Abstract
Fasting reduces gastrointestinal cellular proliferation rates through G1 cycle blockade and can promote cellular protection of normal but not cancer cells through altered cell signaling including down-regulation of insulin-like growth factor 1 (IGF-1). Consequently, the purpose of this study was to determine the effects of fasting on delayed-type chemotherapy-induced nausea and vomiting in dogs receiving doxorubicin. This prospective randomized crossover study involved intended administration of two doses of doxorubicin. Cancer-bearing dogs were randomized to be fasted for 24 hours beginning at 6 P.M. the night before the first or second doxorubicin administration, and all treatments were administered within an hour before or after 12 P.M. Dogs were fed normally before the alternate dose. Circulating IGF-1 concentrations were determined from serum samples obtained immediately before each doxorubicin treatment. Data from 35 doses were available from 20 dogs enrolled. Dogs that were fasted exhibited a significantly lower incidence of vomiting, when compared to fed dogs (10% compared to 67%, P = .020). Furthermore, among the 15 dogs that completed crossover dosing, vomiting was abrogated in four of five dogs that experienced doxorubicin-induced vomiting when fed normally (P = .050). No differences in other gastrointestinal, constitutional, or bone marrow toxicities or serum IGF-1 levels were observed.
Introduction
Despite significant advances in anti-emetic drug therapy, chemotherapy-induced nausea and vomiting (CINV) remains a significant problem in the practice of clinical oncology [1]. CINV ranks among the most distressing side effects of chemotherapy and therefore contributes to patient non-compliance, treatment curtailment, and poor nutritional status. CINV is commonly classified into one of three categories: acute-onset CINV that occurs within 24 hours of initial administration of chemotherapy, delayed-type CINV occurring 1 to 5 days after initial treatment, and anticipatory CINV in patients whose emetic episodes are triggered by senses, thoughts, or anxiety associated with prior chemotherapy.
Various mechanisms for delayed-type CINV have been proposed, including disruption of the blood-brain barrier, disruption of gastrointestinal motility and/or changes in its permeability, influence of endogenous adrenal hormones, and accumulation of emetogenic chemotherapy metabolites [2]. Damage to intestinal crypt cells after exposure to cytotoxic drugs can result in delayed-type CINV through release of 5-hydroxytryptamine 3, substance P, and cholecystokinin. When bound to 5-hydroxytryptamine 3 and neurokinin-1 receptors, these mediators stimulate the terminal ends of vagal afferents that transmit signals to the vomiting center [3]. The considerable morbidity associated with CINV has prompted prophylactic treatment with serotonin antagonists, corticosteroids, dopamine antagonists, and neurokinin-1 inhibitors to become commonplace in clinical practice. Unfortunately, approximately 75% of human breast cancer patients still report some symptoms of delayed-type CINV when treated with doxorubicin-containing chemotherapy protocols [4], [5]. Acute CINV due to doxorubicin administration is also common in human patients but is less frequent than the delayed type [5]. CINV has been reported in 30% to 40% of dogs receiving doxorubicin but is almost exclusively comprised of the delayed type, with one study reporting 91% of all vomiting occurring after 48 hours [6].
Although doxorubicin is classified as a non–cell cycle–specific agent, experimental studies have determined that selective lethal cellular toxicity occurs when cells are in S-phase, whereas cells in G1 appear to be least sensitive [7], [8], [9]. Interestingly, animal studies have determined that proliferative activity of gastrointestinal cells is subject to circadian fluctuation that is largely driven by patterns of food consumption [10]. Furthermore, studies have demonstrated that fasting can dramatically reduce gastrointestinal cellular proliferation rates through G1 cycle blockade, and refeeding of mice after a period of fasting results in peak levels of S cellularity that can exceed four times those of fasted mice [11].
Proliferative activity begins to decrease within 24 hours of initiating fasting, and after refeeding, maximum proliferation usually exceeds baseline in most tissues of the gastrointestinal tract within 24 hours [10], [11]. Taken together, these data provide evidence that patterns of food consumption around the time of chemotherapy administration could contribute to delayed-type CINV in clinical cancer patients.
Fasting has also been shown to increase cellular resistance to stress, inducing a protective effect on normal cells [12], [13]. This protection is believed to be mediated by reduced insulin-like growth factor 1 (IGF-1) signaling and decreased activity of downstream effectors such as Akt, Ras, and the mammalian target of rapamycin (mTOR) [12]. In normal cells, this results in changes in gene expression and promotes resistance to oxidative stress, thought to be one of the major mechanisms of cytotoxicity caused by doxorubicin [14], [15], [16]. In contrast, it appears that the cancer cell's inability to adapt to reduced nutrients results in increased oxidative stress and cell death [17]. Therefore, fasting-induced reduction in IGF-1 not only mediates the protective effects on normal cells in vivo but is also implicated in the chemotherapy sensitization of cancer cells [17], [18]. Thus, fasting may have the potential to modulate the therapeutic index of some chemotherapy drugs.
