Abstract
2-(Trimethylammonium) ethyl (R)-3-methoxy-3-oxo-2-stearamidopropyl phosphate [(R)-TEMOSPho], a derivative of an organic chemical identified from a natural product library, promotes highly efficient megakaryopoiesis. Here, we show that (R)-TEMOSPho blocks osteoclast maturation from progenitor cells of hematopoietic origin, as well as blocking the resorptive function of mature osteoclasts. The inhibitory effect of (R)-TEMOSPho on osteoclasts was due to a disruption of the actin cytoskeleton, resulting from impaired downstream signaling of c-Fms, a receptor for macrophage-colony stimulating factor linked to c-Cbl, phosphoinositol-3-kinase (PI3K), Vav3, and Rac1. In addition, (R)-TEMOSPho blocked inflammation-induced bone destruction by reducing the numbers of osteoclasts produced in mice. Thus, (R)-TEMOSPho may represent a promising new class of antiresorptive drugs for the treatment of bone loss associated with increased osteoclast maturation and activity.
Keywords: antiresorptive drugs, bone destruction, osteoclast, osteoclast maturation, (R)-TEMOSPho
INTRODUCTION
Bone homeostasis is sustained by the coordinated regulation of bone-forming osteoblasts and bone-resorbing osteoclasts (Boyle et al., 2003). Excessive bone resorption by osteoclasts causes pathological bone diseases, such as osteoporosis, periodontitis, and rheumatoid arthritis (Boyle et al., 2003; Harada and Rodan, 2003; Karsenty and Wagner, 2002; Teitelbaum, 2000). Osteoclasts are multinucleated giant cells generated from hematopoietic monocyte/macrophage precursor cells by multiple steps of cell differentiation. The differentiation and function of osteoclast are induced by two critical cytokines: macrophage-colony stimulating factor (M-CSF), and receptor activator of NF-κB ligand (RANKL) (Leibbrandt and Penninger, 2009; Teitelbaum, 2007).
M-CSF mediates the survival and proliferation of precursors of the monocytes and macrophages, as well as their differentiation into mature phagocytes (Ross, 2006). The observation that op/op mice, which fail to express functional M-CSF as a result of a point mutation in the Csf1 gene, are osteopetrotic (Kodama et al., 1991; Wiktor-Jedrzejczak et al., 1990) confirmed the critical role of M-CSF in osteoclast biology. The functional linkage between M-CSF and its transmembrane receptor tyrosine kinase, c-Fms, was established by the observation that mice lacking the gene coding for c-Fms, csf1r, exhibit the same major phenotype as the op/op mice. That is, they have a marked decrease in tissue macrophages and severe osteopetrosis due to a lack of osteoclasts (Dai et al., 2002; Kodama et al., 1991; Marks et al., 1992; Wiktor-Jedrzejczak et al., 1990).
Binding of M-CSF to c-Fms activates receptor autophosphorylation at seven tyrosine residues within the cytoplasmic domain (Faccio et al., 2007; Pixley and Stanley, 2004; Ross, 2006). Several Src homology 2 domain-containing molecules, including phosphoinositol-3-kinase (PI3K) and c-Cbl, are recruited to the autophosphorylated c-Fms to initiate the signaling cascades that lead to cell proliferation, survival, differentiation, and cytoskeletal organization of osteoclast lineage cells (Pixley and Stanley, 2004). M-CSF-dependent cytoskeletal changes in osteoclasts are regulated by the activation of Vav3 and its downstream effector Rac, two key mediators of actin remodeling (Faccio et al., 2005). M-CSF signaling also recruits the adaptor protein complex Grb2/Sos in the cytoplasmic tail of c-Fms, acting as a guanosine exchange factor for Ras, activating the Ras/Raf/Mek/Erk pathway (Ross, 2006) and thereby contributing to the mediation of macrophage proliferation.
It has been reported that 2-(trimethylammonium) ethyl (R)-3-hydroxy-2-stearamidopropyl phosphate (TEHSPho), a lysophosphatidylcholine derivative of a chemical from a natural product library, inhibits osteoclast differentiation and bone resorbing activity of mature osteoclasts through the inhibition of the RANKL-induced activation of ERK, Akt and NF-κB (Kwak et al., 2004). Recently, Lim et al. (2012) described the activity of 2-(trimethylammonium) ethyl (R)-3-methoxy-3-oxo-2-stearamidopropyl phosphate [(R)-TEMOSPho], a novel compound derived from TEHSPho, in megakaryocyte differentiation. They showed that (R)-TEMOSPho induces cell cycle arrest, cell size increase, polyploidization, and megakaryocyte-cell surface marker expression in both leukemia cells and primary CD34+ hematopoietic stem cells.
Here, we characterized the effects of (R)-TEMOSPho on osteoclast differentiation and function. Our results suggest that (R)-TEMOSPho blocks osteoclast maturation and resorptive function through the inhibition of M-CSF signaling, which causes disruption of the actin cytoskeleton. Furthermore, (R)-TEMOSPho was shown to block lipopolysaccharide (LPS)-induced bone destruction in mice, suggesting that (R)-TEMOSPho may be useful for the development of potential therapeutic agents for the treatment of bone diseases.
