Abstract
INTRODUCTION: The present study compared the effect of combination therapy using human apolipoprotein(a) kringle V (rhLK8) to conventional chemotherapy with paclitaxel for human ovarian carcinoma producing high or low levels of vascular endothelial growth factor (VEGF). MATERIALS AND METHODS: Human ovarian carcinoma cells producing high (SKOV3ip1) or low (HeyA8) levels of VEGF were implanted into the peritoneal cavity of female nude mice. Seven days later, mice were randomized into four groups: control (vehicle), paclitaxel [5 mg/kg, weekly intraperitoneal (i.p.) injection], rhLK8 (50 mg/kg, daily i.p. injection), or the combination of paclitaxel and rhLK8. Mice were treated for 4 weeks and examined by necropsy. RESULTS: In mice implanted with SKOV3ip1 cells, rhLK8 treatment had no significant effect on tumor incidence or the volume of ascites but induced a significant decrease in tumor weight compared with control mice. Paclitaxel significantly reduced tumor weight and ascites volume, and combination treatment with paclitaxel and rhLK8 had an additive therapeutic effect. Similarly, in HeyA8 mice, the effect of combination treatment on tumor weight and tumor incidence was statistically significantly greater than that of paclitaxel or rhLK8 alone. Immunohistochemical analysis showed a significant decrease in microvessel density and a marked increase of apoptosis in tumor and tumor-associated endothelial cells in response to combination treatment with paclitaxel and rhLK8. CONCLUSION: Collectively, these results suggest that antiangiogenic therapy with rhLK8 in combination with taxane-based conventional chemotherapy could be effective for the treatment of ovarian carcinomas, regardless of VEGF status.
Introduction
Ovarian cancer is the leading cause of death from gynecological malignancies [1]. Each year, more than 190,000 new cases of ovarian cancer are diagnosed worldwide, which accounts for approximately 4% of all cancers diagnosed in women [2]. Efforts to develop screening or diagnostic tools for early detection of ovarian cancer have not been successful; therefore, a significant number of patients are diagnosed in advanced stages, requiring cytoreductive and systemic therapies such as palliative surgery and chemotherapy, respectively. However, despite an initial favorable response to chemotherapy [3], [4], the heterogeneity and genetic instability of ovarian cancer cells [5] often leads to the development of drug resistance [6], [7], resulting in increased cancer-related mortality. The prognosis of patients with advanced ovarian cancer has not improved over the last few decades [8], and the development of novel therapeutic strategies is therefore of critical importance [9]. Targeting relatively more homogenous and genetically stable host organ microenvironments is a new strategy that was introduced to overcome the drug resistance of tumor cells and produce better therapeutic outcomes [10], [11]. The establishment of a vascular network supplying oxygen and nutrients to tumor cells is one of the most important and critical steps of tumor progression [12]. Consequently, several clinical trials have tested the efficacy of antiangiogenic molecules for blocking the interaction between tumor cells and host factors for angiogenesis, but no significant improvement has been achieved regarding the prognosis of ovarian cancer [2], [13].
Kringle domains are found in many proteins with a diverse array of functions including growth factors and proteases of the blood coagulation and fibrinolysis pathways [14]. Several kringle domains in these proteins have been identified as inhibitors of angiogenesis, even though their parental proteins are not involved in angiogenesis. One of these molecules, angiostatin, includes the first three (or four) kringle domains of human plasminogen and has shown inhibitory effects on angiogenesis in vitro and tumor growth in vivo in preclinical settings [15]; however, in clinical trials, angiostatin did not show significant anticancer effects or improve clinical outcomes [16]. Human apolipoprotein(a) (apo(a)) also consists of tandemly repeated kringle domains homologous to plasminogen kringle IV (KIV), a single copy plasminogen kringle V homolog (KV), and an inactive protease domain. Previously, we demonstrated that the KIV9, KIV10, and KV domains of human apo(a), called LK68, inhibit angiogenesis and tumor growth [17]. In addition, recombinant human apo(a) KV (referred to as rhLK8) also showed antiangiogenic activity that was almost equivalent to that of LK68 [18]. Recently, we showed the therapeutic efficacy of targeting tumor-associated vasculature with rhLK8 in experimental primary and metastatic (bone) prostate carcinoma animal models [19], and on the basis of the results of the preclinical study, rhLK8 has been successfully translated into the phase I clinical trials. Studies determining the indication of the treatment are being expanded.
In this study, we examined the biologic effect of human apo(a) KV (rhLK8) on human ovarian cancer cells growing in the peritoneal cavity of female nude mice. Furthermore, we examined the antiangiogenic mechanism of action of rhLK8 and showed that combination treatment with human apo(a) KV and paclitaxel significantly inhibited tumor growth by inducing apoptosis of tumor cells and tumor-associated endothelial cells.
Materials and Methods
Human Ovarian Cancer Cell Lines and Human Apolipoprotein(a) Kringle V (rhLK8)
Two human ovarian cancer cell lines were selected for this study: SKOV3ip1, which expresses high levels of vascular endothelial growth factor (VEGF) and is associated with increased ascites formation, and HeyA8, which is characterized by low VEGF expression and no ascites formation. Those cell lines are kind gifts of Dr Isaiah J. Fidler (The University of Texas MD Anderson Cancer Center, Houston, TX) [20]. Cells were maintained in monolayer cultures in Eagle's minimal essential medium supplemented with 10% FBS (Life Technologies, Inc, Grand Island, NY), l-glutamine, pyruvate, non-essential amino acids, two-fold vitamins, and penicillin-streptomycin (Invitrogen, Carlsbad, CA) and incubated at 37°C in 5% CO2 and 95% air.
The rhLK8 protein was expressed and purified to homogeneity as previously described [21]. Purified rhLK8 proteins were stored in buffer containing 100 mM NaCl and 150 mM l-glycine (pH 4.2). Paclitaxel (Taxol), purchased from Bristol-Myers Squibb (Princeton, NJ), was diluted in saline for intraperitoneal (i.p.) injection.
Animals
Female athymic nude mice (NCI-nu) were purchased from the Animal Production Area of the National Cancer Institute, Frederick Cancer Research Facility (Frederick, MD). The mice were housed and maintained under specific pathogen-free conditions in facilities approved by the American Association for Accreditation of Laboratory Animal Care and in accordance with all current regulations and standards of the US Department of Agriculture, the US Department of Health and Human Services, and the National Institutes of Health. The mice were used in these experiments in accordance with institutional guidelines when they were 8 to 12 weeks old.
