Abstract
Oxidative damage is a common and early feature of Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis (ALS) and other neurodegenerative disorders. Dr. Mark Smith and his colleagues have built the case for oxidative stress being a primary progenitor rather than a secondary end-stage epiphenomenon of neurodegeneration. They proposed that reactive oxygen species contribute to the “age-related cascade of neurodegeneration” whereby accumulative oxidative damage with age promotes other characteristic pathological changes in afflicted brain regions, including protein aggregation, metabolic deficiencies, and inflammation. Nitric oxide (NO) likely plays a critical role in this age-related cascade. NO is a major signaling molecule produced in the central nervous system (CNS) to modulate neurological activity through stimulating cyclic GMP synthesis. However, the same physiological concentrations of NO relevant in cellular signaling may also initiate and amplify oxidative damage by diffusion-limited reactions with superoxide (O2·−) to produce peroxynitrite (ONOO−). This is perhaps best illustrated in ALS where physiological levels of NO promote survival of motor neurons, but the same concentrations can stimulate motor neuron apoptosis and glial cell activation under pathological conditions. While these changes represent a complex mechanism involving multiple cell types in the pathogenesis of ALS, they also reveal general processes underlying neurodegeneration.
Keywords: nitric oxide, peroxynitrite, amyotrophic lateral sclerosis, neurodegeneration, protein nitration
INTRODUCTION
Widespread oxidative damage to proteins, DNA and lipids is found in many neurodegenerative disorders including Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS) and Parkinson’s disease (PD). However, these biomarkers may only be tombstones that signal the end-stage of a chronic disease process. The work of Dr. Mark Smith and his colleagues over the past two decades has established that reactive oxygen and nitrogen species (ROS/RNS) are involved at the earliest stages of neuronal dysfunction, showing that oxidative stress may drive the disease process rather than just kill neurons outright. His hypotheses on the neurodegenerative cascade incorporate two major observations that other theories fail to address. First, oxidative damage occurs in neuronal populations prior to other pathological hallmarks, such as the presence of neurofibrillary fibers in AD (Nunomura et al., 2001; Pratico et al., 2001) For example, oxidized DNA and protein products are observed prior to the deposition of amyloid-beta protein and neuronal loss in patients with Down’s syndrome (Nunomura et al., 2000). Second, ageing is the primary risk factor of progressive neurodegenerative disorders (Katzman, 1986) and associated with a gradual decline in both the ability to scavenge reactive species and to repair tissue injury.
In 1994, our group developed the first antibodies that recognize nitrotyrosine, an indicator of peroxynitrite-mediated protein damage that persists in tissues (Beckman et al., 1994). While we found extensive nitration in atherosclerosis, lung disease and acute inflammatory diseases, we were disappointed to find little evidence of nitration in advanced cases of AD. We provided the antibody to George Perry and Mark Smith, who identified the widespread presence of nitrated protein tyrosine residues in brain tissue surrounding soft plaques in early-stage AD brains (Smith et al., 1997). Importantly, they observed that protein nitration diminishes in advanced stages of the disease. Since then, nitrated proteins have been identified in the early stages of many neurodegenerative diseases from human cases and animal models (Greenacre and Ischiropoulos, 2001; Casoni et al., 2005; Pacher et al., 2007).
The identification of nitrated proteins in AD brains provided the first evidence for the involvement of nitric oxide (NO)-mediated oxidative damage in the disease. NO itself is neither highly reactive nor particularly toxic under physiological or even pathological conditions. However, it can form peroxynitrite (ONOO−) through a diffusion-limited reaction with superoxide, creating a more potent and specific oxidant (Beckman et al., 1990). While levels of peroxynitrite normally remain low due to effective superoxidescavenging by superoxide dismutase (SOD), even small simultaneous increases in NO and superoxide greatly increases peroxynitrite formation (Beckman and Koppenol, 1996). Peroxynitrite can oxidize biological molecules including lipids, DNA, and proteins through either direct reactions or via spontaneous homolysis to yield the two highly reactive free radicals: nitrogen dioxide (·NO2) and carbonate radical (CO3·−). In particular, these radicals derived from peroxynitrite are capable of modifying tyrosine residues in proteins to form nitrotyrosine, which can drastically effect protein structure and function (Ischiropoulos et al., 1992). The toxic actions of peroxynitrite likely result from reactions with numerous cellular targets, but protein nitration represents a major cytotoxic pathway. Extensive discussion regarding the chemistry surrounding the formation of peroxynitrite, protein nitration, and reaction with biological molecules is beyond the scope of this review but has been described in great detail elsewhere (Beckman, 1996; Pacher et al., 2007; Calcerrada et al., 2011).