A feasibility study recently reported 10 people voluntarily fasting for 48 to 140 hours before treatment and for 5 to 56 hours after receiving various different chemotherapeutic agents [19]. Minimal adverse effects were described during fasting, and most subjects maintained that fewer chemotherapy-related toxicities were experienced after cycles for which they fasted at the time of treatment. However, to the authors' knowledge, a formal prospective study has not evaluated the effects of fasting on delayed-type CINV. A reduced incidence of anticipatory and acute CINV in dogs, both of which can contribute to the delayed-type in people, makes the canine species ideal for the study of delayed-type CINV. Herein, we report the findings of a prospective, randomized study using a crossover design to primarily evaluate the effects of fasting on delayed-type CINV in cancer-bearing dogs. Because IGF-1 levels have been implicated as playing an important role in selective chemosensitization in mouse models and could have been affected by fasting, serum IGF-1 concentrations in both fasted and fed dogs were determined from samples collected immediately before doxorubicin administration. The effects of fasting on the incidence and severity of other commonly observed doxorubicin-induced toxicities including diarrhea, decreased activity, and bone marrow suppression were also evaluated.
Materials and Methods
Ethics Statement
The protocol and owner consent form were approved by the University of California, Davis (UC Davis) Veterinary Medical Teaching Hospital Clinical Trials Review Board (No. 11-11-10) in accordance with campus policy regarding trials involving client-owned dogs. Informed owner consent was obtained before enrollment of all patients.
Patient Selection
Cancer-bearing dogs presenting to the UC Davis William R. Pritchard Veterinary Medical Teaching Hospital (VMTH) between February 2012 and June 2013, with the intention of pursuing at least two doses of doxorubicin during the course of their chemotherapy protocol were considered candidates for enrollment. All dogs received an examination by a VMTH oncology clinician before enrollment. To be included, dogs were required to have a physical examination and weight recorded, in addition to a complete blood count (CBC) and chemistry panel (performed within 2 weeks before enrollment). Clinical chemistry panels and CBCs from veterinary clinics other than the VMTH were considered acceptable.
Both therapy-naïve and patients in relapse after standard of care were eligible for entry into this study. In addition, a favorable performance status indicating a high likelihood of receiving two doses of doxorubicin was necessary for inclusion. Dogs were required to be fed twice daily (A.M. and P.M.), or be fed ad lib, as part of the normal husbandry practices in the home. For entry into the study, owners consented to feed a consistent diet throughout the duration of the study.
Dogs were excluded if they had previously received doxorubicin therapy, were believed to be at risk of the multidrug resistance gene-1 (MDR-1) mutation, were normally fed only once daily, experienced a diet change within 1 week of treatment or showed signs of nausea, vomiting, inappetence, or diarrhea within 2 days before receiving a dose of doxorubicin. Dogs receiving concurrent medications with the potential to alter gastrointestinal toxicosis, such as prednisone or nonsteroidal anti-inflammatory drugs, were excluded unless they had received this medication for a minimum of 2 weeks (1 week for prednisone) before scheduled doxorubicin administration with no reported gastrointestinal adverse effects, and they were anticipated to stay on these medications for the duration of the study period. Dogs with gastrointestinal tract involvement, suspicion of gastrointestinal ulceration or brain metastasis, or pre-existing chronic gastrointestinal diseases such as inflammatory bowel disease or pancreatic insufficiency were also excluded.
Treatment Protocol
All included dogs were intended to receive two doses of doxorubicin at either 30 mg/m2 or 1 mg/kg as is standard of care, depending on patient weight. Doxorubicin treatments were administered at least 3 weeks apart. Dogs that remained on the study for their second doxorubicin treatment received the same total milligram dose as the first treatment. Doxorubicin was administered as a 20-minute IV infusion. Pre-medication was given as is standard at UC Davis at least 30 minutes before doxorubicin and included dexamethasone (0.2 mg/kg, IV) for dogs not receiving oral prednisone and diphenhydramine (2 mg/kg, IM or subcutaneously [SQ]) for all dogs.
At the time of enrollment, dogs were randomized into one of two feeding protocols (A or B). Randomization was performed by selecting a blank envelope containing the dog's assignment from a shuffled pile. A crossover design was used such that dogs in group A were fed normally before their first dose of doxorubicin and then fasted for their second dose. Conversely, dogs randomized to group B were fasted for their first dose and then fed normally before their second dose. When dogs were scheduled to fast, no food was given for 24 hours beginning at 6 P.M. the night before doxorubicin administration. All dogs were treated within an hour before or after 12 P.M., and the time of infusion was recorded. A time discrepancy of less than 2 hours between each of the treatments for each dog was necessary for inclusion in the study.
A CBC with differential counts was scheduled 7 to 10 days after each dose of doxorubicin. Additional hematologic and biochemical parameters on each patient were measured throughout the study as clinically indicated (CBC, chemistry panel, and urinalysis).
Concomitant medications for supportive care or other ongoing medical conditions were allowed for patients enrolled in the study except for prophylactic antiemetic or antidiarrheal drugs. Non-steroidal anti-inflammatory drugs (NSAIDS) were allowed as long as the patient had been on this medication for longer than 2 weeks before enrollment. Dogs experiencing grade II or higher nausea or vomiting toxicity score (according to the Veterinary Cooperative Oncology Group—Common Terminology Criteria for Adverse Events [VCOG-CTCAE] v1.0) [20] were treated as clinically indicated with either oral metoclopramide at a target dose of 0.3 mg/kg per os (PO) three times a day or ondansetron at a target dose of 0.3 to 0.5 mg/kg PO twice a day, depending on clinician preference. The same antiemetic was to be used as required for the duration of the study in each individual dog. Dogs that developed grade II diarrhea were to be treated with oral metronidazole at a target dose of 10 to 15 mg/kg PO twice a day.
