggest that the upregulation of miR-760 and miR-186 is associated with replicative senescence in human lung fibroblast cells, and their cooperative action with miR-337-3p and miR-216b may induce replicative senescence through CK2α downregulation-dependent ROS generation.
Keywords: miRNA, human lung fibroblast, protein kinase CK2, reactive oxygen species, replicative senescence
INTRODUCTION
Cellular senescence is involved in regulating the aging process and acts as a barrier against cell immortalization and tumorigenesis in vivo. Cellular senescence can be divided into two different types: replicative and premature senescence. Replicative senescence is an irreversible cell-growth arrest state triggered by telomere attrition after a finite number of cell divisions (Goldstein, 1990). An acute form of senescence called premature senescence can be induced by various stimuli including DNA damage, oxidative stress, and aberrant oncogenic activation, without any detectable telomere shortening (Chen et al., 1998; Robles and Adami, 1998; Serrano et al., 1997; Zhu et al., 1998). DNA damage activates p53, which arrests cell proliferation largely through p21CIP1/WAF1. Cellular senescence is characterized by several molecular and phenotypic characteristics, including large, flat cell morphology, appearance of senescence-associated β-galactosidase (SA-β-gal) activity, and the accumulation of p53 and p21Cip1/WAF1 (Bayreuther et al., 1998; Brown et al., 1997; Dimri et al., 1995).
CK2 downregulation also induces premature senescence in both normal lung fibroblast IMR-90 cells and colon cancer HCT116 cells (Kang et al., 2009; Ryu et al., 2006). Reactive oxygen species (ROS) play an important role in CK2 inhibition-mediated senescence (CIMS). ROS levels increase in CIMS, and ROS elimination prevents CIMS. p53 and p21Cip1/WAF1 are downstream effectors of ROS that induce CIMS (Jeon et al., 2010). Coumestrol, tamoxifen, and four microRNAs (miRNAs), including miR-760, miR-186, miR-337-3p, and miR-216b, promote CIMS through ROS-p53 axis in colon cancer and breast cancer cells (Kim et al., 2012; Lee et al., 2013; 2014). Histone deacetylase SIRT1 and the phosphatidylinositol 3-kinase (PI3K)-AKT-mammalian target of rapamycin (mTOR) pathway are involved in CIMS (Jang et al., 2011; Park et al., 2013). Despite steady progress in probing the roles of signaling molecules in CIMS, little is known about the mechanisms in which CK2 is downregulated during replicative senescence.
miRNAs are a class of small non-coding RNAs ranging in size from 19 to 22 nucleotides. Although miRNAs do not code for proteins, they regulate gene expression at the post-transcriptional level. The binding of miRNA to the 3′-untranslated region (UTR) of target mRNAs promotes targeted-mRNA degradation or translational suppression, depending on the degree of complementarity between the two sequences (Garzon and Calin, 2009; Kim et al., 2009). Growing evidence suggests that miRNAs are associated with essential biological processes, including proliferation, differentiation, development, apoptosis, cancer, and senescence (Ambros, 2004; Bartel, 2009; Choi and Kemper, 2013; Jung and Suh, 2012). To our knowledge, this is the first time it has been shown that miR-760 and miR-186 are upregulated during replicative senescence in human lung fibroblast cells, and the concerted action of miR-760, miR-186, miR-337-3p, and miR-216b is crucial to achieve both downregulation of CK2α and replicative senescence.
MATERIALS AND METHODS
Cell culture
Human diploid fibroblast IMR-90 cells were obtained from ATCC (USA) at a population doubling level (PDL) of 24. IMR-90 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% (v/v) fetal bovine serum under a humidified atmosphere of 5% (v/v) CO2 at 37°C. The PDL of IMR-90 cells was calculated by the formula PD = log(Nf/Ni)/log2, where Nf is the final cell number and Ni is the initial number of seeded cells.
RNA extraction and miRNA real-time, quantitative PCR
RNAs were extracted from young IMR-90 cells (PDL 33) and senescent IMR-90 cells (PDL 55) using TRIzol reagent (Invitrogen, USA). miRNA real-time, quantitative PCR (qPCR) was performed using a TaqMan miRNA reverse transcription (RT) kit and by miRNA assay according to the manufacturer’s instructions with ABI PRISM 7000 HT (Applied Biosystems, USA). The U48 small nucleolar RNA (RNU48) was used as the housekeeping small RNA reference gene. Real-time PCRs were run in triplicate for three different cDNAs.
SA-β-gal activity assay
SA-β-gal activity was measured as described previously (Dimri et al., 1995) with minor modifications. Cells in subconfluent cultures were washed with PBS, fixed in 3% (v/v) formaldehyde in PBS for 10 min at room temperature, and then incubated with a stain solution containing 1 mg/ml of 5-bromo-4-chloro-3-indolyl-β-d-galactoside, 40 mM citric acid-sodium phosphate (pH 6.0), 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 150 mM NaCl, and 2 mM MgCl2 for 24 h at 37°C. Blue-stained cells were counted in at least 10 fields at 400× magnification, and the counts were expressed as the percentage of positive cells.