Emerging evidence suggests that NO and its oxidation products play a central role in both triggering and amplifying oxidative damage in neurodegeneration. In the CNS, NO is produced as a major signaling molecule that participates in the regulation of blood flow, neurotransmission, memory, and synaptic plasticity. However, these same concentrations of NO that serve many pivotal and beneficial neurological functions also can also mediate cytotoxicity and neurodegeneration. Physiological levels of NO can trigger and amplify neurotoxicity under stressful conditions where superoxide is also being generated. These contrasting roles are perhaps best illustrated in models of ALS, where the same concentrations of NO that promote the survival of motor neurons under normal conditions can also induce apoptosis and glial cell activation following a variety of stressors (Estevez et al., 1998a; Estevez et al., 1998b; Barbeito et al., 2004).
In this review, we describe how the levels of NO produced normally for cellular signaling within the CNS is sufficient to trigger neuronal oxidative stress to induce profound changes in the interactions between neurons and glial cells as well as the activation of neuronal death cascades. While the literature often considers the production from inducible nitric oxide synthase (iNOS) -- commonly associated with macrophages and microglia -- as the source of pathological levels of NO, we describe evidence here that physiological concentrations of NO mediating signaling may be subverted early in neurodegenerative processes through reaction with superoxide to initiate neurodegeneration.
ALS AND SUPEROXIDE DISMUTASE
ALS is characterized by the progressive loss of motor neurons in the motor cortex, brain stem, and spinal cord, which results in increasing muscle weakness, paralysis and death within 3–5 years after symptom onset (Rowland and Shneider, 2001). The vast majority of ALS cases are sporadic without a genetic basis, while 2–3% are associated with mutations in the gene for copper, zinc-superoxide dismutase (SOD1) resulting in disease that is generally indistinguishable from sporadic ALS (Rosen et al., 1993). A majority of the mutations to SOD1 result in the expression of an active protein with full superoxide scavenging activity. Furthermore, SOD1 knockout mice do not develop neuron disease (Reaume et al., 1996), demonstrating that loss of SOD activity is not responsible for disease. However, overexpression of many mutant SOD1 forms in transgenic rats and mice results in development of motor neuron disease, which supports a toxic gain of function in mutant SOD1 (Gurney et al., 1994). SOD1 was previously shown to catalyze peroxynitrite-mediated tyrosine nitration (Ischiropoulos et al., 1992), so it was proposed that SOD1 mutations might enhance nitration (Beckman et al., 1993). This claim is supported by observations of increased tyrosine nitration in both human ALS patients and transgenic ALS animal models (Beal et al., 1997; Ferrante et al., 1997), but the initial concept proved overly simplistic.
Despite nearly two decades of work, the basis for SOD1-induced motor neuron death in ALS remains controversial. However, a general consensus exists that the mutations structurally weaken SOD1 to form a toxic partially unfolded intermediate. One of the key characteristics of SOD1 that is frequently overlooked in ALS is the binding of the two metal cofactors, copper and zinc, which are critical for the functioning of the enzyme (Rhoads et al., 2011). Multiple laboratories have now established that the first step in unfolding of SOD1 is the most likely the loss of the structural zinc atom (Trumbull and Beckman, 2009).
We found that many mutant SOD1 proteins can have identical activities as wild type SOD1 when care was taken to ensure that copper and zinc were both present (Crow et al., 1997a). Furthermore, delivery of mutant SOD1, carefully purified to contain both copper and zinc, were equally protective as wild type Cu,Zn-SOD1 to motor neurons deprived of trophic factors (Estevez et al., 1999; Sahawneh et al., 2010). However, the mutant SOD1 proteins were challenging to prepare replete with both metals. Much of the mutant SOD1 protein lacked zinc when expressed in E. coli (Crow et al., 1997a). This led us to investigate the properties of copper-containing, zinc-deficient SOD1, which proved to be extremely toxic to motor neurons in culture (Estevez et al., 1999). The toxicity required both copper to be bound to the protein as well as NO synthesis by the motor neurons. Of particular importance, the loss of zinc from wild type SOD1 was equally toxic as loss of zinc from mutant SOD1. Competitive blockade of tyrosine nitration also prevented the toxicity of zinc-deficient SOD1 in motor neurons. These cell-based data argue that the loss of zinc from SOD1 is sufficient to instigate the same cell death cascades as trophic factor deprivation and Fas-mediated apoptosis discussed later in this review (Sahawneh et al., 2010). Despite the controversy surrounding the role of SOD1 in promoting oxidative damage, extensive work in primary cultures and in vivo shows that copper in SOD1 interacts with NO and peroxynitrite to initiate the death of motor neurons. The role of copper in zinc-deficient SOD1 toxicity in ALS was recently more fully reviewed than here (Trumbull and Beckman, 2009).