MATERIALS AND METHODS
Synthesis and purification of (R)-TEMOSPho
(R)-TEMOSPho was synthesized by the modification of previously described method (Kim et al., 2003) and purified by silica gel flash column chromatography (metylene chloride : methanol 3:1 to 1:1) to yield the pure compound as white solid (636.2 mg, 66%). 1H-NMR (400 Mz, CD3OD) δ 4.65 (br s, 1H), 4.11–4.25 (m, 4H), 3.78 (s, 3H), 3.62–3.64 (m, 2H), 3.22 (s, 9H), 2.25–2.30 (m, 2H), 1.62 (br s, 2H), 1.29–1.33 (m, 28H), 0.90 (t, J = 6.8 Hz, 3H). HRMS (EI) m/z calcd for C27H56N2O7P 551.3825, found: 551.3826.
Reagents
The β-glycerol phosphate, ascorbate-2-phosphate, dexamethasone, p-nitrophenyl phosphate, and hematoxylin solution were purchased form Sigma-Aldrich (USA). Recombinant human MCSF and BMP-2 were purchased from R&D Systems (USA). Recombinant human RANKL was from PeproTech (USA). Alizarin red solution and phalloidin-FITC were obtained from Alpha-Chem (Middlesex, UK) and Invitrogen (USA), respectively. SYBR Green Master kit was from Kapa Biosystems (USA). Primary antibodies include rabbit anti-Vav3 (Upstate Biotechnology, USA), rabbit anti-PI3K (Upstate Biotechnology), rabbit anti-c-Fms (C-20) (Santa Cruze Biotechnology, USA), mouse anti-c-Cbl (BD Biosciences, USA) and mouse anti-phosphotyrosine mAb 4G10 (Millipore, USA).
In vitro osteoclast differentiation
Osteoclasts were prepared from bone marrow cells using a standard method (Suda et al., 1997). In brief, mouse bone marrow cells were isolated from tibiae and femurs of 6-week-old mice by flushing the bone marrow with α-MEM. Cells were cultured in α-MEM containing 10% FBS, 100 U/ml penicillin, and 100 mg/ml streptomycin in the presence of 30 ng/ml M-CSF for 3 days. Subsequently, the osteoclast precursor cells (bone marrow-derived macrophages [BMMs]) were cultured with 30 ng/ml M-CSF and 100 ng/ml RANKL for the indicated time periods. TRAP assays were carried out as previously described (Lee et al., 2006). TRAP-positive (TRAP+) cells that contained more than 3 or 20 nuclei were counted as TRAP+ multinucleated cells (TRAP+ MNCs). TRAP+ mononuclear cells in each well were scored using an inverted microscope.
In vitro osteogenic differentiation
Primary osteoblast precursor cells were isolated from the calvarial bone from newborn mice by enzymatic digestion with α-MEM containing collagenase (Invitrogen, USA) and 0.2% dispase II (Sigma-Aldrich, USA). The precursor cells were plated at a density of 2 × 104 cells/well in 48-well plates and cultured for 7–14 days in osteogenic medium containing 10 mM β-glycerol phosphate, 50 μg/ml ascorbate-2-phosphate, 10−7μM dexamethasone and 25 ng/ml human recombinant BMP-2 (R&D Systems, USA). The culture medium was replaced with fresh osteogenic medium every 3 days. Alkaline phosphatase (ALP) staining was performed by BCIP/NBT color development substrate (Promega, USA) according to the manufacturer’s instructions. ALP activity assay was measured biochemically using a pnitrophenyl phosphate tablets (Sigma-Aldrich, USA). Cells were differentiated for 7 days and harvested. The cells were then lysed with 1% Triton X-100. Lysates were centrifuged at 13,000 × g for 10 min at 4°C. Cell extracts were added to each 96-well plate containing p-nitrophenyl phosphate and incubated for 30 min at room temperature. Finally, the reaction was stopped by the addition of 50 μl of 3 N NaOH. The ALP activity was determined by absorbance measurement at 405 nm. For Alizarin red staining, cells were fixed with 4% paraformaldehyde for 10 min, washed with PBS and stained with 2% Alizarin red S solution (AlphaChem, UK) for 5 min at room temperature. For Alizarin red quantification, 0.5 N HCL and 5% sodium dodecyl sulfate were added to each well. The extracted dye was measured for light absorbance at 562 nm.
Real-time quantitative PCR (RT-qPCR) analysis
Total RNA (2 μg) isolated from cell cultures at various times using the TRIZOL (Takara Bio, Japan) and was used as a template for cDNA synthesis. RT-qPCR was performed with SYBR Green Master kit (Kapa Biosystems, USA). Primers specific for murine TRAP, cathepsinK, matrix metalloproteinase 9 (MMP9), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used. The following primers were used: for TRAP, 5′-CACAATCCATGCAGGAGCAT-3′ and 5′-AAACACACGCAGCTTC-3′; for cathepsin K, 5′-GGAAGAAGACTCACCAGC-3′ and 5′-GCTATATAGCCGCCTCCAG-3′; for MMP9, 5′-CCTGTGTGTTCCCGTTCA-3′ and 5′-CGCTGGAATGATCTAAGC-3′; for GAPDH, 5′-CCAAAGTTGTCATGGATG-3′. and 5′-CCCTTCATTGACCTCAACC-3′. The amplification reaction was conducted for 22–30 cycles, each of at 95°C for 5 min, 58°C for 1 min, and 72°C for 1 min.