Western blotting
Cells in 60-mm dishes were washed with ice-cold PBS, collected by scraping with a rubber policeman, and lysed in 100 μl of ice-cold RIPA buffer [50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 0.5 mM PMSF, 1 μg/ml of aprotinin, 1 μg/ml of leupeptin, 1 μg/ml of pepstatin]. Western blotting was performed as described previously (Lee et al., 2013). Antibodies specific to CK2α, p53, p21Cip1/WAF1, and β-actin were obtained from Santa Cruz Biotechnology (USA), and anti-HA antibody was obtained from Roche (Switzerland). Anti-p53 phospho-serine 392 antibody was from Cell Signaling Technology (USA).
RT-PCR
Total RNA was extracted from HCT116 cells. RNA was reverse-transcribed using gene-specific reverse primers and reverse transcriptase (Takara, Japan), and the resulting cDNAs were PCR-amplified. PCR primer sequences for CK2α were CK2αFwd (5′-GACAAGCTTATGTCGGGACCC-3′) and CK2α Rev (5′-GACAAGCTTTTACTGCTGAGC-3′). The PCR primer sequences used for p53 were p53Fwd (5′-CCTCACCATCA-TCACACTGG-3′) and p53Rev (5′-CCTCATTCAGCTCTCGG-AAC-3′). The PCR primer sequences used for p21Cip1/WAF1 were p21Fwd (5′-GTGAGCGATGGAACTTCGACT-3′) and p21Rev (5′-CGAGGCACAAGGGTACAAGAC-3′). Primers specific to β-actin RNA were used to standardize the amount of RNA in each sample. PCR products were resolved on 1.5% agarose gel. Quantification of RT-PCR bands was performed using densitometry.
Generation of mutant luciferase constructs and luciferase assay
Human CK2α 3′-UTR was cloned into the XhoI/NotI sites of the psiCHECK-2 vector (Kim et al., 2012). Mutant vectors were generated using designed mutagenic oligonucleotide primers from the QuikChange II XL site-directed mutagenesis kit (Stratagene, USA). Each miRNA-binding site mutation was carried out by one complete procedure of mutant synthesis. For the luciferase assay, 1 × 105 cells were transfected along with the CK2α 3′-UTR reporter and four miRNA mimics in a 24-well plate using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s protocol. After 24 h, firefly and Renilla luciferase activities were measured consecutively using Dual Luciferase Assay (Promega, Korea).
Measurement of intracellular ROS
Intercellular ROS level was determined using oxidation-sensitive fluorescent probes CM-H2DCFDA and dihydroethidium (DHE) as described previously (Jeon et al., 2010).
Statistical analysis
Statistical significance of the data was analyzed by one-way ANOVA with SPSS package program (SPSS Inc., USA). The results were considered significant if the P value was less than 0.05. Duncan’s multiple-range test was also performed to test if the differences between the groups were identified at α = 0.05.
RESULTS
miR-760 and miR-186 are upregulated during replicative senescence in lung fibroblast IMR-90 cells
Previously, we demonstrated that mimics of miR-760, miR-186, miR-337-3p, and miR-216b together downregulated CK2α expression and prompted premature senescence in human colon cancer cells (Kim et al., 2012). To determine how the expression patterns of these miRNAs are affected by replicative senescence, we repeatedly passed lung fibroblast IMR-90 cells until a senescence-like state was observed. Most cells at PDL 55 stained positive for SA-β-gal, whereas only a few stained positive for SA-β-gal in early passage (PDL 33) cells (Fig. 1A). Western blot analysis revealed that the level of CK2α protein decreased in senescent cells (Fig. 1B), which corroborates previous results (Ryu et al., 2006). The protein amounts of p53 and p21Cip1/WAF1 increased in senescent cells. We validated the four miRNAs in cells using real-time qPCR. In comparison with proliferating IMR-90 cells (PDL33), miR-760 and miR-186 in senescent IMR-90 cells (PDL 55) increased by 180% and 240%, respectively (Fig. 1C). miR-216b and miR-337-3p have been previously shown to be present at increased levels in senescent WI-38 human diploid fibroblast cells and in human peripheral blood mononuclear cells, respectively (Marasa et al., 2010; Noren Hooten et al., 2010). However, miR-337-3p expression did not increase in senescent IMR-90 cells (Fig. 1C). miR-216b was not detected in IMR-90 cells under our experimental conditions.
Fig. 1.
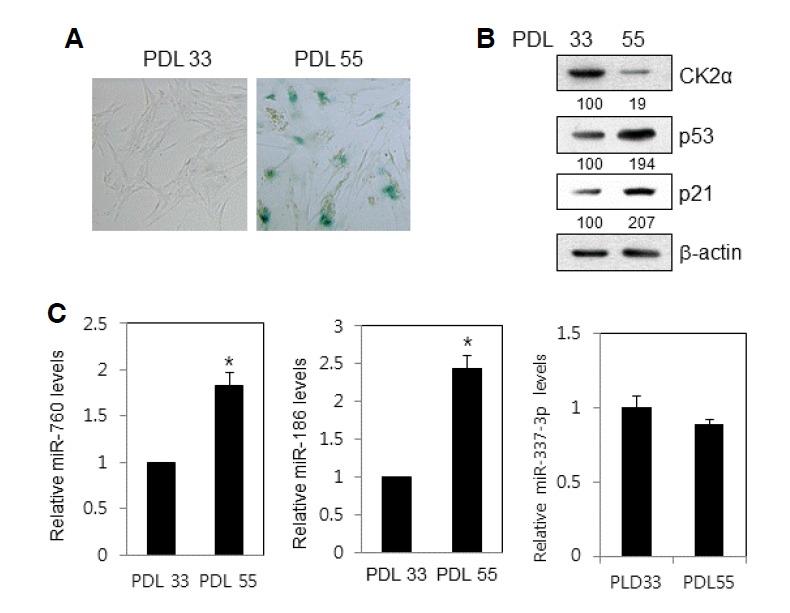
Upregulation of miR-760 and miR-186 expression in replicatively senescent IMR-90 cells. (A) After fixation in 2% formaldehyde/0.2% glutaraldehyde in PBS, IMR-90 cells of PDL 33 and PDL 55 were stained with 1 mg/ml of 5-bromo-4-chloro-3-indolyl-β-d-galactoside. Representative images were obtained at 20× magnification. (B) Cell lysates were electrophoresed on a 12% (w/v) SDS-polyacrylamide gel and visualized by Western blotting with appropriate antibodies. Quantification of each band was carried out by a densitometer analysis. The β-actin was used as a control. (C) Total RNA was isolated from IMR-90 cells of PDL 33 and PDL 55 and subjected to analysis by qPCR analysis to determine the relative levels of miR-760, miR-186, miR-337-3p, and miR-216b, using RNU48 for normalization. Data represent standard error of the mean from three independent experiments. Data are shown as the means ± SEM. *P < 0.05.