Orthotopic Implantation of Ovarian Cancer Cells in Animal Models
To establish peritoneal ovarian tumors, SKOV3ip1 or HeyA8 cells were harvested from subconfluent cultures by a brief exposure to 0.25% trypsin and 0.02% EDTA. The cells were washed once in serum-free medium and resuspended in Ca2 +-/Mg2 +-free Hank's balanced salt solution. Cell viability was determined by trypan blue exclusion. Only single-cell suspensions of more than 95% viability were used for injection. SKOV3ip1 (1 × 106) or HeyA8 (2.5 × 105) cells in 200 μl of Ca2 +-/Mg2 +-free Hank's balanced salt solution were injected into the peritoneal cavity of female nude mice as previously described [22].
Therapy Experiments
Seven days after cell implantation into the peritoneal cavity, the mice were randomized into four treatment groups (n = 10 mice per group) as follows: (1) control group, daily i.p. injection of vehicle (100 mM NaCl and 150 mM l-glycine, pH 4.2) and weekly i.p. injection of saline; (2) paclitaxel group, weekly i.p. injection of paclitaxel (5 mg/kg) and daily i.p. injection of vehicle; (3) rhLK8 group, daily i.p. injection of rhLK8 (50 mg/kg) and weekly i.p. injection of saline; and (4) combination group, daily i.p. injection of rhLK8 (50 mg/kg) and weekly i.p. injection of paclitaxel (5 mg/kg). Treatments were continued for 4 weeks.
Necropsy and Preparation of Tissues
After 4 weeks of treatment, mice were killed by CO2 inhalation and examined by necropsy. Body weight, tumor incidence, tumor weight, and volume of ascites were recorded. Tumor tissues were embedded in OCT compound (Miles, Inc, Elkhart, IN) and rapidly frozen in liquid nitrogen or fixed in 10% buffered formalin for 24 hours and processed for paraffin blocks.
Immunohistochemical Analyses of Proliferating Cell Nuclear Antigen, Microvessel Density (CD31/platelet endothelial cell adhesion molecule-1 (PECAM-1)), Bcl-2, Bax, and VEGF
Paraffin-embedded tissues were used for identification of proliferating cell nuclear antigen (PCNA)–positive cells as previously described [23]. Frozen tissues used for identification of CD31/PECAM-1 were sectioned (8-10 μm) and fixed in cold acetone. Immunohistochemistry (IHC) procedures for PCNA and CD31/PECAM-1 were performed, and all antibodies and reagents for IHC were purchased from sources exactly as described previously [24]. Control samples exposed to secondary antibody alone showed no specific staining.
For immunohistochemical staining of Bcl-2, Bax, and VEGF, paraffin sections (4-6 μm thick) were mounted on positively charged Superfrost slides (Fisher Scientific, Co, Houston, TX) and dried overnight. Sections were deparaffinized in xylene, dehydrated with a graded series of alcohol [100%, 95%, and 80% ethanol/double-distilled H2O (vol/vol)], rehydrated in phosphate-buffered saline (PBS; pH 7.5), and then microwaved for 5 minutes to improve “antigen retrieval.” The slides were rinsed twice with PBS, and endogenous peroxidase activity was blocked with 3% hydrogen peroxidase in PBS for 12 minutes. Nonspecific reactions were blocked by incubating the sections in a solution containing 5% normal horse serum and 1% normal goat serum for 20 minutes at room temperature (RT). Then, the slides were incubated overnight at 4°C with a 1:50 dilution of polyclonal antibodies against Bcl-2, Bax, or VEGF (Santa Cruz Biotechnology, Dallas, TX). The samples were then rinsed three times with PBS and incubated in HRP-conjugated goat anti-rabbit IgG at the appropriate dilutions for 60 minutes at RT. After rinsing with PBS, the slides were incubated for 5 minutes with DAB (Life Technologies, Inc), rinsed with distilled water, counterstained with Gill's hematoxylin for 1 minute, and mounted with Universal Mount (Life Technologies, Inc).
For quantification of PCNA expression, the number of positive cells was quantified in 10 random fields at × 200 magnification. To quantify mean vessel density, 10 random fields at × 100 magnification were examined for each tumor, and the microvessels within those fields were counted. A single microvessel was defined as a discrete cluster of cells stained positive for CD31 and the presence of lumen [25].
Immunofluorescence Double Staining for CD31 (Endothelial Cells) and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) (Apoptotic Cells)
TUNEL assay was performed following CD31/PECAM-1 immunofluorescent staining as described previously [26]. The TUNEL assay was performed using a commercially available apoptosis detection kit (Promega, Madison, WI) with the following modifications. Samples were fixed with 4% paraformaldehyde for 10 minutes at RT, washed twice with PBS for 5 minutes, and then incubated with 0.2% Triton X-100 for 15 minutes at RT. After two 5-minute washes with PBS, the samples were incubated with equilibration buffer (from kit) for 10 minutes at RT. The equilibration buffer was drained, and the reaction buffer containing equilibration buffer, nucleotide mix, and terminal deoxynucleotidyl transferase (TdT) enzyme was added to the tissue sections and incubated in a humid atmosphere at 37°C for 1 hour in the dark. The reaction was terminated by immersing the samples in 2 × SSC for 15 minutes. Samples were washed three times for 5 minutes to remove unincorporated fluorescein-labeled deoxyuridine triphosphate (dUTP). The samples were incubated with 300 μg/ml Hoechst 33342 for 10 minutes at RT. Fluorescence bleaching was minimized by treating slides with a ProLong anti-fade reagent (Life Technologies, Inc). The apoptotic index of tumor-associated endothelial cells was determined by co-localization of CD31 and TUNEL staining. Endothelial cells and DNA fragmentation in apoptotic cells were identified by red and green fluorescence, respectively, and apoptotic endothelial cells were identified by yellow fluorescence within the nucleus. Apoptotic tumor cells and tumor-associated endothelial cells were identified and counted in five random fields at × 400. Images were captured by an Olympus BX-51 microscope (Olympus America, Inc, Center Valley, PA).
Statistical Analyses
Tumor incidence, tumor weight, ascites volume (Mann-Whitney U test), the number of PCNA-positive cells, and microvessel density (MVD; CD31/PECAM-1) (unpaired Student's t test) were compared in each treatment group. All values are expressed as means ± SD except where indicated.