Actin ring formation
BMMs were cultured with M-CSF and RANKL for 4 days with or without (R)-TEMOSPho (10 μM). For actin ring staining, cells were fixed in 3.7% paraformaldehyde for 15 min, permeabilized in 0.1% Triton X-100 for 5 min, and rinsed in PBS. The cells were incubated in phalloidin-FITC (Invitrogen, USA) for 30 min.
Resorption pit assay
BMMs were plated on dentine slices (provided by M. Takami, Showa University, Japan) and treated with (R)-TEMOSPho (10 μM) for 6 days. After incubation, attached cells were completely removed from the surface of dentine slices by abrasion with a cotton tip, and their resorption pits were visualized by staining with hematoxylin solution. Photographs were taken under a light microscope and total area of resorption pits were measured and analyzed with the Image Pro-Plus program 4.5 (Media Cybernetics, USA).
Western blot analysis
For immunoprecipitation, BMMs were cultured with M-CSF and RANKL for 2 days with or without (R)-TEMOSPho. Pre-osteoclasts were starved of serum and cytokines for 5 h and then stimulated with M-CSF for 5 min. Cells were washed twice with cold-PBS and lysed in 2x-HNTG buffer (40 mM HEPES [pH7.4], 200 mM NaCl, 2% Triton X-100, 20% glycerol, 1 mM sodium orthovanadate, 1 mM sodium fluoride, protease inhibitor), Lysates (800 μg) were incubated with 2 μg of primary antibody for overnight at 4°C. Protein G beads were then added and incubated with rotation for 1h at 4°C. Washed beads were resuspended in 2× SDS loading buffer, boiled for 5 min, centrifuged, and subjected to SDS-PAGE. Primary antibodies include rabbit anti-Vav3, rabbit anti-PI3K, rabbit anti-c-Fms (C-20), mouse anti-c-Cbl, and mouse anti-phosphotyrosine 4G10.
LPS-induced bone destruction
All animal study protocols were approved by the Animal Care Committee of Ewha Laboratory Animal Genomics Center. Mice (C57BL/6 mice, male, 6–7 weeks old) were administered with a local calvarial injection of LPS (12.5 mg/kg body weight, Sigma-Aldrich) twice with an intervening 48 h. (R)-TEMOSPho or saline was calvaria injected daily for 4 days. All mice were killed at 5 days after the first LPS injection. For histological analysis, calvaria were fixed in 4% paraformaldehyde for 3 days, decalcified with 0.5 M EDTA for 8 days, embedded in paraffin, cut into 5 μm sections, and then stained for TRAP and hematoxylin.
Statistical analysis
Data are expressed as mean ± S.D. from at least three independent experiments. Statistical differences were analyzed by student’s t-test. P < 0.05 was considered statistically significant.
RESULTS
Inhibitory effects of (R)-TEMOSPho on osteoclast formation
To determine whether the (R)-TEMOSPho could affect osteoclastogenesis, BMMs cultured in M-CSF and RANKL were incubated with (R)-TEMOSPho. RANKL-induced osteoclast formation was inhibited by incubation with (R)-TEMOSPho in a dose-dependent manner (Fig. 1A). The formation of large (> 200 μm) osteoclasts containing more than 20 nuclei was greatly diminished by treatment with the (R)-TEMOSPho; however, we observed that the number of TRAP+ mononuclear cells were higher in (R)-TEMOSPho-treated cells (Fig. 1B). Interestingly, (R)-TEMOSPho-treated osteoclasts formed an irregular or condensed shape with a smaller cell size compared to that of control (Fig. 1C). These results suggest that the (R)-TEMOSPho may regulate the formation of large osteoclasts, but not the differentiation of TRAP− to TRAP+ osteoclast precursors. In contrast, treatment of the (R)-TEMOSPho in osteogenic cultures of calvarial pre-osteoblasts did not inhibit either osteoblast differentiation or calcium mineralization (Fig. 2).
Fig. 1.
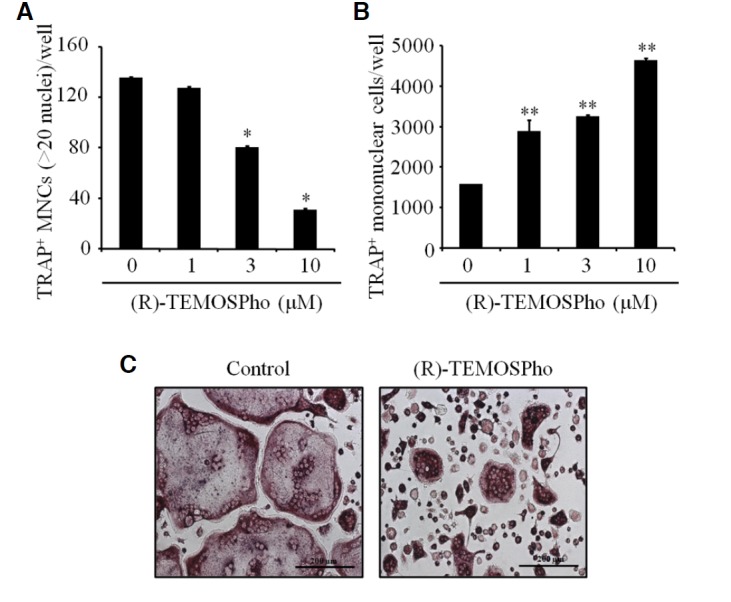
Effects of (R)-TEMOSPho on osteoclast differentiation. (A) BMMs were cultured with M-CSF (30 ng/ml) and RANKL (100 ng/ml) for 4 days with or without increasing concentration of (R)-TEMOSPho as indicated. The numbers of TRAP+ MNCs containing more than 20 nuclei per well are shown in the graph. Data represent mean ± S.D. *P < 0.001. (B) As in (A), except that number of TRAP+ mononuclear cells were counted. Data represent mean ± S.D. **P < 0.01. (C) BMMs were cultured with MCSF and RANKL for 4 days with or without of (R)-TEMOSPho (10 μM). On day 4, the cells were stained for TRAP activity. Scale bar denotes 200 μm.