miR-760, miR-186, miR-337-3p, and miR-216b jointly down-regulate CK2α expression in lung fibroblast IMR-90 cells
To test the possible role of miR-760 and miR-186 in the regulation of CK2α, proliferating IMR-90 cells (PDL 33) were transfected with mimics of both miRNAs. Western blot analysis revealed that transfection with both mimics had a negligible effect on the CK2α protein levels in IMR-90 cells. Furthermore, three mimics of miR-760, miR-186, and miR-337-3p had no significant effects on the protein amount of CK2α. However, quantification by densitometry analysis revealed that transfection with all four of the miRNA mimics decreased the CK2α protein amount by 60% in IMR-90 cells compared with that of the samples transfected with control miRNA (Fig. 2A). To confirm the inhibitory effects of the four miRs on CK2α expression, proliferating IMR-90 cells were transfected with antisense inhibitors of these miRNAs. None of the possible combinations of the three miRNA inhibitors increased the protein level of CK2α (data not shown). Simultaneous knockdown of the four miRNAs by antisense inhibitors increased the CK2α protein amount up to 2.4-fold in IMR-90 cells. These data suggest that the four miRNAs are generated, which suppress CK2α expression in proliferating lung fibroblast cells, and that the antisense inhibitors function as effective repressors for these miRNAs (Fig. 2B).
Fig. 2.
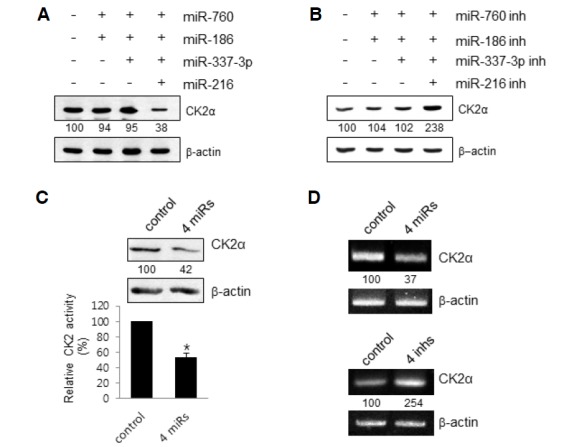
Effect of miR-760, miR-186, miR-337-3p and miR-216b on CK2α expression in proliferating IMR-90 cells. (A) IMR-90 cells (PDL 33) were transfected with control miRNA or different combinations of mimics the four miRNAs. After 2 days, effects of these mimics on the protein level of CK2α were determined by Western blot analysis. Quantification of each band was carried out by a densitometer analysis. The β-actin was used as a control. (B) IMR-90 cells (PDL 33) were transfected with different combinations of antisense inhibitors (inh) the four miRNAs. After 2 days, effects of different combinations of these mimics on the protein level of CK2α were determined by Western blot analysis. (C) IMR-90 cells (PDL 33) were transfected with control miRNA or the four mimics (4 miRs) for 2 days. Cell lysates were utilized in kinase assays using specific CK2 substrate peptides. 32P incorporation into the substrate peptide was measured by scintillation counting. Data are shown as the means ± SEM. *P < 0.05. (D) IMR-90 cells (PDL 33) were transfected with the four mimics (upper panel) or four inhibitors (bottom panel) for two days. Total RNA was extracted from cells and reverse-transcribed using CK2α-specific primers and reverse transcriptase. Primers for β-actin RNA were used as a control. PCR products were resolved on a 1.5% (w/v) agarose gel.
When CK2 activity was assessed using a CK2 peptide substrate, extracts from the IMR-90 cells transfected with the four mimics comprised 40% less CK2 activity compared to the control extract (Fig. 2C). RT-PCR analysis revealed that cotransfection of IMR-90 cells with the four mimics diminished the level of CK2α mRNA by 60%, whereas co-transfection with the four miRNA inhibitors raised the CK2α mRNA amount up to 2.5-fold (Fig. 2D). Taken together, these results suggest that the four miRNAs cooperatively act to suppress CK2α expression by stimulating degradation of CK2α mRNA in proliferating lung fibroblast cells.