MOTOR NEURON APOPTOSIS MEDIATED BY PEROXYNITRITE
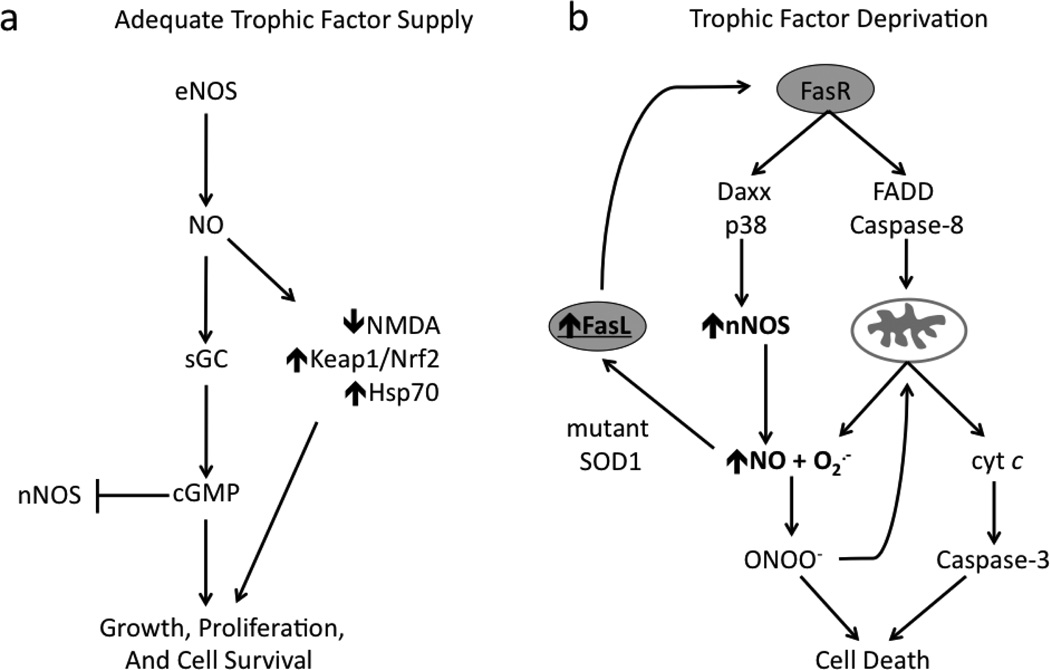
The two faces of NO in motor neuron biology are evidenced through support for survival as well as induction of apoptosis. The survival of motor neurons in culture requires appropriate of several trophic factors (Hughes et al., 1993; Oppenheim, 1996). In the presence of brain-derived neurotrophic factor (BDNF), motor neurons cultured from embryonic rat spinal cord tissue constitutively express endothelial NOS (eNOS), which supports motor neuron survival through binding of NO to the heme group of soluble guanylate cyclase thereby stimulating cyclic GMP (cGMP) synthesis (Estevez et al., 1998b) (Figure 1A). Inhibition of NO synthesis under these same conditions decreases motor neuron survival, while protection is afforded by either by generating steady-state concentrations of <100 nM exogenous NO or by the addition of cell-permeant cGMP analogs.
Figure 1. The neuroprotective and neurotoxic effects of NO in motor neurons.
(A) Motor neurons supported by trophic factors produce NO through constitutive expression of eNOS. NO exerts neuroprotective effects via two mechanisms: stimulation of sGC/cGMP system and induced expression of antioxidant and pro-survival pathways. nNOS expression is blocked in part by sequestration of free intracellular calcium via cGMP and inhibition of calcium influx through NMDA receptor. (B) Trophic factor deprivation in motor neurons leads to activation of the Fas pathway associated with induction of nNOS and increased NO production. Membrane-bound or soluble FasL binding to Fas receptor activates both Daxx and FADD components of the pathway. Downstream of Daxx, NO produced from p38-induced nNOS expression reacts with superoxide (O2·−) to form of peroxynitrite (ONOO−). FADD activates caspase- and mitochondrial-dependent mechanisms of apoptosis. Fas pathway activation can also occur in mutant SOD1-expressing motor neurons exposed to exogenous NO with adequate trophic factor support, suggesting unique susceptibility to Fas- or NO-triggered cell death in familial ALS. Feed-forward amplification of the Fas/NO loop may contribute to the slow, progressive demise of motor neurons.