Fig. 2.
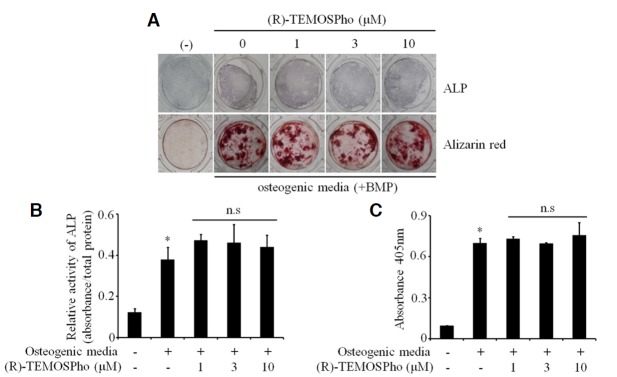
Effects of (R)-TEMOSPho on osteoblast differentiation and function. (A) Pre-osteoblasts from calvaria were grown to confluence and then cultured in osteogenic differentiation medium with and without (R)-TEMOSPho as indicated. Subsequently, alkaline phosphatase (ALP) staining and mineralized nodules staining (Alizarin red) were conducted at 7 and 14 days after culture, respectively. The figure of alkaline phosphatase staining shows no effects of the (R)-TEMOSPho on osteoblast differentiation. Mineralized nodule formation revealed by Alizarin red staining showed no inhibitory effect of (R)-TEMOSPho. (B) ALP activities were measured by using a spectrophotometer at 405 nm. (C) Alizarin red staining was quantified by using a spectrophotometer at 562 nm. Data represent mean ± S.D. *P < 0.01 versus control. n.s, not significant.
To determine the stage of osteoclast formation at which the (R)-TEMOSPho acts, osteoclastogenic cultures were treated with (R)-TEMOSPho for various time periods. Exposure to the (R)-TEMOSPho during the last 2 days of osteoclast differentiation was sufficient to inhibit osteoclast formation (Figs. 3A and 3B), suggesting that (R)-TEMOSPho may affect the later stage of differentiation, i.e., cell-cell fusion of pre-osteoclasts. Importantly, there were no significant differences in the inducible levels of osteoclastogenic markers including MMP9, TRAP, and cathepsin K (Fig. 3C). These results support that (R)-TEMOSPho alters osteoclast formation in a later stage of osteoclastogenesis, but has no effect on the generation of TRAP+ osteoclast precursors.
Fig. 3.
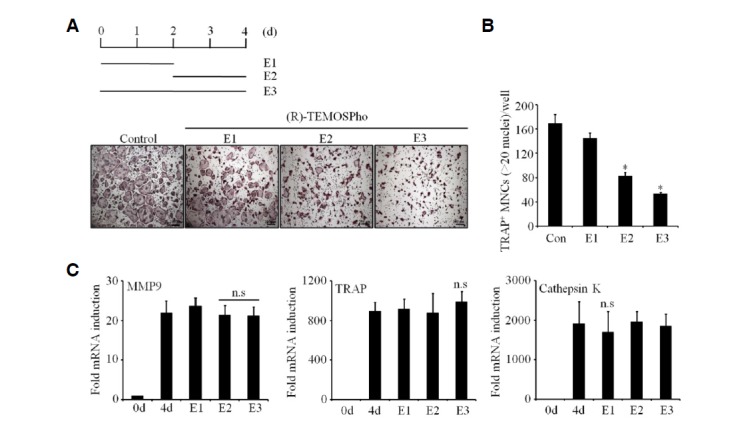
(R)-TEMOSPho inhibits osteoclast maturation in the terminal differentiation stage. (A) BMMs cultured with M-CSF (30 ng/ml) and RANKL (100 ng/ml) were treated with (R)-TEMOSPho (10 μM) for the first 2 days (E1), the last 2 days (E2) or the entire 4 days (E3). The cells were stained for TRAP activity. Scale bar denotes 200 μm. (B) The numbers of TRAP+ MNCs containing more than 20 nuclei per well are shown in the graph. Data represent mean ± S.D. *P < 0.001. (C) Same as in (A), cells were analyzed for TRAP, MMP9, and cathepsin K expression by RT-qPCR. GAPDH served as loading control.