Simultaneous binding of miR-760, miR-186, miR-337-3p, and miR-216b to CK2α 3′-UTR is necessary for suppression of CK2α expression in lung fibroblast IMR-90 cells
To determine the specificity between miR-760, miR-186, miR-337-3p, and miR-216b and target sites within CK2α 3′-UTR, a luciferase reporter vector was mutated at each target element, the “seed” match region, in the CK2α 3′-UTR (Figs. 3A and 3B). Transfection of IMR-90 cells (PDL 33) with the four miRNA mimics decreased the activity of the wild-type luciferase reporter by 60% compared with samples transfected with control miRNA. However, the four mimics could not suppress the luciferase activity of any mutant reporters (Fig. 3C). Therefore, these results clearly indicated that all four miRNAs must simultaneously bind to the CK2α 3′-UTR to repress CK2α expression in lung fibroblast cells.
Fig. 3.
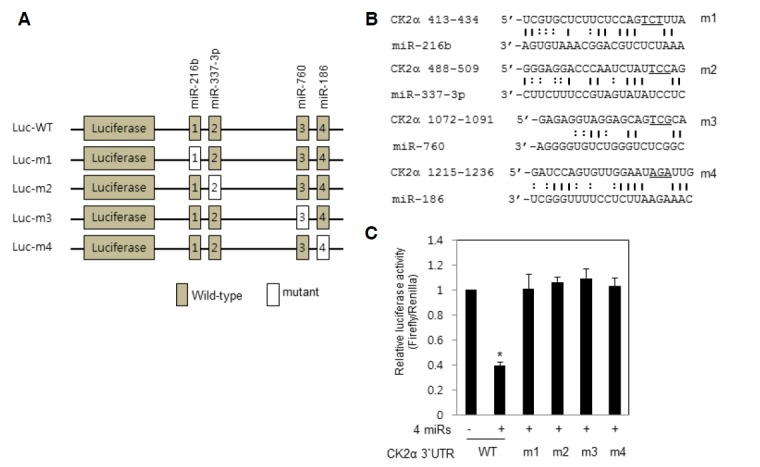
Simultaneous binding of miR-760, miR-186, miR-337-3p and miR-216b to CK2α 3′-UTR is necessary for suppression of CK2α expression in IMR-90 cells. (A) The luciferase reporter vector (pGL3) containing wild-type (WT) or mutant 3′-UTR of the CK2α. Individual miR-binding sites were mutated in the CK2α 3′-UTR vector. (B) Mutant vectors (m1, m2, m3, and m4) were generated using designed mutagenic oligonucleotide primers. Mutations in the core sequences are underlined. Watson-Crick and wobble base (G-U) pairings are indicated by solid and dashed vertical lines, respectively. (C) Luciferase reporter vectors, either WT or mutant (m1, m2, m3, and m4) were co-transfected with either control miRNAs or all four miRNA mimics into IMR-90 cells (PDL 33) at a final concentration of 100 nM. Luciferase activity was measured 24 h after transfection and normalized to Renilla. Data are shown as the means ± SEM. *P < 0.05.
miR-760, miR-186, miR-337-3p, and miR-216b accelerate premature senescence in proliferating IMR-90 cells
To examine the role of the four miRNAs in cellular senescence in lung fibroblast cells, we knocked down CK2α in proliferating IMR-90 cells (PDL 33) by co-treatment with the four miRNA mimics. To assess the effect of miRNA knockdown on senescence, after transfection for two days, the transfectants were then stained for SA-β-gal activity. IMR-90 cells (PDL 33) transfected with the four mimics displayed a higher rate of SA-β-gal staining compared with control cells (Fig. 4A). Western blot data showed that the expression amounts of p53 and p21Cip1/WAF1 increased in proliferating IMR-90 cells treated with the four miRNA mimics in comparison with the control cells. Quantification by densitometry analysis revealed that the protein level of p53 and p21Cip1/WAF1 increased by 170% and 280%, respectively (Fig. 4B). Since it has been reported that CK2 phosphorylates serine 392 on p53 (Meek et al., 1990), we investigated whether the four miRNAs regulated the phosphorylation state of serine 392. Immunoblotting analysis using a serine 392 phosphorylation-specific antibody revealed that CK2-mediated p53 phosphorylation decreased regardless of p53 upregulation (Fig. 4B). Cells treated with the four miRNA mimics showed an increase in the mRNA levels of p21Cip1/WAF1, but not in p53, suggesting that CK2 inhibition upregulated p21Cip1/WAF1 expression at the transcriptional level (Fig. 4C). To examine whether the four miRNAs stimulate ROS generation in proliferating IMR-90 cells, cells were incubated with CM-H2DCFDA or DHE. Transfection with the four mimics considerably increased ROS levels in proliferating IMR-90 cells (Fig. 4D). Co-treatment with miR-760 and miR-186 did not show any senescence markers, including SA-β-gal staining, in proliferating IMR-90 cells (Fig. 4E). Thus, these data demonstrate that the four miRNAs promote premature senescence via ROS generation and p53 stabilization in proliferating IMR-90 cells.
Fig. 4.