Dogs were removed from the study if a significant toxicity occurred that precluded continuation of doxorubicin administration at the same dose or if deemed to be clinically necessary for any other reason. Dogs were removed from study at any time if review of the medical record indicated a dog did not meet eligibility criteria, if a dog did not receive the drug/agent at the prescribed dose, if progressive disease occurred, if the dog required a significant diet change, or if the owner requested withdrawal from the trial for any reason.
As was required at UC Davis for client-owned animals, the study design and treatment protocol were evaluated by the Clinical Trials Review Board at the UC Davis VMTH and were granted approval.
Response and Toxicity Assessment
One week after each dose of doxorubicin, owners were asked to score their pet's toxicity on a visual analog scale similar to that reported in Rau et al. [6]. Gastrointestinal toxicity was scored by the owners 1 week after administration of doxorubicin using the visual analog scale as previously published [6]. The mark placed by owners on each scale was given a number between 0 and 4 and corresponded to the VCOG-CTCAE v1.0 toxicity scoring [20]. If owners marked between whole numbers, then a value equal to the proportion along the scale was given. Neutropenia and thrombocytopenia were assessed from CBC values obtained 7 to 10 days after doxorubicin administration and given a grade using the VCOG-CTCAE v1.0 scheme [20]. Gastrointestinal, constitutional, and hematologic variables were evaluated as both continuous and categorical data. Each mark corresponded to a score from the VCOG-CTCAE v1.0 scheme, yielding a numerical value from 0 (no toxicity) to 4 (life threatening toxicity). Specific categories assessed included appetite, nausea, vomiting, diarrhea, and activity. The owner of one dog performed daily evaluations of toxicity rather than one evaluation at the end of the week. In this case, the highest score for each category was assigned for that dose. In the one dog that was hospitalized due to toxicity, scores were recorded based on the owner's evaluation but were then updated with information from the medical record during the hospital stay.
Bone marrow toxicity was scored using CBC results obtained 7 to 10 days after each dose of doxorubicin according to the VCOG-CTCAE v1.0 criteria for neutropenia and thrombocytopenia.
Serum IGF-1 Measurement
Blood samples (~ 3.0 ml) were obtained by jugular venipuncture before doxorubicin treatment. Samples were allowed to clot and were then centrifuged, enabling serum to be drawn off and promptly frozen at − 80°C until analysis. Samples were stored in this manner until all were collected. Serum IGF-1 concentrations were measured using an IGF-1 ELISA (ALPCO Diagnostics, Salem, NH). This assay uses two specific and high affinity antibodies against human IGF-1. The first is coated on the 96-well microtiter plate, to which the serum sample was added. The second is biotinylated, resulting in color development after the addition of streptavidin-peroxidase-enzyme conjugate that was proportional to the IGF-1 level in the serum sample.
Statistical Analysis
Statistical analyses consisted of Fisher exact and exact Mann-Whitney tests on first dose toxicity data. For paired data, the McNemar test and the Wilcoxon-signed rank test were used to evaluate incidence and severity of toxicity, respectively.
Results
Twenty-seven client-owned, cancer-bearing dogs were enrolled (Figure 1). Six dogs were withdrawn from the study after randomization but before administration of any doxorubicin. One of these six dogs was removed due to the finding of preexisting cardiotoxicity, one was euthanized before receiving doxorubicin, two owners were non-compliant with the feeding protocol, and the remaining two dogs developed concurrent illness before doxorubicin administration that precluded their involvement in the study. In addition, one dog was euthanized due to disease progression shortly after receiving the first dose of doxorubicin before toxicity data could be collected.
Figure 1.
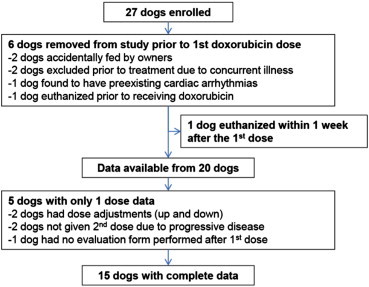
Enrollment and exclusions. Flow chart provides details on dogs enrolled and exclusions from measured end points.
Of the remaining 20 dogs (10 group A and 10 group B), 15 successfully crossed over and completed the second intended dose of doxorubicin on the study. Consequently, 15 dogs had complete gastrointestinal toxicity data available for both “fed” and “fasted” treatments. These dogs were represented by six from group A (fed first, fasted second) and nine from group B (fasted first, fed second). Of the five dogs for which data were available for one dose of doxorubicin only, four dogs were in group A with three being withdrawn after the first “fed” dose. The remaining dog in group A had recorded toxicity data from the second fasted dose only. One of these five dogs with only one data set was randomized to group B and was subsequently withdrawn after the first fasted dose. Figure 1 outlines the reasons for lack of complete data from these five dogs. In each group, A and B, similar characteristics were observed in regards to age, sex, weight, breed, and tumor type (Table 1). All 20 dogs had lymphoma, and patient details reflected that of previous reports on dogs with this cancer type [21]. In addition, there were similar proportions of dogs receiving doxorubicin at the 1 mg/kg dose and 30 mg/m2 dose between group A and group B.