Effects of (R)-TEMOSPho on actin ring formation and bone resorption
Since (R)-TEMOSPho-treated osteoclasts formed an irregular or condensed shape with a smaller cell size (Fig. 1C), we examined the morphological impact of the (R)-TEMOSPho on actin ring formation by staining the cells with FITC-phalloidin and TRAP. Consistent with their abnormal shape, the formation of an actin ring was severely disrupted in the presence of (R)-TEMOSPho (Fig. 4A), suggesting that the (R)-TEMOSPho could affect formation of the actin ring, a cytoskeletal structure that is essential for osteoclasts functions, such as bone resorption.
Fig. 4.
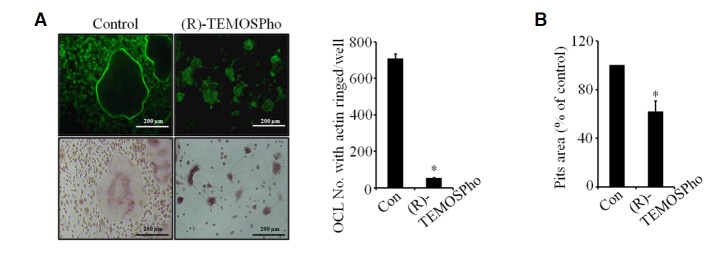
(R)-TEMOSPho impairs cytoskeletal organization and resorption function. (A) BMMs were cultured with M-CSF (30 ng/ml) and RANKL (100 ng/ml) for 4 days with or without (R)-TEMOSPho (10 μM). Cells were stained for F-actin (upper panels) and TRAP (lower panels). The actin cytoskeleton was visualized by Alexa-Fluor-488 phalloidin staining. (B) BMMs were plated on dentine slices with M-CSF and RANKL with or without (R)-TEMOSPho for 6 days. Osteoclasts were removed and the bone slices stained with hematoxylin. The total area of resorption pits was measured with Image-Pro Plus 4.5 (Media cybernetics). Data represent mean ± S.D. *P < 0.01. Scale bar denotes 200 μm.
To determine the functional implications of the (R)-TEMOSPho in osteoclasts, the capacity of osteoclasts to resorb mineralized matrix in the presence of the (R)-TEMOSPho was examined. The resorbed area on dentine slices was reduced by the addition of the (R)-TEMOSPho (Fig. 4B), suggesting that (R)-TEMOSPho regulates the function of osteoclasts.
(R)-TEMOSPho blocks M-CSF receptor signaling
M-CSF signaling plays an important role in actin cytoskeletal reorganization in osteoclasts (Fuller et al., 1993; Sakai et al., 2006). Thus, we asked whether (R)-TEMOSPho may affect MCSF signaling. We first examined M-CSF-induced tyrosine phosphorylation of the c-Fms receptor. Treatment of (R)-TEMOSPho markedly suppressed c-Fms phosphorylation by MCSF in pre-osteoclasts (Fig. 5A). Moreover, tyrosine phosphorylation of c-Cbl and PI3K downstream of the c-Fms receptor were suppressed by treatment of (R)-TEMOSPho (Figs. 5B and 5C). Furthermore, the activation of Vav3 and Rac1, two key mediators of actin reorganization, was inhibited by (R)-TEMOSPho (Figs. 5D and 5E). Overall, these data indicated that (R)-TEMOSPho blocked the phosphorylation of the c-Fms receptor, resulting in the inhibition of Vav3 and Rac1, which are required for M-CSF-dependent actin reorganization.
Fig. 5.
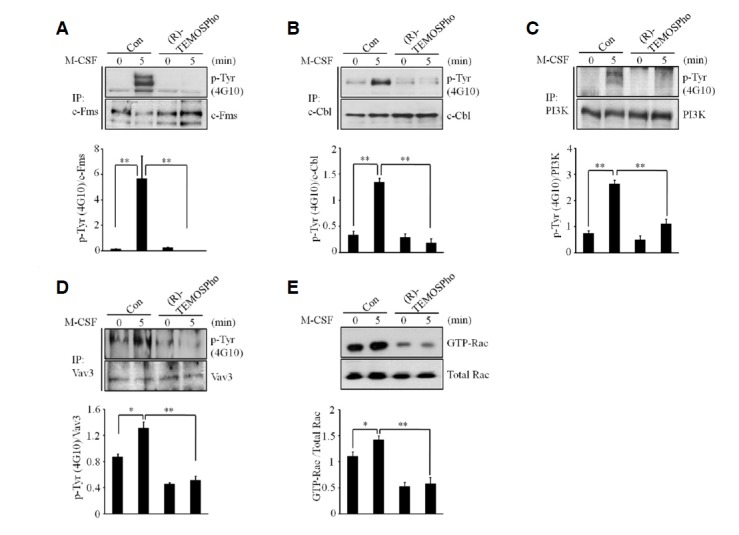
(R)-TEMOSPho inhibits M-CSF-induced c-Fms signaling in pre-osteoclasts. (A–D) BMMs were cultured with M-CSF and RANKL for 2 days with or without of (R)-TEMOSPho (10 μM). After serum starvation for 5 h, pre-osteoclasts were stimulated with or without MCSF (30 ng/ml) for 5 min. Cell lysates were immunoprecipitated with a c-Fms antibody (A), c-Cbl antibody (B), PI3K antibody (C) and Vav3 antibody (D) followed by Western blotting for anti-phosphotyrosine antibody 4G10. Levels of c-Fms, c-Cbl, PI3K and Vav3 are shown with respect to loading control. The phospho-specific bands were quantified and normalized by the intensities of the corresponding nonphospho bands (n = 3, *P < 0.05, **P < 0.01). (E) Pre-osteoclasts were stimulated with M-CSF for 5 min, and activated forms of Rac were detected by GST pull down assay. The chemiluminescence signals for GTP-Rac were quantified and normalized according to those of Rac (n= 3, *P < 0.05, **P < 0.01).