Results
Effects of rhLK8 on the Tumor Growth
We determined the biologic effects of rhLK8 and paclitaxel on the growth of SKOV3ip1 human ovarian cancer cells producing high levels of VEGF injected into the peritoneal cavity of female nude mice. Paclitaxel significantly reduced tumor weight [0.04 g (0-0.2 g) vs 0.98 g (0.66-1.63g); median (range), P < .01)] and ascites [0.1 ml (0-0.2 ml) vs 0.9 ml (0.5-1.6 ml); median (range), P < .05] compared to control mice. No significant differences in tumor incidence or ascites volume were detected between control mice and mice treated with rhLK8 alone; however, rhLK8 significantly decreased tumor weight compared to the control [Table 1; 0.65 g (0.01-1.3 g) vs 0.98 g (0.66-1.63 g); median (range), P < .05]. Combination treatment with paclitaxel and rhLK8 had an additive effect on reducing tumor weight [control group 0.98 g (0.66-1.63 g) vs combination group 0.01 g (0-0.14 g); median (range), P < .01)] and the volume of ascites [control group 0.9 ml (0.5-1.6 ml) vs 0 ml (0-0.2 ml); median (range), P < .05].
Table 1.
Therapy of Human Ovarian Cancers Growing in the Peritoneal Cavity of Female Nude Mice.
| Treatment Groups | Tumor Incidence | Tumor Weight (g) [Median (range)] | Ascites (ml) [Median (range)] | |
|---|---|---|---|---|
| SKOV3ip1 | Control | 10/10 | 0.98 (0.66-1.63) | 0.9 (0.5-1.6) |
| Paclitaxel | 9/10 | 0.04 (0-0.20)** | 0.1(0-0.2)* | |
| rhLK8 | 10/10 | 0.65 (0.01-1.30)* | 0.5 (0-1.9) | |
| rhLK8 + paclitaxel | 6/10 | 0.01 (0-0.14)** | 0 (0-0.2)* | |
| HeyA8 | Control | 10/10 | 4.0 (0.2-7.2) | NA |
| Paclitaxel | 8/10 | 2.1 (0-3.6)* | NA | |
| rhLK8 | 7/10 | 1.0 (0-6.0)* | NA | |
| rhLK8 + paclitaxel | 5/9* | 0.3 (0-2.4)** | NA |
Note: 1 × 106 SKOV3ip1 cells or 2.5 × 105 HeyA8 cells were implanted into the peritoneum of female nude mice. Seven days later, treatment with vehicle, paclitaxel (5 mg/kg, weekly i.p. injection), and rhLK8 (50 mg/kg, daily i.p. injection) alone or in combination with paclitaxel was started and continued for 4 weeks. Tumor incidence, tumor weight, and ascites volume were determined. *P < .05; **P < .01. NA, not applicable.
The biologic effects of rhLK8 were also examined in mice injected with HeyA8 human ovarian cancer cells producing low levels of VEGF (Table 1). In these mice, tumor weight was significantly reduced by treatment with paclitaxel or rhLK8 alone compared to that in control mice [2.1 g (0-3.6 g) vs 4.0 g (0.2-7.2 g), P < .05 and 1.0 g (0-6.0 g) vs 4.0 g (0.2-7.2 g), P < .05, respectively]. Combination treatment with paclitaxel and rhLK8 had a significant and synergistic effect on decreasing tumor incidence (55.6 % vs 100%, P < .05) and tumor weight [0.3 g (0-2.4 g) vs 4.0 g (0.2-7.2 g), P < .01]. No substantial differences in the body weight of mice were observed among treatment groups (data not shown).
VEGF levels were ~ 10-fold higher in SKOV3ip1 cells than in HeyA8 cells. Treatment of cells with rhLK8 for 48 hours had no significant effect on VEGF levels in SKOV3ip1 or in HeyA8 cells (Figure W1).
Determination of Cellular Proliferation and MVD
Cellular proliferative index was determined according to the number of PCNA-positive cells, and MVD was determined by the number of CD31-positive cells. The number of PCNA-positive cells was significantly lower in paclitaxel-treated SKOV3ip1 tumors than in control mice (64.4 ± 17.3 vs 108.4 ± 24.7, P < .01), whereas no significant reduction was observed in response to rhLK8 treatment (74.0 ± 17.6 vs 108.4 ± 24.7, P > .05). The most significant decrease in the number of PCNA-positive cells was observed in SKOV3ip1 tumors treated with the combination of paclitaxel and rhLK8 (41.0 ± 12.8 vs 108.4 ± 24.7, P < .01; Table 2 and Figure 1A). In HeyA8 tumors, treatment with paclitaxel or rhLK8 alone did not significantly decrease the number of PCNA-positive cells (88.6 ± 16.9 vs 98.4 ± 16.1, P > .05 and 76.1 ± 20.0 vs 98.4 ± 16.1, P > .05, respectively); however, combination treatment significantly reduced the number of PCNA-positive cells (55.9 ± 14.2 vs 98.4 ± 16.1, P < .01; Table 2 and Figure 1B).
Table 2.
Cellular Proliferation, Mean Vessel Density, and Apoptosis of Tumor-Associated Endothelial Cells in Human Ovarian Cancers Growing in the Peritoneal Cavity of Female Nude Mice.
| Treatment Groups | PCNA (Mean ± SD) | CD31 (Mean ± SD) | TUNEL+CD31+ (Mean ± SD) | |
|---|---|---|---|---|
| SKOV3ip1 | Control | 108.4 ± 24.7 | 84.0 ± 27.5 | 0.6 ± 1.0 |
| Paclitaxel | 64.4 ± 17.3** | 73.1 ± 20.4 | 4.0 ± 2.1* | |
| rhLK8 | 74.0 ± 17.6 | 44.0 ± 9.7** | 11.7 ± 4.0** | |
| rhLK8 + paclitaxel | 41.0 ± 12.8** | 29.4 ± 5.7** | 31.3 ± 9.4*** | |
| HeyA8 | Control | 98.4 ± 16.1 | 57.1 ± 18.5 | 0.2 ± 0.4 |
| Paclitaxel | 88.6 ± 16.9 | 40.0 ± 15.7* | 2.7 ± 1.6* | |
| rhLK8 | 76.1 ± 20.0 | 27.0 ± 6.1** | 7.3 ± 3.4** | |
| rhLK8 + paclitaxel | 55.9 ± 14.2** | 14.3 ± 5.0*** | 26.4 ± 10.2*** |
Note: After 4 weeks of treatment, SKOV3ip1 or HeyA8 tumors were harvested and stained with PCNA, CD31/PECAM, and/or TUNEL. For quantification of PCNA, mean vessel density, and apoptotic endothelial cells, the number of positive cells was quantified in 10 random fields at × 200 magnification and × 100 magnification and five random fields at × 400, respectively. *P < .05; **P < .01; ***P < .001.