Table 1.
Patient Characteristics of Dogs in Group A (Fed First) Compared to Dogs in Group B (Fasted First).
| Characteristics | All | Group A | Group B | |
|---|---|---|---|---|
| Number | 20 | 10 | 10 | |
| Age (years) | Median | 7.5 | 9 | 7 |
| Range | 3-14 | 5-14 | 3-12 | |
| Sex | MC | 11 | 6 | 5 |
| FS | 7 | 3 | 4 | |
| F | 2 | 1 | 1 | |
| Weight (kg) | Median | 24.4 | 23.85 | 27.1 |
| Range | 3.9-38.8 | 9.5-38.8 | 3.9-37.3 | |
| Breed | Mix | 10 | 5 | 5 |
| Welsh Corgie | 2 | 2 | 0 | |
| Lab | 2 | 1 | 1 | |
| Basset Hound | 2 | 1 | 1 | |
| Other | 4 | 1 | 3 | |
| Tumor type | Lymphoma (B cell) | 14 | 8 | 6 |
| Lymphoma (T cell) | 4 | 1 | 3 | |
| Lymphoma (unknown) | 2 | 1 | 1 | |
| Doxorubicin dose | 1 mg/kg | 9 | 5 | 4 |
| 30 mg/m2 | 11 | 5 | 6 | |
Since dogs could be withdrawn from the study due to excessive toxicity after their initial treatment, we first wanted to analyze toxicities experienced by all dogs after their first dose. It was suspected that an inherent bias toward study withdrawal could occur in dogs experiencing toxicity after the first dose; therefore, bias might occur if in fact dogs in one group were more likely to experience delayed-type CINV. In fact, of the three dogs in group A that were removed from the study after their first dose, all three experienced vomiting after this initial “fed” dose. The dog in group B (fasted first) that was withdrawn did not experience vomiting after this first dose. Included in this initial analysis were 9 dogs that were fed before their first treatment (group A dogs) and 10 dogs that were fasted before their first treatment (group B dogs; Table 2). A significant difference between vomiting incidence in dogs was observed, with 6 of 9 (67%) fed before treatment experiencing vomiting compared to 1 of 10 (10%) that fasted (P = .020). Of those who were fed before treatment, vomiting scores consisted of three dogs with grade 0.5, two dogs with grade 1, and one dog with grade 3 vomiting on a continuous scale. The single dog that vomited after fasting before administration had grade 1 toxicity. Interestingly, the owner of this dog reported that the animal had eaten trimmings of horse hooves before the episode on day 5 after receiving doxorubicin. The difference in mean vomiting scores between dogs fed and fasted before their first treatment was also found to be significant (0.72 compared to 0.10, P = .017).
Table 2.
Toxicity Data.
| First Dose Data |
Crossover Data |
|||||||
|---|---|---|---|---|---|---|---|---|
| Fed |
Fasted |
P Value |
Fed |
Fasted |
P Value |
|||
| Toxicity | (%) | (%) | Incidence | Scores | (%) | (%) | Incidence | Scores |
| Vomiting | 6/9 | 1/10 | .020 | .017 | 5/15 | 1/15 | .050 | .313 |
| (67) | (10) | (33) | (6.7) | |||||
| Nausea | 4/9 | 4/10 | 1.000 | .810 | 6/15 | 6/15 | 1.000 | .531 |
| (44) | (40) | (40) | (40) | |||||
| Appetite | 3/9 | 3/10 | 1.000 | .703 | 5/15 | 5/15 | 1.000 | 1.000 |
| (33) | (30) | (33) | (33) | |||||
| Diarrhea | 7/9 | 7/10 | 1.000 | .103 | 6/15 | 10/15 | .130 | .613 |
| (78) | (70) | (40) | (67) | |||||
| Activity | 6/9 | 8/10 | .629 | .636 | 7/15 | 10/15 | .540 | .672 |
| (67) | (80) | (47) | (67) | |||||
| Neutropenia | 4/9 | 1/10 | .141 | .098 | 4/14 | 4/14 | 1.000 | 1.000 |
| (44) | (10) | (29) | (29) | |||||
| Thrombocytopenia | 3/9 | 1/10 | .303 | .512 | 3/14 | 2/14 | .700 | 1.000 |
| (33) | (10) | (21) | (14) | |||||
| Mean IGF-1 (ng/ml) | 183 | 164 | .910 | |||||
Paired data were then evaluated from the 15 dogs for which it was available. Given the likelihood of a bias among these dogs toward individuals that were less likely to vomit (given their continued presence on the study after their first dose), we were most interested in the dogs whose toxicity changed between treatments. Ten of 15 dogs did not exhibit vomiting after being fasted or fed. Among the five dogs that vomited, one dog vomited after both fasted and fed doses, and the remaining four dogs vomited only when fed before treatment (P = .050). Of these four dogs, three were in group A and one in group B. However, the majority of dogs exhibited only mild vomiting and there was no significant difference in severity of vomiting (P = .31).