Therapeutic effects of (R)-TEMOSPho on inflammation-induced bone loss
To investigate the effects of (R)-TEMOSPho on the pathological formation of osteoclasts during inflammation, subcutaneous tissue over the periosteum of mouse calvaria were injected with vehicle alone (PBS), and LPS together with or without the addition of (R)-TEMOSPho at 1-day intervals for 4 days, with the exception that the initial injection of LPS was followed by injection with PBS. Whole calvaria was fixed in 4% paraformaldehyde and used for TRAP staining (Fig. 6A). The formation of TRAP+ MNCs was greatly suppressed in (R)-TEMOSPho-treated mice (Fig. 6B), and the extent of bone erosion was notably reduced by (R)-TEMOSPho treatment (Fig. 6C), suggesting that (R)-TEMOSPho effectively inhibited inflammation-induced bone destruction. Together, these results suggest that using (R)-TEMOSPho targeting osteoclasts may represent a promising new strategy for antirsorptive drug development.
Fig. 6.
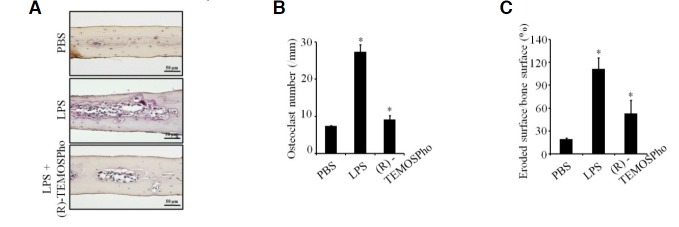
(R)-TEMOSPho suppresses LPS-induced osteoclast formation and bone destruction. (A–C) C57BL/6 mice were subcutaneously injected over the calvaria with LPS (12.5 mg/kg), twice at an interval of 48 h, with or without (R)-TEMOSPho (3 mg/kg) for 4 consecutive days. Histological sections of calvaria were stained with TRAP and hematoxylin (A). After TRAP staining, the number of osteoclast (B) and eroded surface (C) were analyzed. Data represent mean± S.D. *P < 0.01 (n = 5). Scale bar denotes 50 μm.
DISCUSSION
Osteoclast formation and function is a multi-step process that involves proliferation and recruitment of osteoclast progenitors, commitment to osteoclast precursors, fusion into multinucleated cells, and activation (Boyle et al., 2003; Suda et al., 1999). Signaling of M-CSF/c-Fms axis leads to cell proliferation, differentiation and cytoskeletal organization of osteoclast lineage cells (Pixley and Stanley, 2004). In this study, we have shown that (R)-TEMOSPho may target M-CSF/c-Fms signaling, thereby suppressing osteoclast maturation, actin ring formation, and bone resorption. Thus, our study suggests that the use of (R)-TEMOSPho may be a promising treatment option for pathological bone disorders, through the inhibition of osteoclasts.
Our results indicate that (R)-TEMOSPho inhibited osteoclast maturation by targeting a later stage of osteoclast formation. In support of this hypothesis, (R)-TEMOSPho had no effect on the expression levels of TRAP, cathepsin K and MMP 9, which are well known osteoclast marker genes (Crabtree and Olson, 2002; Hogan et al., 2003). This is further supported by experiments in vitro which showed (R)-TEMOSPho did not affect the proliferation of osteoclast progenitor cells (data not shown), nor the generation of TRAP+ mononuclear pre-osteoclasts. Therefore, we can conclude that while (R)-TEMOSPho had a profound effect on the formation of TRAP+ multinucleated osteoclasts, it did not affect the formation of TRAP+ mononuclear cells. This effect of (R)-TEMOSPho is likely a result of inhibition of the fusion of mononuclear pre-fusion osteoclasts into large osteoclasts.
In addition, we also found that osteoclasts developed in the presence of (R)-TEMOSPho showed an irregular or condensed shape with a smaller cell size, whereas the shape of the control-treated osteoclasts was round with a regular edge and a larger size. By actin ring staining, we found that peripheral actin rings were rarely formed in cells treated with (R)-TEMOSPho, suggesting that (R)-TEMOSPho impairs cytoskeleton integrity or formation of actin ring, which is essential for bone resorption by activated osteoclasts (Burgess et al., 1999; Vaananen et al., 2000). Consistent with this notion, (R)-TEMOSPho-treated osteoclasts exhibited a markedly decreased capacity for bone resorption in vitro. These results suggest that impaired osteoclast formation and bone resorption by (R)-TEMOSPho are attributable to cytoskeleton disorganization.