Figure 1.
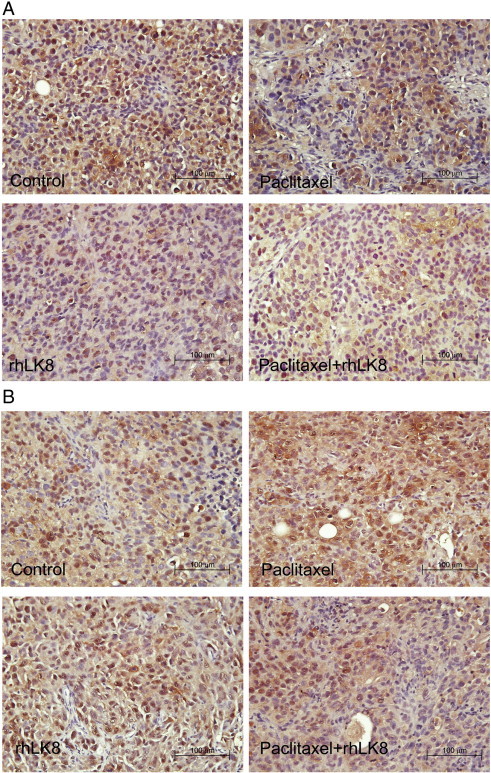
Analysis of cellular proliferation. (A) In SKOV3ip1 tumors, paclitaxel significantly decreased the number of proliferative (PCNA-positive) cells, and this effect was enhanced in response to combination treatment with paclitaxel and rhLK8. (B) In HeyA8 tumors, treatment with paclitaxel alone or rhLK8 alone did not change the cellular proliferative index (PCNA-positive cells), but combination treatment with paclitaxel and rhLK8 significantly decreased the number of PCNA-positive cells.
No significant differences in MVD were detected between control and paclitaxel-treated SKOV3ip1 tumors (84.0 ± 27.5 vs 73.1 ± 20.4, P > .05); however, treatment with rhLK8 alone and, in particular, the combination of rhLK8 and paclitaxel significantly decreased MVD in SKOV3ip1 tumors as compared with the controls (44.0 ± 9.7 vs 84.0 ± 27.5, P < .01 and 29.4 ± 5.7 vs 84.0 ± 27.5, P < 0.01, respectively; Table 2 and Figure 2A). In HeyA8 tumors, MVD was significantly reduced by treatment with paclitaxel compared with the control group (40.0 ± 15.7 vs 57.1 ± 18.5, P < .05) and to a greater extent with rhLK8 alone (27.0 ± 6.1 vs 57.1 ± 18.5, P < .01) or the combination of paclitaxel and rhLK8 (14.3 ± 5.0 vs 57.1 ± 18.5, P < .001; Table 2 and Figure 2B).
Figure 2.
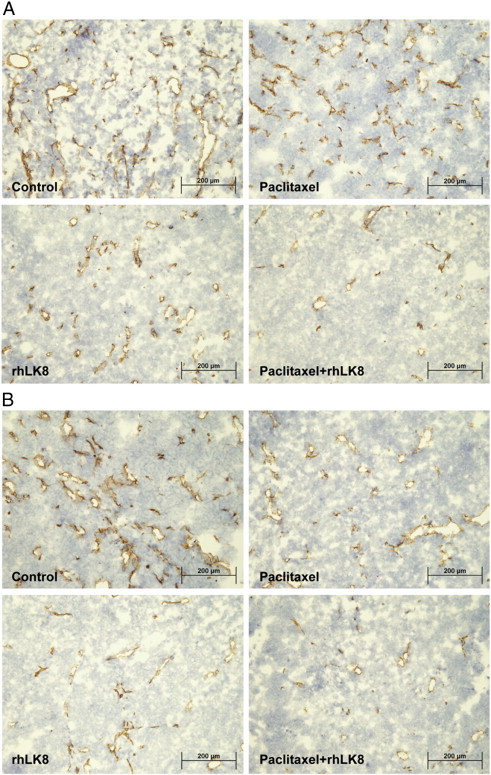
MVD of tumors by immunohistochemical staining of CD31. (A) In SKOV3ip1 tumors, rhLK8 significantly reduced the number of CD31-positive cells, and this effect was enhanced by combination treatment with rhLK8 and paclitaxel. (B) In HeyA8 tumors, treatment with paclitaxel alone significantly decreased MVD as determined by the number of CD31-positive cells, and this effect was intensified by rhLK8 treatment and most significantly by the combination of paclitaxel and rhLK8.
Determination of Apoptosis of Tumor Cells and Tumor-Associated Endothelial Cells by Co-Localization of CD31 and TUNEL
Immunofluorescence double staining of CD31 (red) and TUNEL (green) was performed to evaluate apoptosis of tumor cells and tumor-associated endothelial cells in response to the different treatments. Apoptosis of endothelial cells is indicated by co-localization, detected by a yellow signal. In SKOV3ip1 tumors (Table 2 and Figure 3A), few tumor cells or tumor-associated endothelial cells were apoptotic in the control group. Paclitaxel treatment significantly induced apoptosis in tumor-associated endothelial cells compared with the control group (4.0 ± 2.1 vs 0.6 ± 1.0, P < .05). A more significant increase in apoptosis was induced by rhLK8 alone (11.7 ± 4.0 vs 0.6 ± 1.0; P < .01), and the combination of the two drugs enhanced this effect (31.3 ± 9.4 vs 0.6 ± 1.0, P < .001). A similar trend was observed in HeyA8 tumors (Table 2 and Figure 3B), in which paclitaxel significantly induced apoptosis compared to the control group (2.7 ± 1.6 vs 0.2 ± 0.4, P < .05), and the effect was enhanced by rhLK8 (7.3 ± 3.4 vs 0.2 ± 0.4, P < .01) or the combination of the two drugs (26.4 ± 10.2 vs 0.2 ± 0.4, P < .001). In the SKOV3ip1 and HeyA8 tumor models, apoptosis of tumor cells was induced only in the paclitaxel treatment group and not in the rhLK8 treatment group, whereas the combination of paclitaxel and rhLK8 intensified the apoptosis of tumor cells (Figure 3). On the basis of the findings by Oltval and colleagues [27] suggesting that the Bax/Bcl-2 ratio governs the sensitivity of cells to apoptotic stimuli, we analyzed the expression of Bax and Bcl-2 proteins in the tumor tissues and observed that there were no significant changes in Bcl-2 expression in the tumor tissues from control and treatment groups, but the expression of BAX was significantly increased in the tumor tissues from combination treatment group of mice (Figure W2).