When nausea incidence was evaluated between dogs fed and those fasted before their first dose, 4 of 9 (44%) that were fed and 4 of 10 (40%) that were fasted experienced nausea. This difference was not statistically significant (P = 1). Nausea scores after the first dose of doxorubicin in dogs that were fed included one dog with grade 1, two dogs with grade 2, and one dog with grade 4 toxicity. In dogs that fasted before their first dose, nausea scores reported were two dogs with grade 1 and one dog each with grade 2 and grade 4 toxicity. No significant difference in nausea scores was observed (P = .81). Additionally, no difference in the incidence of nausea or nausea scores was detected when evaluating all paired data (Table 2).
Second, we evaluated the difference in other gastrointestinal and constitutional toxicity observed between treatments when dogs were fed and fasted. No significant difference between the incidence and scores of appetite, diarrhea, or activity was evident between treatments when first dose or paired data were analyzed (Table 2). Similarly, no differences in the incidence or scores of neutropenia or thrombocytopenia were detected (Table 2). Lastly, there was no significant difference in IGF-1 concentration between when dogs were fed or fasted before treatment (Table 2 and Figure 2).
Figure 2.
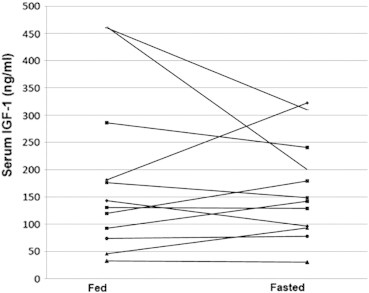
IGF-1 concentration changes. Pretreatment serum IGF-1 levels were determined by ELISA. Each line represents the change in IGF-1 concentration for an individual dog between “fed” and “fasted treatments.
Discussion
To the authors' knowledge, this study is the first randomized prospective clinical evaluation of the effects of fasting on the incidence of CINV in cancer-bearing patients. Here, we reported our findings assessing the impact of fasting for 18 hours before and 6 hours after doxorubicin chemotherapy in cancer-bearing dogs. Our data suggest that fasting for 24 hours significantly reduces the incidence of vomiting in dogs treated with doxorubicin but did not appear to affect nausea or other potential adverse effects commonly seen in doxorubicin-treated cancer-bearing dogs.
The effect of fasting on the modulation of digestive tract cellular proliferation has long been known [10], [11]. Theoretically, by blocking gastrointestinal cells in the G1 phase with fasting, these cells should be less sensitive to the effects of doxorubicin, which is preferentially toxic to cells in the S phase [8], [9]. In addition to the effects of fasting on the cell cycle, it also appears that protection is elicited in part by other mechanisms that likely alter gene expression [18]. In one study, protection of mice from doxorubicin toxicity by fasting before treatment appeared to be mediated by a reduction in circulating IGF-1 levels such that administration of IGF-1 abolished the protective effect of fasting [18]. Furthermore, mouse embryonic fibroblasts grown to confluence in vitro and then treated with doxorubicin were found to be protected from cell killing by IGF-1 receptor deletion compared to cells that overexpressed IGF-1 receptor [18]. In this case, the proliferation rate was kept relatively constant by the confluence of the cells in culture and therefore cytoprotection appeared to be independent of the cell cycle.
Supporting the notion that fasting before chemotherapy might result in reduced clinical toxicity are several studies in mice illustrating that cellular stress resistance is elicited by fasting [13], [18]. In one report, etoposide administered at 80 mg/kg killed 43% of control mice compared to 6% of mice that were fasted for 48 hours before administration [13]. Reduced IGF-1 signaling was also found to protect primary glia but not glioma cells against high-dose cyclophosphamide in vitro, supporting the notion of differential stress resistance, whereby normal cells but not cancer cells experience this protective effect [18]. Recently, fasting cycles alone have also been shown to cause cytotoxicity and chemotherapy sensitization of cancer cells in vitro and in mouse models [17].
In a feasibility study reporting on 10 people with various cancer types and stages, who had voluntarily fasted for 48 to 140 hours before and for 5 to 56 hours after chemotherapy, patient complaints during fasting included mild dizziness, hunger, and headaches, which did not interfere with daily function [19]. Any weight loss was rapidly recovered after cessation of fasting. All 10 human patients who undertook fasting around the time of treatment described the lack of nausea, vomiting, diarrhea, abdominal cramps, and mucositis after cycles of chemotherapy. At least one of these symptoms was reported in five of six patients after cycles where no fasting was performed [19]. While the previous study showed that fasting was feasible and safe in human cancer patients receiving chemotherapy, it was not a prospective design and the exceedingly long fasting periods seem unlikely to be acceptable for many patients in clinical practice.
Dogs may serve as an excellent model to study the clinical applications of fasting to ameliorate delayed-type CINV in cancer patients. The relative lack of doxorubicin-associated anticipatory and acute CINV in dogs, compared with people, ensures that delayed-type CINV specifically can be studied in dogs without any appreciated cumulative effects of the other two types, as occurs in people [2]. Furthermore, client-owned dogs are more likely to have a consistent diet, allowing minimal variation in potentially confounding factors between doses within each patient. People, in the absence of fasting, have a much more diverse diet and individuals possess the ability to decide the type, frequency, and amount of food consumed on any particular day.