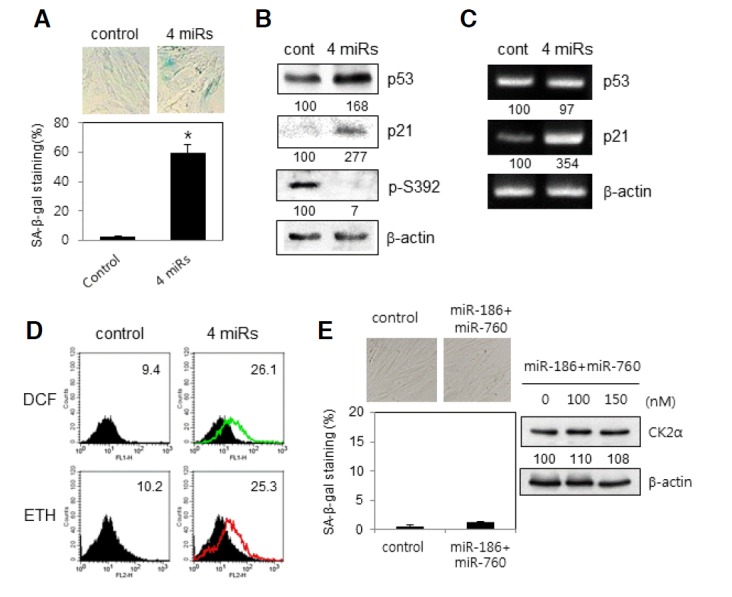
Induction of premature senescence by miR-760, miR-186, miR-337-3p and miR-216b in proliferating IMR-90 cells. IMR-90 cells (PDL 33) were transfected with the four mimics (A–D) or two mimics of miR-760 and miR-186 (E) for 2 days. (A) Cells were stained with 5-bromo-4-chloro-3-indolyl-β-d-galactoside, and representative images were obtained at 20× magnification (upper panel). The percentage of positively stained cells was measured (bottom panel). Data are shown as the means ± SEM. *P < 0.05. (B) Cell lysates were electrophoresed on a 12% (w/v) SDS-polyacrylamide gel and visualized by Western blotting with appropriate antibodies. Quantification of each band was carried out by a densitometer analysis. (C) Total RNA was extracted from cells and reverse transcribed using p21 and p53-specific primers. PCR products were resolved on a 1.5% (w/v) agarose gel. (D) Cells were incubated with CM-H2DCFDA or DHE as described in “Materials and Methods.” Fluorescence intensity was determined by flow cytometry analysis. The mean fluorescence intensity is shown in the upper right corner of the histograms. (E) Cells were stained with 5-bromo-4-chloro-3-indolyl-β-d-galactoside, and representative images were obtained at 20× magnification (left upper panel). The percentage of positively stained cells was measured (left bottom panel). Cell lysates were electrophoresed on a SDS-polyacrylamide gel and visualized by Western blotting (right panel).
CK2α overexpression restrains miR-760, miR-186, miR-337-3p, and miR-216b-induced premature senescence in lung fibroblast cells
We explored if CK2 activation suppresses the senescence induced by the four miRNAs in proliferating fibroblast cells. For this, IMR-90 cells (PDL 33) were transfected with the four miRNA mimics in the presence of pcDNA-HA-CK2α. As shown in Fig. 5A, co-transfection of cells with CK2α suppressed an increase in SA-β-gal activity in the cells treated with the four mimics. Further, co-treatment of cells with CK2α repressed the upregulation of p53 and p21Cip1/WAF1 as well as ROS generation activity in the cells treated with the four mimics (Figs. 5B and 5C). Therefore, these data intensely suggest that the four miRNAs stimulate these events, including senescence, ROS generation, and p53 accumulation, through CK2α downregulation in proliferating IMR-90 cells.
Fig. 5.
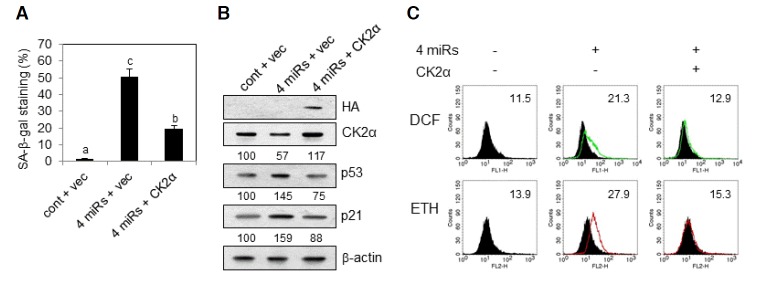
Effect of CK2α overexpression on miR-760, miR-186, miR-337-3p and miR-216b-induced premature senescence in lung fibroblast cells. IMR-90 cells (PDL 33) were co-transfected with control miRNA (cont) or the four mimics (4 miRs) in the presence of empty vector (vec) or pcDNA-HA-CK2α for 2 days. (A) Cells were stained with 5-bromo-4-chloro-3-indolyl-β-d-galactoside, and the percentage of positively stained cells was measured. Data are shown as the means ± SEM. Bars that do not share a common letter (a, b, and c) are significantly different among groups at P < 0.05. (B) Cell lysates were electrophoresed on a SDS-polyacrylamide gel and visualized by Western blotting. Quantification of each band was carried out by a densitometer analysis. (C) Cells were incubated with CM-H2DCFDA or DHE as described in “Materials and Methods.” Fluorescence intensity was determined by flow cytometry analysis. The mean fluorescence intensity is shown in the upper right corner of the histograms.