Figure 3.
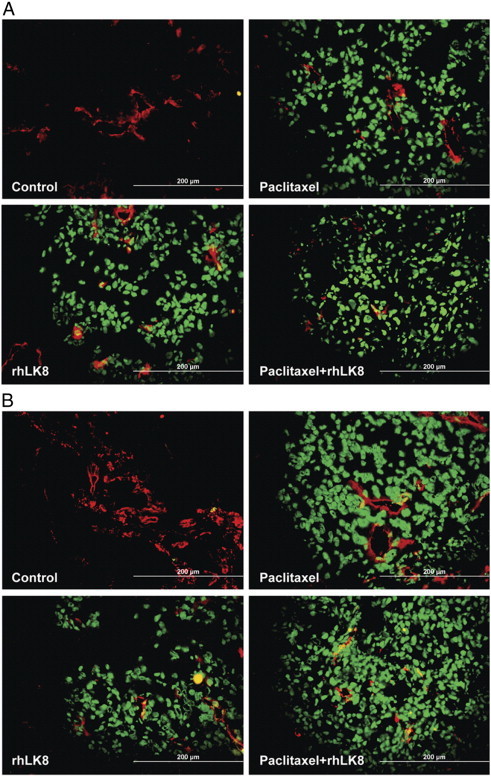
Apoptosis of tumor cells and tumor-associated endothelial cells. Tumor specimens from (A) SKOV3ip1 or (B) HeyA8 tumors were subjected to immunofluorescence double labeling, and the co-localization of signals for CD31 and TUNEL was determined. Treatment with paclitaxel alone significantly induced apoptotic (TUNEL-positive, green) tumor cells and tumor-associated endothelial cells (TUNEL-positive and CD31-positive, yellow), and this effect was intensified by treatment with rhLK8 alone. Combination treatment (paclitaxel plus rhLK8) had the most significant effect on the induction of apoptosis.
To investigate the effects of rhLK8 or paclitaxel treatment, as a single agent or combination of two drugs, on the expression of VEGF in the tumor tissues, immunohistochemical staining of VEGF was performed. VEGF expression in SKOV3ip1 tumors was significantly higher than that in HeyA8 tumors, compared to tumors of control groups (Figure W3). Treatment of mice with either paclitaxel or rhLK8 did not significantly alter the expression of VEGF; however, expression of VEGF in tumors of HeyA8 was slightly increased in tumors of mice treated with either paclitaxel or rhLK8 (Figure W3B). Treatment with the combination of paclitaxel and rhLK8 significantly decreased the expression of VEGF (Figure W3B).
Discussion
Ovarian tumors possess a rich vascular network that is highly dependent on VEGF-mediated angiogenesis [28], [29]. Therefore, many angiogenesis inhibitors have been evaluated in the preclinical and clinical settings for the treatment of ovarian carcinoma [30]. One of the most extensively studied vascular targeting molecules is bevacizumab (Avastin), which neutralizes circulating VEGF and suppresses angiogenesis [31]. Recent phase III clinical trials in first-line ovarian cancers showed that bevacizumab prolonged progression-free survival when administered in combination with chemotherapy [32]. However, the effect of anti-VEGF therapy on overall survival is limited and it is often associated with several clinical toxicities [33], [34]. Moreover, tumor cells can escape from prolonged anti-VEGF therapy by producing other proangiogenic factors [35]. Therefore, the development of antiangiogenic drugs that are effective independent of the VEGF status of tumors is critical.
Our results clearly showed the efficacy of antiangiogenic therapy with rhLK8 in combination with paclitaxel on the proliferation of human ovarian carcinoma cells producing high or low levels of VEGF in a xenograft mouse model. We examined two human ovarian cancer cell lines with significantly different VEGF levels and expected to find differences in the biologic activity of the VEGF axis. Tumors derived from SKOV3ip1 cells grew relatively slower, produced higher levels of VEGF, induced the development of ascites, and showed higher MVD, whereas HeyA8 cells formed larger tumors with lower VEGF expression levels that did not produce ascites and showed lower MVD. Treatment with paclitaxel or rhLK8 as a single agent significantly reduced tumor size but not tumor incidence in both models. Immunohistochemical analysis of tumor specimens revealed that paclitaxel treatment inhibited tumor cell proliferation, whereas rhLK8 showed minimal effects; however, rhLK8 treatment significantly induced apoptosis of tumor-associated endothelial cells and decreased MVD, whereas paclitaxel had no or a minimal effect. Although rhLK8 significantly reduced tumor size in a limited period of time (~ 4 weeks) by inducing apoptosis of tumor-associated endothelial cells, leading to the induction of apoptosis of nearby tumor cells nourished by the same vasculature, it did not affect tumor cell proliferation. These findings suggest that the cytostatic nature of angiogenesis inhibitors, including rhLK8, may limit their ability to control the growth of cancer cells, and combination therapy with chemotherapeutic agents may be necessary to enhance their therapeutic efficacy and to prolong the median survival of patients with ovarian cancer. In this study, we found that antiangiogenic and antitumor efficacy was dramatically improved in mice treated with the combination of paclitaxel and rhLK8. Our results are in agreement with an increasing body of work demonstrating that the combination of angiogenesis inhibitors with chemotherapeutic drugs significantly improves treatment outcomes compared to single agent therapy. Tumor blood vessels are irregular, dilated, tortuous, and leaky, which leads to elevated tumor interstitial fluid pressure and thus inefficient delivery of chemotherapeutic agents [36]. Antiangiogenic therapy may induce the transient normalization of tumor vasculature, which enhances the delivery of chemotherapeutic agents such as paclitaxel by decreasing interstitial pressure, leading to an increase in therapeutic efficacy [37]. In addition, rhLK8 may attenuate the survival pathway of tumor-associated endothelial cells, which makes proliferating tumor-associated endothelial cells more sensitive to anticycling drug, paclitaxel. The improved therapeutic outcomes induced by combination therapy with rhLK8 appear not to be limited to taxane-based chemotherapy. Our preliminary data showed that combination therapy of rhLK8 with gemcitabine or irinotecan (or 5-fluorouracil) improved treatment outcomes than the corresponding treatment as a single agent in the human pancreatic and colon carcinoma animal models, respectively (Kim JS et al., unpublished data). In this context, combination of rhLK8 with other chemotherapeutic agents such as carboplatin or cisplatin, which have been regarded as the primary treatment option of advanced ovarian cancer together with paclitaxel, was also expected to show improved treatment outcomes, although appropriate preclinical and/or clinical evaluation of the combination therapy will be critically required.