In contrast, deprivation of trophic factors increases NO production via upregulation of neuronal NOS (nNOS) in cultured motor neurons. Trophic factor deprivation also stimulates co-generation of superoxide, which promotes the endogenous production of peroxynitrite. This led to increased nitrotyrosine immunoreactivity and induction of apoptosis within 24 hours in greater than 60% of cultured neurons (Estevez et al., 1998a). Interestingly, NO-dependent activation of cGMP was later shown to prevent apoptosis in motor neurons in part by blocking the expression of nNOS through sequestration of free intracellular calcium (Estevez et al., 2002) (Figure 1A). Inhibition of NO production in trophic factor-deprived motor neurons prevented increases in nitrotyrosine immunoreactivity and cell death (Estevez et al., 1998a). This protection was lost by the addition of an NO donor maintaining a steady-state concentration of <100 nM, the same concentration that shown protection in models described above. Lower steady-state NO concentrations (5–10 nM) in culture media induce less neuron death at 24 h but are still toxic. After 3 days, lower NO levels can produce cell death to the same extent compared to higher concentrations at 24 hr (Estevez et al., 1998a; Estevez et al., 1998b). Importantly, these rates of NO production showed no toxicity to motor neurons adequately supplied with trophic factors and do not potentiate trophic factor-deprived motor neuron death. Furthermore, intracellular scavenging of superoxide was also protective and prevented the toxicity of exogenous nitric oxide (Estevez et al., 1998b). Together, these results suggest that NO itself is neither sufficient nor limiting to produce motor neuron apoptosis.
Meanwhile, activation of Fas death receptors was identified in motor neuron death during trophic factor deprivation (Raoul et al., 1999). Further investigation showed that Fas-triggered death occurred via two parallel pathways involving induction of nNOS in association with established FADD and caspase-8-mediated mitochondrial cascades of apoptosis (Chinnaiyan et al., 1995; Muzio et al., 1996; Scaffidi et al., 1998; Raoul et al., 2002). This pathway was activated in the presence of low-level extracellular steady-state NO (as low as 10–20 nM) in mutant SOD1-expressing motor neurons with adequate trophic support, but not in nontransgenic or wild-type SOD1-expressing cultures (Raoul et al., 2006), suggesting a unique sensitivity of ALS-associated SOD mutations. Exogenous NO triggered upregulation of membrane-bound Fas ligand (FasL) with subsequent activation of Fas receptor-mediated death cascades in addition to upregulation of nNOS and further NO synthesis (Figure 1B). Antagonistic anti-Fas antibodies and expression of a Daxx-dominant negative form were protective, providing further evidence that NO produced from nNOS upregulation alone was insufficient to induce neuronal death. The amplification of this Fas/NO positive feedback loop was necessary to induce cell death suggesting that its chronic activation may contribute to the slow, progressive motor neuron loss in ALS. Additionally, motor neurons with positive immunoreactivity for several elements of this pathway including FasL, Daxx, and p38 kinase have also been observed in presymptomatic transgenic animals and sporadic human ALS (Bendotti et al., 2004; Raoul et al., 2006), suggesting a common molecular pathway between familial ALS and ALS patients without SOD1 mutations.
The involvement of NO, nNOS, and Fas pathways also extend to motor neuron death in vivo. Upregulation of nNOS occurs in motor neurons prior to iNOS in both ALS patients and pre-symptomatic transgenic animal models (Sasaki et al., 2001a; Chen et al., 2010; Moreno-Lopez et al., 2011), consistent with nNOS having the potential to contribute to motor neuron loss. Motor neuron death following axonal injury is also associated with induction of nNOS and increased nitrotyrosine immunoreactivity (Wu, 1993; Wu et al., 1994a; Wu et al., 1994b; Martin et al., 1999; Martin et al., 2005). Inhibition of NOS offers protection to motor neurons against ventral root aversion, while SOD1 deficiency increases motor neuron vulnerability to the same injury (Wu and Li, 1993; Reaume et al., 1996). Axotomy-induced motor neuron apoptosis was also prevented in Fas-knockout mice and transgenic mice expressing dominant negative form of FADD (Ugolini et al., 2003; Martin et al., 2005). Together, these results suggest that NO/Fas-mediated pathways involved in the induction of apoptosis in cultured motor neurons are also active in vivo and play a role in the degeneration of adult motor neurons.