Antisense inhibitors against miR-760, miR-186, miR-337-3p, and miR-216b do not rejuvenate replicatively senescent IMR-90 cells
We examined whether antisense inhibitors of the four miRNAs rejuvenate replicatively senescent IMR-90 cells (PDL 53). The basal level of SA-β-gal activity was too low to be detected in proliferating IMR-90 cells (Fig. 6A). Treatment with the four anti-sense inhibitors apparently reduced ROS amount (by 30%) as well as the expression of p53 (by 57%) and p21Cip1/WAF1 (by 90%) proteins in proliferating cells (Figs. 6B and 6C). This had relevance to the increased protein and mRNA amounts of CK2α by the four inhibitors (Fig. 6C). However, treatment with the four antisense inhibitors did not have an influence on SA-β-gal activity, ROS production, and expression levels of CK2α, p53, and p21Cip1/WAF1 in replicatively senescent IMR-90 cells (Figs. 6A–6C). These results suggest that the antisense inhibitors are unable to inactivate these four miRNAs in replicatively senescent cells.
Fig. 6.
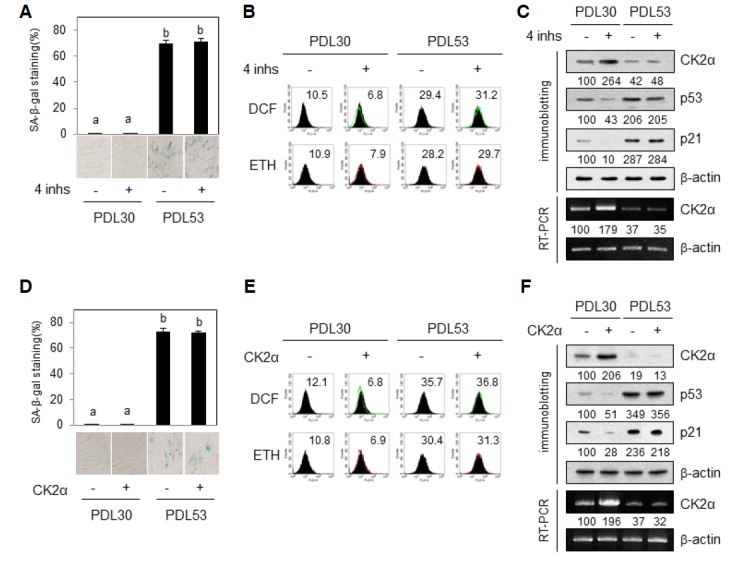
Effect of antisense inhibitors for miR-760, miR-186, miR-337-3p and miR-216b on replicatively senescent IMR-90 cells. Proliferating (PDL 30) or replicatively senescent (PDL 53) IMR-90 cells were transfected with four miRNAs antisense inhibitors (inhs) (A–C) or pcDNA-HA-CK2α (D–F) for 2 days. (A, D) Cells were stained with 5-bromo-4-chloro-3-indolyl-β-d-galactoside, and the percentage of positively stained cells was measured. Data are shown as the means ± SEM. Groups with different letters are significantly different from one another (p < 0.05). Groups with the same letter are not significantly different. (B, E) Cells were incubated with CM-H2DCFDA or DHE as described in “Materials and Methods”. Fluorescence intensity was determined by flow cytometry analysis. The mean fluorescence intensity is shown in the upper right corner of the histograms. (C, F) Cell lysates were electrophoresed on a SDS-polyacrylamide gel and visualized by Western blotting (upper panels). Total RNA was extracted from cells and reverse-transcribed using CK2α-specific primers. Primers for β-actin RNA were used as a control. PCR products were resolved on a 1.5% (w/v) agarose gel (bottom panels).
To investigate the reason why the antisense inhibitors do not stimulate expression of CK2α, we examined the effect of CK2α transfection on replicatively senescent cells. Although the basal level of SA-β-gal activity was too low to be detected in proliferating cells, transfection with pcDNA-HA-CK2α reduced ROS amount (by 40%) and expression of p53 and p21Cip1/WAF1 proteins (by 50% and 70%, respectively) in proliferating cells (Figs. 6D–6F). However, transfection with pcDNA-HA-CK2α did not decrease SA-β-gal activity, ROS production, and the protein levels of p53 and p21Cip1/WAF1 in replicatively senescent IMR-90 cells (Figs. 6D–6F). Transfection with pcDNA-HA-CK2α did not increase the protein and mRNA levels of CK2α in senescent cells (Fig. 6F). Taken together, these results suggest that transfection of replicatively senescent cells and/or expression of the exogenous CK2α gene in replicatively senescent IMR-90 cells is extremely inefficient.
DISCUSSION
It has been suggested that miRNAs regulate diverse biological processes, such as development, differentiation, apoptosis, proliferation, and senescence, by controlling gene expression, although little is known about how miRNAs contribute to human aging. We recently reported that miR-760, miR-186, miR-337-3p, and miR-216b function as novel stimulators of premature senescence in cancer cells through the negative regulation of CK2α expression (Kim et al., 2012). To our knowledge, this paper is the first to show that replicative senescence can also be mediated by these four miRNAs. In the present study, we found that miR-760 and miR-186 were indeed upregulated in replicatively senescent lung fibroblast IMR-90 cells. This was accompanied by a decrease in CK2α expression during replicative senescence in IMR-90 cells (Fig. 1). Other groups have reported that miR-337-3p and miR-216b increase during senescence (Marasa et al., 2010; Noren Hooten et al., 2010). In this study, however, miR-337-3p expression did not increase in senescent IMR-90 cells, and miR-216b was not detected by our experimental conditions.