Paclitaxel significantly reduced the volume of ascites in SKOV3ip1 tumor–bearing mice but rhLK8 alone did not. However, the effect of rhLK8 on decreasing MVD was more significant than that of paclitaxel. These results are in agreement with prior findings suggesting that ascites formation may depend on the levels of VEGF (originally referred to as vascular permeability factor) produced by tumor cells rather than MVD [38], because paclitaxel-treated tumors were smaller (and displayed a lower tumor burden) than those treated with rhLK8 alone. Interestingly, rhLK8 as a single agent significantly reduced tumor size, and this effect was more pronounced in HeyA8 tumors producing low levels of VEGF. rhLK8 appears not to target tumor cells but inhibits activated endothelial cells. Therefore, antitumor efficacy of rhLK8 was independent on the VEGF expression of the corresponding tumor cells. Because SKOV3ip1 produced the profound amount of biologically active VEGF, they have more biologic redundancy in the survival of tumor-associated endothelial cells than HeyA8, which depends on more tight and narrower angiogenesis activity. Therefore, when rhLK8 inhibits angiogenesis of tumor-associated vasculature, there would be more impact on the HeyA8 tumors. This may explain the synergistic therapeutic effect of rhLK8 on HeyA8 cells. Collectively, these results suggest that VEGF may not be a determinant of the response of certain cancers to antiangiogenic therapy with rhLK8.
In this study, expression of VEGF was increased in HeyA8 tumors of mice treated with paclitaxel or rhLK8. This may be from the local hypoxic condition induced by impaired tumor vasculature caused by either destruction of proliferating tumor-associated endothelial cells by paclitaxel or suppressed angiogenesis by rhLK8. Because intrinsic level of expression in SKOV3ip1 tumors was high, it was not altered by the local hypoxia induced by the treatment with either paclitaxel or rhLK8. Decreased expression of VEGF in tumors of mice treated by the combination of paclitaxel and rhLK8 may reflect the decreased biologic activity or, further, viability of tumor cells from additive or synergistic effects on the induction of the apoptosis of tumor-associated endothelial cells.
Previously, we showed that combination treatment with paclitaxel and rhLK8 could be an effective therapy for prostate cancer metastatic to the bone [19], as well as other solid tumors including colorectal carcinoma, pancreatic carcinoma, renal cell carcinoma, and melanoma (data not shown); however, macroscopic (tumor incidence and tumor size) or microscopic (cellular proliferation, MVD, or apoptosis) responses to therapy with paclitaxel and rhLK8 were not observed in orthotopic animal models of human lung cancer, PC14 cells, which produce high levels of VEGF, and pleural effusion (Kim JS et al., unpublished data).
The mechanisms mediating the different responses to rhLK8 treatment between tumors growing in different organs are not clear. One possible explanation is that rhLK8 may have a differential effect on the biologic properties of tumor-associated endothelial cells, because the specific features of endothelial cells have been reported to be organ-dependent [39], [40]. Furthermore, the interaction of rhLK8 with glucose-regulated protein 78 on the endothelial cell surface plays an important role in rhLK8-mediated apoptosis of endothelial cells (Ahn JH et al., unpublished data), although the impact of this interaction in the endothelial cells of certain organs or that of interactions with other target protein(s) or receptor(s) on the organ-specific therapeutic outcomes mediated by rhLK8 remains unclear. Moreover, tumor cell features such as the activation of some oncogenes and interactions with components of the tumor microenvironment, such as immune cells, may affect the angiogenic phenotype of the tumors [41]. Therefore, the effects of rhLK8 on those factors cannot be ruled out. This possibility is supported by the finding that plasminogen kringle 5, which has significant sequence homology with rhLK8, can exert its antitumor activity either by inhibiting the recruitment of tumor-associated macrophages or by promoting the recruitment of neutrophils or NKT lymphocytes [42], [43].
In conclusion, our results suggest that antiangiogenic therapy with rhLK8 in combination with taxane-based conventional chemotherapy could be a promising therapeutic approach to the treatment of patients with ovarian cancer. Furthermore, the level of VEGF expressed or produced by tumor cells may not be the absolute determinant as the indication of antivascular therapy with rhLK8. Human apo(a) KV, rhLK8, has recently entered phase I clinical trials in patients with cancer. The safety and therapeutic outcomes of the combination of rhLK8 with conventional chemotherapy should also be assessed.
Appendix A. Supplementary data
Figure W1.
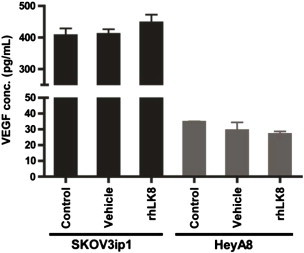
Effect of rhLK8 on VEGF production by human ovarian cancer cells.
Figure W2.
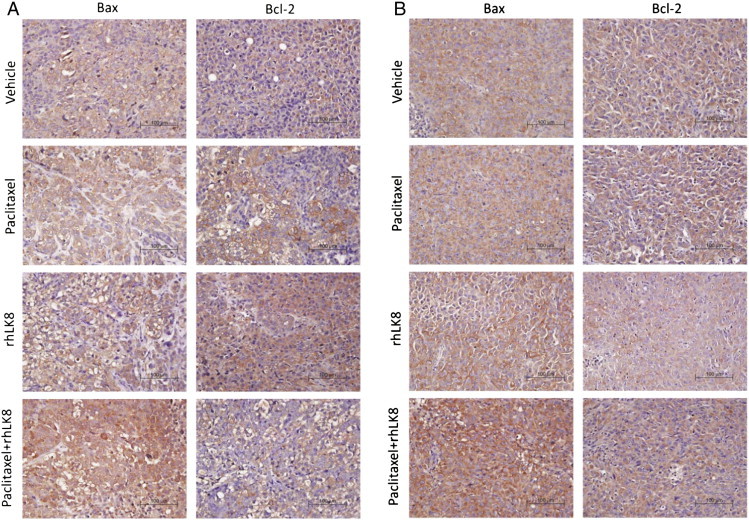
Effects of rhLK8 and/or paclitaxel treatment on the Bcl-2 and Bax expression.
Figure W3.