When all available first dose data were analyzed alone, a significant increase in vomiting incidence and severity was observed in dogs that were fed (67% incidence) compared to dogs that were fasted before doxorubicin treatment (10% incidence). The limitations of this analysis however is that without longitudinal paired data (i.e., data from a “fasted” and a “fed” dose in the same dog), we lose the internal control values for each dog. This leaves our data open to confounding from an immeasurable number of variables that might increase or decrease each dog's risk of vomiting. However, if dogs were more likely to have experienced toxicity after doxorubicin when they were fed normally, it is possible that dogs randomized to group A (fed first) would be more likely to be withdrawn from the study before their second dose than dogs in group B. Removal of these dogs that vomited after their first dose would exclude them from the paired analysis completely, perhaps creating a bias toward group A dogs that are less likely to vomit in general. In fact, of the four dogs that were withdrawn from the study before their second dose, three were fed first and all three experienced vomiting. While we saw a higher incidence of vomiting in dogs fed before their first doxorubicin dose when compared to historical reports with this agent, feeding was not standardized in previous studies. When all dogs in our study were evaluated together, the overall incidence of vomiting in 19 dogs for which first dose data were available was 36.8% (7 of 19) and is similar to data from dogs receiving placebo in a previous prospective randomized study [6].
In our paired data, the incidence of gastrointestinal and constitutional side effects after “fed” (control) doses of doxorubicin was similar to that previously reported when evaluated in a similar manner [6]. While 33% of dogs vomited after the “fed” treatment (5 of 15), only 6.7% (1 of 15) of dogs vomited after the “fasted” treatment. In other words, fasting appeared to abrogate vomiting in four of five dogs that otherwise vomited when they were fed normally before doxorubicin treatment. It was interesting that in the only dog that experienced vomiting after being fasted the owner noted that this was likely secondary to dietary indiscretion (dog had eaten horse hoof trimmings). While the authors considered that dietary indiscretion could warrant exclusion from analysis, it was felt that exclusion of this case was not justified and might inappropriately bias the results.
Interestingly, despite the difference in vomiting incidence between dogs fed and fasted before doxorubicin treatment, the incidence of inappetence, nausea, diarrhea, and lethargy appeared to be very similar between treatments. The reason for this inconsistency is unknown. A plausible argument for the lack of change in nausea and lethargy would simply be that owners often struggle to accurately observe changes in these subjective signs, resulting in only the objective aberrations being noted. Similar to our findings, Rau et al. reported no perceived reduction in nausea incidence or severity with prophylactic maropitant administration, despite significant decreases in vomiting [6]. It is also possible that some parts of the gastrointestinal tract may be more or less susceptible to the protective state induced by fasting. In mice, colonic mucosa does appear to respond to refeeding by increasing proliferation above baseline feeding rates in a much more exaggerated manner than mucosa of the jejunum and ileum [11]. However, these peak proliferation rates reach their maximum around 24 hours after refeeding, which would be approximately 30 hours after doxorubicin administration in our dogs. While some doxorubicin is likely still present in serum at that time due to a terminal elimination half life of around 20 hours, the concentration is very low [22]. To the authors' knowledge, differences in stress resistance elicited by fasting in various anatomic locations have not been thoroughly determined. Nevertheless, it is quite possible that the timing of refeeding after fasting may be critical. In contrast, the case series including 10 human cancer patients described reduced nausea, vomiting, and diarrhea with fasting before chemotherapy [19]. Although, patient self-reporting of side effects raises the possibility of bias or placebo effect in human patients volunteering for such a study. No effect of fasting on myelotoxicity was expected, and although we evaluated only a limited number of patients at a single time point, our results failed to show any significant differences in neutropenia or thrombocytopenia between “fed” and “fasted” treatments.
Circulating IGF-1 levels have been found to negatively correlate with the protective effect of 72-hour fasting against chemotherapy toxicity in mice [18]. In rats, IGF-1 concentration begins to drop after 24 hours of fasting, but significant decreases from baseline are not apparent until 48 hours [23]. As previously discussed, alterations in cell cycle and decreased IGF-1 signaling have both been implicated as mechanisms for differential stress resistance in mouse models [17], [18]. However, their individual or collective contributions to CINV simply cannot be accurately evaluated in murine models that do not exhibit vomiting. Using clinical canine patients, we observed a significant difference in the incidence of vomiting in dogs that were fasted when compared to fed dogs. However, in the measurement of serum IGF-1 using an ELISA as has previously been reported in dogs [24], [25], we found no significant difference in serum IGF-1 concentration in dogs with paired data from both “fed” and “fasted” treatments, which is in agreement with two previous canine studies that have reported that fasting for 18 to 20 hours does not alter serum IGF-1 or IGFBP concentrations [26], [27]. The lack of a significant decrease in IGF-1 levels in our dogs after an 18-hour fast suggests that extending the duration of fasting might be necessary to significantly reduce the IGF-1 concentration before chemotherapy and consequently to see a maximum clinical benefit. However, the reduction in vomiting incidence despite the lack of a significant decrease in serum IGF-1 concentration may indicate that this effect is independent of IGF-1 signaling.