Nevertheless, we believe that miR-337-3p and miR-216b are involved in downregulating CK2α in lung fibroblast cells for the following reasons. First, different combinations of two or three miRNA mimics or antisense inhibitors had no significant effects on the protein level of CK2α in proliferating IMR-90 cells. All four miRNA mimics and all four antisense inhibitors were necessary for downregulation and upregulation of the CK2α protein, respectively (Figs. 2A and 2B). Second, mutation in any of the four miRNA-binding sequences within the CK2α 3′-UTR apparently reduced the ability of all four miRNAs to inhibit luciferase activity, indicating that all four miRNAs should bind to the target sequences at the same time for efficient CK2 downregulation in lung fibroblast cells (Fig. 3). Third, only the mixture of the four miRNAs, but not different combinations of three miRNA mimics, induced prematurely senescent phenotypes in proliferating IMR 90 cells (Fig. 4). Finally, the four miRNA mimics or four inhibitors were required for the regulation of CK2α mRNA stability (Fig. 2D). It is well established that a single mRNA molecule can be targeted by multiple miRNAs, and studies focusing on the combinatorial actions of miRNAs have begun to emerge (Mavrakis et al., 2011). These results suggest that CK2α gene expression can be controlled by a miRNA network including miR-760, miR-186, miR-337-3p, and miR-216b in lung fibroblasts.
p53 is a key player in tumor suppression, as it regulates cell cycle arrest, apoptosis, and cellular senescence. The critical role of p53 in the prevention of tumor development is suggested to be the result of a p53 mutation in approximately 50% of human cancer incidences. Thus, understanding the mechanism of p53 regulation is very important for cancer therapy (Rivlin et al., 2011). p53 is normally maintained at low levels in the absence of stress. This is primarily due to continuous ubiquitination by ubiquitin E3 ligase Mdm2 and subsequent degradation. The stabilization of p53 is largely mediated by posttranslational modification. Recently, it has been reported that miRNAs also stabilizes p53. For example, miR-192, miR-194, and miR-215 result in Mdm2 downregulation and p53 accumulation in multiple myeloma (Pichiorri et al., 2010). In the present study, we provide a possible pathway for p53 stabilization in human lung fibroblast cells: miR-760, miR-186, miR-337-3p, and miR-216b may accumulate p53 protein indirectly through negative regulation of CK2α during replicative senescence (Fig. 4).
CK2 is a second messenger-independent serine/threonine kinase. The holoenzyme of CK2 is a heterotetramer composed of two catalytic (α) and two regulatory (β) subunits. CK2 has high expression in various tumors, including leukemia and breast, colon, lung, ovarian, and pancreatic tumors (Duncan and Litchfield, 2008; Ruzzene and Pinna, 2010). Therefore, in many cases, the dysregulation of CK2 expression serves as a prognostic indicator of cancer. On the other hand, cellular senescence is an important anti-cancer defense. However, the exact mechanisms underlying the initiation and maintenance of senescence are still obscure. In the present study, we found that the four miRNAs promoted ROS production and senescence in lung fibroblast cells, and CK2α overexpression antagonized this event (Figs. 4 and 5). Therefore, we conclude that the four miRNAs promote cellular senescence through the ROS-p53-p21Cip1/WAF1 pathway in a CK2α downregulation-dependent manner in lung fibroblast cells. Aging is characterized by accelerated cellular senescence and progressive dysfunction of organs including the lungs. A more complete understanding of CK2 regulation mechanisms in lung fibroblast cells will provide new therapeutic options for the restoration of lung function in the elderly.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2010-0024102).
REFERENCES
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayreuther K., Rodemann H.P., Hommel R., Dittmann K., Albiez M., Francz P.I. Human skin fibroblasts in vitro differentiate along a terminal cell lineage. Proc. Natl. Acad. Sci. USA. 1998;85:5112–5116. doi: 10.1073/pnas.85.14.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.P., Wei W., Sedivy J.M. Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science. 1997;277:831–834. doi: 10.1126/science.277.5327.831. [DOI] [PubMed] [Google Scholar]
- Chen Q.M., Bartholomew J.C., Campisi J., Acosta M., Reagan J.D., Ames B.N. Molecular analysis of H2O2-induced senescent-like growth arrest in normal human fibroblasts: p53 and Rb control G1 arrest but not cell replication. Biochem. J. 1998;332:43–50. doi: 10.1042/bj3320043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S.E., Kemper J.K. Regulation of SIRT1 by micro-RNAs. Mol. Cells. 2013;36:385–392. doi: 10.1007/s10059-013-0297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri G.P., Lee X., Basile G., Acosta M., Scott G., Roskelley C., Medrano E.E., Linskens M., Rubelj I., Pereira-Smith O., et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J.S., Litchfield D.W. Too much of a good thing: the role of protein kinase CK2 in tumorigenesis and prospects for therapeutic inhibition of CK2. Biochim. Biophys. Acta. 2008;1784:33–47. doi: 10.1016/j.bbapap.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Garzon R., Calin G.A. Croce CM. MicroRNAs in cancer. Annu. Rev. Med. 2009;60:167–179. doi: 10.1146/annurev.med.59.053006.104707. [DOI] [PubMed] [Google Scholar]