M-CSF binds to its receptor, c-Fms, and induces receptor auto phosphorylation at seven tyrosine residues within the cytoplasmic domain (Faccio et al., 2007; Pixley and Stanley, 2004). Several SH2 domain-containing molecules, such as PI3K (Grey et al., 2000) and c-Cbl (Adapala et al., 2010), are recruited to the phospho-Tyr residues of c-Fms upon M-CSF binding, initiating the signaling cascades that lead to cytoskeletal reorganization (Faccio et al., 2007). In this context, we found that tyrosine phosphorylation of c-Fms upon M-CSF stimulation was markedly reduced by treatment of (R)-TEMOSPho. Furthermore, we confirmed that treatment of (R)-TEMOSPho inhibits M-CSF-induced phosphorylation of PI3K and c-Cbl. In osteoclasts, Vav proteins regulate cytoskeleton organization by activating small GTPases of the Rho family (Etienne-Manneville and Hall, 2002; Faccio et al., 2005; Hall, 1998; Razzouk et al., 1999; Ridley, 2001a; 2001b; Schmidt and Hall, 2002;). Vav3-deficient preosteoclasts display abnormal morphology, deficiency in actin cytoskeleton organization, and attenuated bone resorptive activity (Faccio et al., 2005). In the current study, we found that phosphorylation of Vav3 was reduced by treatment of (R)-TEMOSPho in the presence of M-CSF signaling. Furthermore, (R)-TEMOSPho also decreased the activation of Rac1 during MCSF stimulation. Our results show that blocking c-Fms phosphorylation by (R)-TEMOSPho inhibits actin formation and bone resorption. Importantly, the inhibitory pathway downstream c-Fms by which (R)-TEMOSPho acts appears to be PI3K-c-Cbl pathway, as well as Vav3-Rac1 pathway.
In summary, our findings indicate that inhibitory effects of (R)-TEMOSPho on osteoclasts (i) result from both the inhibition of the fusion of pre-osteoclasts and the disruption of cytoskeletal integrity in osteoclasts; (ii) that these effects are a consequence of the inhibition of M-CSF signaling through blocking the tyrosine phosphorylation of c-Fms; and (iii) that the inhibition of c-Fms downstream signaling pathways related to maintenance of actin ring integrity, and that alterations in these processes can inhibit bone resorption. Therefore, we conclude that (R)-TEMOSPho might be representative of a promising new class of antiresorptive drugs for treatment of bone-related diseases.
Acknowledgments
We thank Dr. M. Takami at Showa University for providing the dentin slices. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIP) (No. 2013R1A2A1A05005153; No. 2012R1A5A10 48236; No. 2012M3A9C5048708).
REFERENCES
- Adapala N.S., Barbe M.F., Langdon W.Y., Tsygankov A.Y., Sanjay A. Cbl-phosphatidylinositol 3 kinase interaction differentially regulates macrophage colony-stimulating factor-mediated osteoclast survival and cytoskeletal reorganization. Ann. N Y Acad. Sci. 2010;1192:376–384. doi: 10.1111/j.1749-6632.2009.05346.x. [DOI] [PubMed] [Google Scholar]
- Boyle W.J., Simonet W.S., Lacey D.L. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- Burgess T.L., Qian Y., Kaufman S., Ring B.D., Van G., Capparelli C., Kelley M., Hsu H., Boyle W.J., Dunstan C.R., et al. The ligand for osteoprotegerin (OPGL) directly activates mature osteoclasts. J. Cell Biol. 1999;145:527–538. doi: 10.1083/jcb.145.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree G.R., Olson E.N. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109:S67–79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- Dai X.M., Ryan G.R., Hapel A.J., Dominguez M.G., Russell R.G., Kapp S., Sylvestre V., Stanley E.R. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–120. doi: 10.1182/blood.v99.1.111. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S., Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Faccio R., Teitelbaum S.L., Fujikawa K., Chappel J., Zallone A., Tybulewicz V.L., Ross F.P., Swat W. Vav3 regulates osteoclast function and bone mass. Nat. Med. 2005;11:284–290. doi: 10.1038/nm1194. [DOI] [PubMed] [Google Scholar]
- Faccio R., Takeshita S., Colaianni G., Chappel J., Zallone A., Teitelbaum S.L., Ross F.P. M-CSF regulates the cytoskeleton via recruitment of a multimeric signaling complex to c-Fms Tyr-559/697/721. J. Biol. Chem. 2007;282:18991–18999. doi: 10.1074/jbc.M610937200. [DOI] [PubMed] [Google Scholar]
- Fuller K., Owens J.M., Jagger C.J., Wilson A., Moss R., Chambers T.J. Macrophage colony-stimulating factor stimulates survival and chemotactic behavior in isolated osteoclasts. J. Exp. Med. 1993;178:1733–1744. doi: 10.1084/jem.178.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey A., Chen Y., Paliwal I., Carlberg K., Insogna K. Evidence for a functional association between phosphatidylinositol 3-kinase and c-src in the spreading response of osteoclasts to colony-stimulating factor-1. Endocrinology. 2000;141:2129–2138. doi: 10.1210/endo.141.6.7480. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Harada S., Rodan G.A. Control of osteoblast function and regulation of bone mass. Nature. 2003;423:349–355. doi: 10.1038/nature01660. [DOI] [PubMed] [Google Scholar]