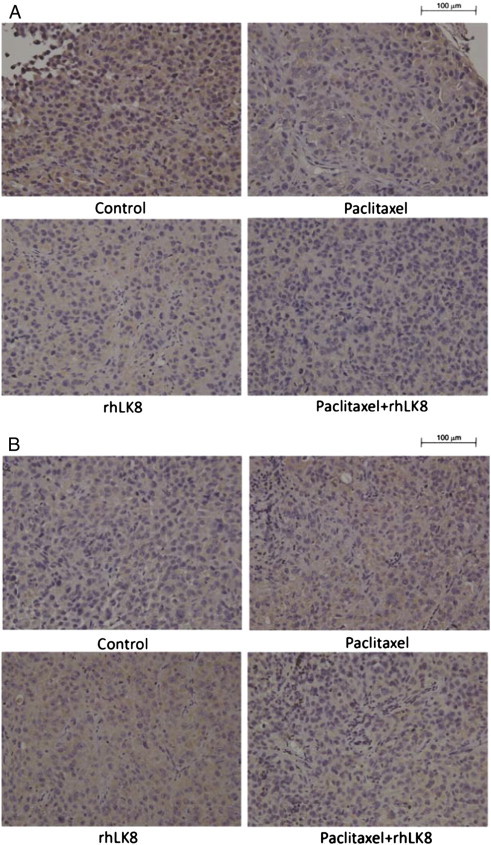
Effects of rhLK8 and/or paclitaxel treatment on the expression of VEGF in the tumor tissues.
Footnotes
This study was financially supported by the Green Cross Corporation, a grant from the Korea Health 21 R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A050905), a grant from the KRIBB Research Initiative Program, and a Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2012R1A1A2007994). The study sponsors had no involvement in the study design; the collection, analysis, and interpretation of the data; the writing of the manuscript; or in the decision to submit the manuscript for publication. In addition, the authors have no potential conflicts of interest to disclose.
This article refers to supplementary materials, which are designated by Figures W1 to W3 and are available online at www.transonc.com.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.tranon.2014.04.005.
Contributor Information
Jang-Seong Kim, Email: jangskim@kribb.re.kr.
Sun Jin Kim, Email: sunkim@mdanderson.org.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Kumaran GC, Jayson GC, Clamp AR. Antiangiogenic drugs in ovarian cancer. Br J Cancer. 2009;100:1–7. doi: 10.1038/sj.bjc.6604767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cannistra SA. Cancer of the ovary. N Engl J Med. 1993;329:1550–1559. doi: 10.1056/NEJM199311183292108. [DOI] [PubMed] [Google Scholar]
- 4.du Bois A, Lück HJ, Meier W, Adams HP, Möbus V, Costa S, Bauknecht T, Richter B, Warm M, Schroder W. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003;95:1320–1329. doi: 10.1093/jnci/djg036. [DOI] [PubMed] [Google Scholar]
- 5.Fidler IJ, Hart IR. Biological diversity in metastatic neoplasms: origins and implications. Science. 1982;217:998–1003. doi: 10.1126/science.7112116. [DOI] [PubMed] [Google Scholar]
- 6.McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, Clarke-Pearson DL, Davidson M. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 7.Bookman MA, McGuire WP, III, Kilpatrick D, Keenan E, Hogan WM, Johnson SW, O'Dwyer P, Rowinsky E, Gallion HH, Ozols RF. Carboplatin and paclitaxel in ovarian carcinoma: a phase I study of the Gynecologic Oncology Group. J Clin Oncol. 1996;14:1895–1902. doi: 10.1200/JCO.1996.14.6.1895. [DOI] [PubMed] [Google Scholar]
- 8.Vaughan S, Coward JI, Bast RC, Jr., Berchuck A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R, Etemadmoghadam D. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer. 2011;11:719–725. doi: 10.1038/nrc3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 10.Fidler IJ. Modulation of the organ microenvironment for treatment of cancer metastasis. J Natl Cancer Inst. 1995;87:1588–1592. doi: 10.1093/jnci/87.21.1588. [DOI] [PubMed] [Google Scholar]
- 11.Kim SJ, Uehara H, Karashima T, Shepherd DL, Killion JJ, Fidler IJ. Blockade of epidermal growth factor receptor signaling in tumor cells and tumor-associated endothelial cells for therapy of androgen-independent human prostate cancer growing in the bone of nude mice. Clin Cancer Res. 2003;9:1200–1210. [PubMed] [Google Scholar]
- 12.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 13.Wenham RM. Ovarian cancer: a bright future. Cancer Control. 2011;18:4–5. doi: 10.1177/107327481101800101. [DOI] [PubMed] [Google Scholar]
- 14.Cao Y, Cao R, Veitonmaki N. Kringle structures and antiangiogenesis. Curr Med Chem Anticancer Agents. 2002;2:667–681. doi: 10.2174/1568011023353705. [DOI] [PubMed] [Google Scholar]
- 15.O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 16.Kurup A, Lin CW, Murry DJ, Dobrolecki L, Estes D, Yiannoutsos CT, Mariano L, Sidor C, Hickey R, Hanna N. Recombinant human angiostatin (rhAngiostatin) in combination with paclitaxel and carboplatin in patients with advanced non-small-cell lung cancer: a phase II study from Indiana University. Ann Oncol. 2006;17:97–103. doi: 10.1093/annonc/mdj055. [DOI] [PubMed] [Google Scholar]
- 17.Kim JS, Chang JH, Yu HK, Ahn JH, Yum JS, Lee SK, Jung KH, Park DH, Yoon Y, Byun SM. Inhibition of angiogenesis and angiogenesis-dependent tumor growth by the cryptic kringle fragments of human apolipoprotein(a) J Biol Chem. 2003;278:29000–29008. doi: 10.1074/jbc.M301042200. [DOI] [PubMed] [Google Scholar]
- 18.Kim JS, Yu HK, Ahn JH, Lee HJ, Hong SW, Jung KH, Chang SI, Hong YK, Joe YA, Byun SM. Human apolipoprotein(a) kringle V inhibits angiogenesis in vitro and in vivo by interfering with the activation of focal adhesion kinases. Biochem Biophys Res Commun. 2004;313:534–540. doi: 10.1016/j.bbrc.2003.11.148. [DOI] [PubMed] [Google Scholar]
- 19.Lee HJ, Yu HK, Papadopoulos JN, Kim SW, He J, Park YK, Yoon Y, Kim JS, Kim SJ. Targeted antivascular therapy with the apolipoprotein(a) kringle V, rhLK8, inhibits the growth and metastasis of human prostate cancer in an orthotopic nude mouse model. Neoplasia. 2012;14:335–343. doi: 10.1593/neo.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang S, Van Arsdall M, Tedjarati S, McCarty M, Wu W, Langley R, Fidler IJ. Contributions of stromal metalloproteinase-9 to angiogenesis and growth of human ovarian carcinoma in mice. J Natl Cancer Inst. 2002;94:1134–1142. doi: 10.1093/jnci/94.15.1134. [DOI] [PubMed] [Google Scholar]