- Goldstein S. Replicative senescence: the human fibroblast comes of age. Science. 1990;249:1129–1133. doi: 10.1126/science.2204114. [DOI] [PubMed] [Google Scholar]
- Jang S.Y., Kim S.Y., Bae Y.S. p53 deacetylation by SIRT1 decreases during protein kinase CK2 downregulation-mediated cellular senescence. FEBS Lett. 2011;585:3360–3366. doi: 10.1016/j.febslet.2011.09.027. [DOI] [PubMed] [Google Scholar]
- Jeon S.M., Lee S.J., Kwon T.K., Kim K.J., Bae Y.S. NADPH oxidase is involved in protein kinase CK2 downregulation-mediated senescence through elevation of the level of reactive oxygen species in human colon cancer cells. FEBS Lett. 2010;584:3137–3142. doi: 10.1016/j.febslet.2010.05.054. [DOI] [PubMed] [Google Scholar]
- Jung H.J., Suh Y. MicroRNA in aging: from discovery to biology. Curr. Genomics. 2012;13:548–557. doi: 10.2174/138920212803251436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J.Y., Kim J.J., Jang S.Y., Bae Y.S. The p53-p21Cip1/WAF1 pathway is necessary for cellular senescence induced by the inhibition of protein kinase CK2 in human colon cancer cells. Mol. Cells. 2009;28:489–494. doi: 10.1007/s10059-009-0141-9. [DOI] [PubMed] [Google Scholar]
- Kim V.N, Han J., Siomi M.C. Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell Biol. 2009;10:126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- Kim S.Y., Lee Y.H., Bae Y.S. miR-186, miR-216b, miR-337-3p, and miR-760 cooperatively induce cellular senescence by targeting α subunit of protein kinase CKII in human colorectal cancer cells. Biochem. Biophys. Res. Commun. 2012;429:173–179. doi: 10.1016/j.bbrc.2012.10.117. [DOI] [PubMed] [Google Scholar]
- Lee Y.H., Yuk H.J., Park K.H., Bae Y.S. Coumestrol induces senescence through protein kinase CKII inhibition-mediated reactive oxygen species production in human breast cancer and colon cancer cells. Food Chem. 2013;141:381–388. doi: 10.1016/j.foodchem.2013.03.053. [DOI] [PubMed] [Google Scholar]
- Lee Y.H., Kang B.S., Bae Y.S. Premature senescence in human breast cancer and colon cancer cells by tamoxifen-mediated reactive oxygen species generation. Life Sci. 2014;97:116–122. doi: 10.1016/j.lfs.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Marasa B.S., Srikantan S., Martindale J.L., Kim M.M., Lee E.K., Gorospe M., Abdelmohsen K. MicroRNA profiling in human diploid fibroblasts uncovers miR-519 role in replicative senescence. Aging. 2010;2:333–343. doi: 10.18632/aging.100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrakis K.J., Van Der Meulen J., Wolfe A.L., Liu X., Mets E., Taghon T., Khan A.A., Setty M., Rondou P., Vandenberghe P., et al. A cooperative microRNA-tumor suppressor gene network in acute T-cell lymphoblastic leukemia (T-ALL) Nat. Genet. 2011;43:673–678. doi: 10.1038/ng.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek D.W., Simon S., Kikkawa U., Eckhart W. The p53 tumour suppressor protein is phosphorylated at serine 389 by casein kinase II. EMBO J. 1990;9:3253–3260. doi: 10.1002/j.1460-2075.1990.tb07524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren Hooten N., Abdelmohsen K., Gorospe M., Ejiogu N., Zonderman A.B., Evans M.K. microRNA expression patterns reveal differential expression of target genes with age. PLoS One. 2010;5:e10724. doi: 10.1371/journal.pone.0010724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.H., Kim J.J., Bae Y.S. Involvement of PI3KAKT-mTOR pathway in protein kinase CKII inhibition-mediated senescence in human colon cancer cells. Biochem. Biophys. Res. Commun. 2013;433:420–425. doi: 10.1016/j.bbrc.2013.02.108. [DOI] [PubMed] [Google Scholar]
- Pichiorri F., Suh S.S., Rocci A., De Luca L., Taccioli C., Santhanam R., Zhou W., Benson D.M., Jr, Hofmainster C., Alder H., et al. Downregulation of p53-inducible microRNAs 192, 194, and 215 impairs the p53/MDM2 autoregulatory loop in multiple myeloma development. Cancer Cell. 2010;18:367–381. doi: 10.1016/j.ccr.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rivlin N., Brosh R., Oren M., Rotter V. Mutations in the p53 tumor suppressor gene: important milestones at the various steps of tumorigenesis. Genes Cancer. 2011;2:466–474. doi: 10.1177/1947601911408889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles S.J., Adami G.R. Agents that cause DNA double strand breaks lead to p16INK4a enrichment and the premature senescence of normal fibroblasts. Oncogene. 1998;16:1113–1123. doi: 10.1038/sj.onc.1201862. [DOI] [PubMed] [Google Scholar]
- Ruzzene M., Pinna L.A. Addiction to protein kinase CK2: a common denominator of diverse cancer cells? Biochim. Biophys. Acta. 2010;1804:499–504. doi: 10.1016/j.bbapap.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Ryu S.W., Woo J.H., Kim Y.H., Lee Y.S., Park J.W., Bae Y.S. Down-regulation of protein kinase CK2 is associated with cellular senescence. FEBS Lett. 2006;580:988–994. doi: 10.1016/j.febslet.2006.01.028. [DOI] [PubMed] [Google Scholar]
- Serrano M., Lin A.W., McCurrach M.E., Beach D., Lowe S.W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Zhu J., Woods D., McMahon M., Bishop J.M. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 1998;12:2997–3007. doi: 10.1101/gad.12.19.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]