- Hogan P.G., Chen L., Nardone J., Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- Karsenty G., Wagner E.F. Reaching a genetic and molecular understanding of skeletal development. Dev. Cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Kim Y.A., Chung H.M., Park J.S., Choi W., Min J., Park N.H., Kim K.H., Jhon G.J., Han S.Y. Synthesis of novel lysophosphatidylcholine analogues using serine as chiral template. J. Org. Chem. 2003;68:10162–10165. doi: 10.1021/jo034969s. [DOI] [PubMed] [Google Scholar]
- Kodama H., Nose M., Niida S., Yamasaki A. Essential role of macrophage colony-stimulating factor in the osteoclast differentiation supported by stromal cells. J. Exp. Med. 1991;173:1291–1294. doi: 10.1084/jem.173.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak H.B., Lee S.W., Li Y.J., Kim Y.A., Han S.Y., Jhon G.J., Kim H.H., Lee Z.H. Inhibition of osteoclast differentiation and bone resorption by a novel lysophosphatidylcholine derivative, SCOH. Biochem. Pharmacol. 2004;67:1239–1248. doi: 10.1016/j.bcp.2003.10.032. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Rho J., Jeong D., Sul J.Y., Kim T., Kim N., Kang J.S., Miyamoto T., Suda T., Lee S.K., et al. v-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat. Med. 2006;12:1403–1409. doi: 10.1038/nm1514. [DOI] [PubMed] [Google Scholar]
- Leibbrandt A., Penninger J.M. RANKL/RANK as key factors for osteoclast development and bone loss in arthropathies. Adv. Exp. Med. Biol. 2009;649:100–113. doi: 10.1007/978-1-4419-0298-6_7. [DOI] [PubMed] [Google Scholar]
- Limb J.K., Song D., Jeon M., Han S.Y., Han G., Jhon G.J., Bae Y.S., Kim J. 2-(Trimethylammonium)ethyl (R)-3-methoxy-3-oxo-2-stearamidopropyl phosphate promotes megakaryocytic differentiation of myeloid leukaemia cells and primary human CD34(+) haematopoietic stem cells. J. Tissue Eng. Regen. Med. 2012 doi: 10.1002/term.1628. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Marks S.C., Jr, Wojtowicz A., Szperl M., Urbanowska E., MacKay C.A., Wiktor-Jedrzejczak W., Stanley E.R., Aukerman S.L. Administration of colony stimulating factor-1 corrects some macrophage, dental, and skeletal defects in an osteopetrotic mutation (toothless, tl) in the rat. Bone. 1992;13:89–93. doi: 10.1016/8756-3282(92)90365-4. [DOI] [PubMed] [Google Scholar]
- Pixley F.J., Stanley E.R. CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol. 2004;14:628–638. doi: 10.1016/j.tcb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Razzouk S., Lieberherr M., Cournot G. Rac-GTPase, osteoclast cytoskeleton and bone resorption. Eur. J. Cell Biol. 1999;78:249–255. doi: 10.1016/S0171-9335(99)80058-2. [DOI] [PubMed] [Google Scholar]
- Ridley A.J. Rho family proteins: coordinating cell responses. Trends Cell Biol. 2001a;11:471–477. doi: 10.1016/s0962-8924(01)02153-5. [DOI] [PubMed] [Google Scholar]
- Ridley A.J. Rho GTPases and cell migration. J. Cell Sci. 2001b;114:2713–2722. doi: 10.1242/jcs.114.15.2713. [DOI] [PubMed] [Google Scholar]
- Ross F.P. M-CSF, c-Fms, and signaling in osteoclasts and their precursors. Ann. N Y Acad. Sci. 2006;1068:110–116. doi: 10.1196/annals.1346.014. [DOI] [PubMed] [Google Scholar]
- Sakai H., Chen Y., Itokawa T., Yu K.P., Zhu M.L., Insogna K. Activated c-Fms recruits Vav and Rac during CSF-1-induced cytoskeletal remodeling and spreading in osteoclasts. Bone. 2006;39:1290–1301. doi: 10.1016/j.bone.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587–1609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- Suda T., Jimi E., Nakamura I., Takahashi N. Role of 1 alpha,25-dihydroxyvitamin D3 in osteoclast differentiation and function. Methods Enzymol. 1997;282:223–235. doi: 10.1016/s0076-6879(97)82110-6. [DOI] [PubMed] [Google Scholar]
- Suda T., Takahashi N., Udagawa N., Jimi E., Gillespie M.T., Martin T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999;20:345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- Teitelbaum S.L. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- Teitelbaum S.L. Osteoclasts: what do they do and how do they do it? Am. J. Pathol. 2007;170:427–435. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Väänänen H.K., Zhao H., Mulari M., Halleen J.M. The cell biology of osteoclast function. J. Cell Sci. 2000;113:377–381. doi: 10.1242/jcs.113.3.377. [DOI] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W., Bartocci A., Ferrante A.W., Jr, Ahmed-Ansari A., Sell K.W., Pollard J.W., Stanley E.R. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc. Natl. Acad. Sci. USA. 1990;87:4828–4832. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]