A limitation of our study is the small sample size. This may have resulted in insufficient power to prove a significant difference in toxicity in the 15 dogs with paired data. The predominant reason most owners gave for declining enrollment in the trial was the perception that withholding food from their dog would cause them (their dog and frequently also the owner) distress. Therefore, while most studies suggest that fasting for longer than 24 hours is necessary to observe maximum protection against toxicity, this may be difficult clinically without thorough elucidation and education of the potential benefits.
Our study did not attempt to investigate the potential therapeutic effects of fasting on tumor control and chemotherapy sensitization, although clinical studies evaluating this in the future may be warranted. Data from this report, however, would suggest that a lengthier fasting period is necessary in dogs to significantly reduce circulating IGF-1 levels and possibly elicit the therapeutic effect that has been suggested in murine studies. In addition, clinical studies evaluating the benefit of fasting in reducing toxicity from other chemotherapy agents are critical. Importantly, some chemotherapy agents such as those in the platinum family possess the greatest cytotoxic effect when exposure is in the G1 phase and thus could cause increased toxicity to intestinal epithelial cells. In such a case, fasting could differentially increase CINV for this class of agents [8], [28]. Furthermore, investigation into any potential additive effect of fasting combined with prophylactic antiemetic therapy is necessary to determine if this protective effect can be enhanced further, especially since prophylactic antiemetic therapy is routinely prescribed.
Delayed-type CINV remains a significant concern for both human and canine cancer patients. Our findings suggest that fasting for 18 hours before and 6 hours after doxorubicin chemotherapy reduces the risk of vomiting in doxorubicin-treated cancer-bearing dogs. When first dose data alone were reviewed, a significantly reduced vomiting incidence and severity were detected in dogs fasted before treatment compared to those that were fed. While it is clear that many dogs vomited neither after the “fed” nor the “fasted” doses, analysis of paired data revealed that in dogs that vomited after only one dose, this tended to be from the “fed” dose. Taken together, these data suggest that some dogs may benefit from fasting before doxorubicin, especially dogs that have vomited after treatment in the past. We contend that the dog serves as an excellent model to further investigate the optimal parameters and clinical efficacy of fasting for reduction of chemotherapy side effects in people.
Footnotes
Financial support: Funded by the Center for Companion Animal Health, School of Veterinary Medicine and NCRR K01 RR031272 [currently supported by the Office of Research Infrastructure Programs (8K01OD011111-02)].
References
- 1.Lohr L. Chemotherapy-induced nausea and vomiting. Cancer J. 2008;14:85–93. doi: 10.1097/PPO.0b013e31816a0f07. [DOI] [PubMed] [Google Scholar]
- 2.Roila F, Donati D, Tamberi S, Margutti G. Delayed emesis: incidence, pattern, prognostic factors and optimal treatment. Support Care Cancer. 2002;10:88–95. doi: 10.1007/s005200100295. [DOI] [PubMed] [Google Scholar]
- 3.Bayo J, Fonseca PJ, Hernando S, Servitja S, Calvo A, Falagan S, García E, González I, Miguel MJ, Pérez Q. Chemotherapy-induced nausea and vomiting: pathophysiology and therapeutic principles. Clin Transl Oncol. 2012;14:413–422. doi: 10.1007/s12094-012-0818-y. [DOI] [PubMed] [Google Scholar]
- 4.Shih V, Wan HS, Chan A. Clinical predictors of chemotherapy-induced nausea and vomiting in breast cancer patients receiving adjuvant doxorubicin and cyclophosphamide. Ann Pharmacother. 2009;43:444–452. doi: 10.1345/aph.1L437. [DOI] [PubMed] [Google Scholar]
- 5.Hickok JT, Roscoe JA, Morrow GR, King DK, Atkins JN, Fitch TR. Nausea and emesis remain significant problems of chemotherapy despite prophylaxis with 5-hydroxytryptamine-3 antiemetics: a University of Rochester James P. Wilmot Cancer Center Community Clinical Oncology Program Study of 360 cancer patients treated in the community. Cancer. 2003;97:2880–2886. doi: 10.1002/cncr.11408. [DOI] [PubMed] [Google Scholar]
- 6.Rau SE, Barber LG, Burgess KE. Efficacy of maropitant in the prevention of delayed vomiting associated with administration of doxorubicin to dogs. J Vet Intern Med. 2010;24:1452–1457. doi: 10.1111/j.1939-1676.2010.0611.x. [DOI] [PubMed] [Google Scholar]
- 7.Barranco SC. Cellular and molecular effects of adriamycin on dividing and nondividing cells. Pharmacol Ther. 1984;24:303–319. doi: 10.1016/0163-7258(84)90007-x. [DOI] [PubMed] [Google Scholar]