- 21.Kang KY, Kim SG, Kim WK, You HK, Kim YJ, Lee JH, Jung KH, Kim CW. Purification and characterization of a recombinant anti-angiogenic kringle fragment expressed in Escherichia coli: Purification and characterization of a tri-kringle fragment from human apolipoprotein (a) (kringle IV (9)–kringle IV (10)–kringle V) Protein Expr Purif. 2006;45:216–225. doi: 10.1016/j.pep.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 22.Thaker PH, Yazici S, Nilsson MB, Yokoi K, Tsan RZ, He J, Kim SJ, Fidler IJ, Sood AK. Antivascular therapy for orthotopic human ovarian carcinoma through blockade of the vascular endothelial growth factor and epidermal growth factor receptors. Clin Cancer Res. 2005;11:4923–4933. doi: 10.1158/1078-0432.CCR-04-2060. [DOI] [PubMed] [Google Scholar]
- 23.Bruns CJ, Solorzano CC, Harbison MT, Ozawa S, Tsan R, Fan D, Abbruzzese J, Traxler P, Buchdunger E, Radinsky R. Blockade of the epidermal growth factor receptor signaling by a novel tyrosine kinase inhibitor leads to apoptosis of endothelial cells and therapy of human pancreatic carcinoma. Cancer Res. 2000;60:2926–2935. [PubMed] [Google Scholar]
- 24.Jeong SJ, Shin HJ, Kim SJ, Ha GH, Cho BI, Baek KH, Kim CM, Lee CW. Transcriptional abnormality of the hsMAD2 mitotic checkpoint gene is a potential link to hepatocellular carcinogenesis. Cancer Res. 2004;64:8666–8673. doi: 10.1158/0008-5472.CAN-03-3455. [DOI] [PubMed] [Google Scholar]
- 25.Lu C, Kamat AA, Lin YG, Merritt WM, Landen CN, Kim TJ, Spannuth W, Arumugam T, Han LY, Jennings NB. Dual targeting of endothelial cells and pericytes in antivascular therapy for ovarian carcinoma. Clin Cancer Res. 2007;13:4209–4217. doi: 10.1158/1078-0432.CCR-07-0197. [DOI] [PubMed] [Google Scholar]
- 26.Baker CH, Kedar D, McCarty MF, Tsan R, Weber KL, Bucana CD, Fidler IJ. Blockade of epidermal growth factor receptor signaling on tumor cells and tumor-associated endothelial cells for therapy of human carcinomas. Am J Pathol. 2002;161:929–938. doi: 10.1016/S0002-9440(10)64253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oltval ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 28.Ramakrishnan S, Subramanian IV, Yokoyama Y, Geller M. Angiogenesis in normal and neoplastic ovaries. Angiogenesis. 2005;8:169–182. doi: 10.1007/s10456-005-9001-1. [DOI] [PubMed] [Google Scholar]
- 29.Masoumi Moghaddam S, Amini A, Morris DL, Pourgholami MH. Significance of vascular endothelial growth factor in growth and peritoneal dissemination of ovarian cancer. Cancer Metastasis Rev. 2012;31:143–162. doi: 10.1007/s10555-011-9337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teoh D, Secord AA. Antiangiogenic agents in combination with chemotherapy for the treatment of epithelial ovarian cancer. Int J Gynecol Cancer. 2012;22:348–359. doi: 10.1097/IGC.0b013e31823c6efd. [DOI] [PubMed] [Google Scholar]
- 31.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 32.Raja FA, Chopra N, Ledermann JA. Optimal first-line treatment in ovarian cancer. Ann Oncol. 2012;23(Suppl. 10):x118–x127. doi: 10.1093/annonc/mds315. [DOI] [PubMed] [Google Scholar]
- 33.Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB., III Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 34.Okines A, Cunningham D. Current perspective: bevacizumab in colorectal cancer—a time for reappraisal? Eur J Cancer. 2009;45:2452–2461. doi: 10.1016/j.ejca.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 35.Relf M, LeJeune S, Scott PA, Fox S, Smith K, Leek R, Moghaddam A, Whitehouse R, Bicknell R, Harris AL. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor β-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res. 1997;57:963–969. [PubMed] [Google Scholar]
- 36.Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev. 2005;15:102–111. doi: 10.1016/j.gde.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 38.Yoneda J, Kuniyasu H, Crispens MA, Price JE, Bucana CD, Fidler IJ. Expression of angiogenesis-related genes and progression of human ovarian carcinomas in nude mice. J Natl Cancer Inst. 1998;90:447–454. doi: 10.1093/jnci/90.6.447. [DOI] [PubMed] [Google Scholar]
- 39.Langley RR, Ramirez KM, Tsan RZ, Van Arsdall M, Nilsson MB, Fidler IJ. Tissue-specific microvascular endothelial cell lines from H-2K(b)-tsA58 mice for studies of angiogenesis and metastasis. Cancer Res. 2003;63:2971–2976. [PubMed] [Google Scholar]
- 40.Ribatti D, Nico B, Vacca A, Roncali L, Dammacco F. Endothelial cell heterogeneity and organ specificity. J Hematother Stem Cell Res. 2002;11:81–90. doi: 10.1089/152581602753448559. [DOI] [PubMed] [Google Scholar]
- 41.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 42.Perri SR, Nalbantoglu J, Annabi B, Koty Z, Lejeune L, Francois M, Di Falco MR, Béliveau R, Galipeau J. Plasminogen kringle 5-engineered glioma cells block migration of tumor-associated macrophages and suppress tumor vascularization and progression. Cancer Res. 2005;65:8359–8365. doi: 10.1158/0008-5472.CAN-05-0508. [DOI] [PubMed] [Google Scholar]
- 43.Perri SR, Martineau D, Francois M, Lejeune L, Bisson L, Durocher Y, Galipeau J. Plasminogen kringle 5 blocks tumor progression by antiangiogenic and proinflammatory pathways. Mol Cancer Ther. 2007;6:441–449. doi: 10.1158/1535-7163.MCT-06-0434. [DOI] [PubMed] [Google Scholar]