- 8.Grdina DJ, Sigdestad CP, Peters LJ. Cytotoxic effect in vivo of selected chemotherapeutic agents on synchronized murine fibrosarcoma cells. Br J Cancer. 1980;42:677–683. doi: 10.1038/bjc.1980.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SH, Kim JH. Lethal effect of adriamycin on the division cycle of HeLa cells. Cancer Res. 1972;32:323–325. [PubMed] [Google Scholar]
- 10.Burholt DR, Etzel SL, Schenken LL, Kovacs CJ. Digestive tract cell proliferation and food consumption patterns of Ha/ICR mice. Cell Tissue Kinet. 1985;18:369–386. doi: 10.1111/j.1365-2184.1985.tb00668.x. [DOI] [PubMed] [Google Scholar]
- 11.Hagemann RF, Stragand JJ. Fasting and refeeding: cell kinetic response of jejunum, ileum and colon. Cell Tissue Kinet. 1977;10:3–14. doi: 10.1111/j.1365-2184.1977.tb00278.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee C, Longo VD. Fasting vs dietary restriction in cellular protection and cancer treatment: from model organisms to patients. Oncogene. 2011;30:3305–3316. doi: 10.1038/onc.2011.91. [DOI] [PubMed] [Google Scholar]
- 13.Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, Longo VD. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci U S A. 2008;105:8215–8220. doi: 10.1073/pnas.0708100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henis-Korenblit S, Zhang P, Hansen M, McCormick M, Lee S, Cary M, Kenyon C. Insulin/IGF-1 signaling mutants reprogram ER stress response regulators to promote longevity. Proc Natl Acad Sci U S A. 2010;107:9730–9735. doi: 10.1073/pnas.1002575107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- 16.Doroshow JH. Topoisomerase II inhibitors: Anthracyclines. In: Chabner BA, Longo DL, editors. Cancer Chemotherapy and Biotherapy: Principles and Practice. Lippincott Williams & Wilkins; Philadelphia, PA: 2011. pp. 356–392. [Google Scholar]
- 17.Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Martin-Montalvo A, Pistoia V, Wei M, Hwang S, Merlino A. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med. 2012;4:124ra127. doi: 10.1126/scitranslmed.3003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee C, Safdie FM, Raffaghello L, Wei M, Madia F, Parrella E, Hwang D, Cohen P, Bianchi G, Longo VD. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. 2010;70:1564–1572. doi: 10.1158/0008-5472.CAN-09-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Safdie FM, Dorff T, Quinn D, Fontana L, Wei M, Lee C, Cohen P, Longo VD. Fasting and cancer treatment in humans: A case series report. Aging. 2009;1:1–20. doi: 10.18632/aging.100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veterinary Co-operative Oncology Group—Common Terminology Criteria for Adverse Events (VCOG-CTCAE) v1.0. A consensus document from the VCOG. Vet Comp Oncol. 2004;2:194–213. doi: 10.1111/j.1476-5810.2004.0053b.x. [DOI] [PubMed] [Google Scholar]
- 21.Vail DM, Pinkerton ME, Young KM. Canine lymphoma and lymphoid leukemias. In: Withrow SJ, Vail DM, Page RL, editors. Small Animal Clinical Oncology. Elsevier Saunders; St Louis, MO: 2013. [Google Scholar]
- 22.Gustafson DL, Rastatter JC, Colombo T, Long ME. Doxorubicin pharmacokinetics: Macromolecule binding, metabolism, and excretion in the context of a physiologic model. J Pharm Sci. 2002;91:1488–1501. doi: 10.1002/jps.10161. [DOI] [PubMed] [Google Scholar]
- 23.Frystyk J, Delhanty PJD, Skjaerbaek C, Baxter RC. Changes in the circulating IGF system during short-term fasting and refeeding in rats. Am J Physiol. 1999;277:E245–E252. doi: 10.1152/ajpendo.1999.277.2.E245. [DOI] [PubMed] [Google Scholar]
- 24.Greer KA, Hughes LM, Masternak MM. Connecting serum IGF-1, body size, and age in the domestic dog. Age. 2011;33:475–483. doi: 10.1007/s11357-010-9182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Queiroga FL, Pérez-Alenza D, Silvan G, Pena L, Lopes CS, Illera JC. Serum and intratumoural GH and IGF-I concentrations: Prognostic factors in the outcome of canine mammary cancer. Res Vet Sci. 2010;89:396–403. doi: 10.1016/j.rvsc.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 26.Maxwell A, Butterwick R, Yateman M, Batt RM, Cotterill A, Camacho-Hubner C. Nutritional modulation of canine insulin-like growth factors and their binding proteins. J Endocrinol. 1998;158:77–85. doi: 10.1677/joe.0.1580077. [DOI] [PubMed] [Google Scholar]
- 27.Tvarijonaviciute A, Tecles F, Carillo JM, Rubio M, Ceron JJ. Serum insulin-like growth factor-1 measurements in dogs: Performance characteristics of an automated assay and study of some sources of variation. Can J Vet Res. 2011;75:312–316. [PMC free article] [PubMed] [Google Scholar]
- 28.Fraval HNA, Roberts JJ. G1 phase Chinese hamster V79-379A cells are inherently more sensitive to platinum bound to their DNA than mid S phase or asynchronously treated cells. Biochem Pharmacol. 1979;28:1575–1580. doi: 10.1016/0006-2952(79)90167-9. [DOI] [PubMed] [Google Scholar]