NO-dependent oxidative damage can also promote apoptosis in motor neurons via mechanisms involving non-neuronal cells. Astrogliosis is associated with increased expression and release of several growth factors and cytokines, including nerve growth factor (NGF) (Vargas et al., 2004). NGF plays an essential role in differentiation and survival of some neuronal populations, but also serves to eliminate damaged neurons and glia (Chao, 2003). NGF mediates apoptosis through the p75 neurotrophin receptor (p75NTR), which induces ceramide-dependent mitochondrial superoxide production and nitrotyrosine immunoreactivity in affected neurons (Barker, 2004; Nykjaer et al., 2005; Pehar et al., 2007). Although p75NTR is presumed lacking in adult motor neurons, expression has been observed following nerve injury and in cases of ALS (Seeburger et al., 1993; Lowry et al., 2001). The re-expression of p75NTR may act as a mechanism to signal or increase susceptibility of damaged motor neurons to undergo apoptosis. Cultured motor neurons expressing p75NTR showed sensitivity to NGF-induced apoptosis with significant immunoreactivity for nitrotyrosine when exposed to low-level (<50 nM) exogenous NO (Pehar et al., 2004). Follow up studies showed that peroxynitrite-mediated nitration of NGF also induced motor neuron apoptosis (Pehar et al., 2006b). Additionally, motor neurons overexpressing ALS-linked SOD1 mutation showed increased susceptibility to NGF-dependent apoptosis occurring in the absence of exogenous NO. This sensitivity was associated with decreased expression of key anti-oxidant elements including Nrf2 expression and glutathione biosynthesis (Pehar et al., 2007).
Importantly, these studies demonstrate that NO-mediated apoptosis occurs in motor neurons via initial induction of nNOS. iNOS is also upregulated in motor neurons from ALS patients and symptomatic transgenic mice, although generally occurs later than increases in nNOS (Moreno-Lopez et al., 2011). NO alone is insufficient to initiate apoptosis in motor neurons, but simultaneous production of superoxide appears to be a critical step in triggering cell death pathways. The above cases demonstrate that trophic factor deprivation, depletion of antioxidant reserves, expression of mutant SOD1, activation of p75 receptor, or other stressors arising from aging or environmental and genetic factors can directly or indirectly increase production of superoxide within motor neurons. Subsequent reaction with NO produced constitutively for physiological purposes can promote oxidative stress and apoptosis via two mechanisms: 1) preventing NO-dependent stimulation of cyclic GMP synthesis and 2) increasing peroxynitrite formation (Estevez et al., 2002).
Although peroxynitrite is known to exert cytotoxicity, among the earliest evidence that it could induce apoptosis was demonstrated in PC12 rat pheochromocytoma cells and cortical cell cultures (Bonfoco et al., 1995; Estevez et al., 1995). Later, we showed that tyrosine-containing peptides could be used to identify the critical role of peroxynitrite-mediated nitration of tyrosine residues in apoptosis of these same cells as well as cultured motor neurons (Ye et al., 2007). These peptides significantly reduced nitrotyrosine immunoreactivity to a similar degree as scavengers of superoxide and peroxynitrite. However, these peptides did not scavenge peroxynitrite, but instead served as competitive inhibitors for tyrosine nitration. These same peptides offered no protection against cell death by hydrogen peroxide or staurosporin (Ye et al., 2007), suggesting that endogenous production of peroxynitrite in motor neurons induces apoptosis through nitration of critical proteins.
ALS AND PROTEIN NITRATION
While peroxynitrite can cause oxidative damage to multiple biological macromolecules, protein nitration represents a major cytotoxic pathway contributing to neurodegenerative disease. The reactions, conditions, and specificity driving peroxynitrite-mediated nitration of protein tyrosine residues has been detailed elsewhere (Koppenol et al., 1992; Beckman, 1996; Radi, 1998; Souza et al., 1999; Ischiropoulos, 2003). Protein nitration is a highly selective process that is limited to specific tyrosine residues on a small number of proteins. Nitration of a single tyrosine residue can induce significant changes in protein structure and function. In the context of neurodegenerative diseases, nitrated proteins have been identified with altered enzyme activity, increased propensity to form aggregates, and ability to elicit immunogenic response (Reynolds et al., 2007). The consequences of protein nitration are particularly relevant in ALS (Crow et al., 1997a; Estevez et al., 1999). Here, we discuss how some of the nitrated proteins found in ALS might effect the development of motor neuron disease.
Structural proteins are major targets for nitration given their high abundance in cells and high proportion of tyrosine residues. Neurofilament proteins are among the most abundant structural proteins found within motor neurons and proper assembly is critical for axon integrity and structure (Hoffman et al., 1987). Mutations in highly conserved regions of neurofilament subunits leads to development of motor neuron disease in humans and transgenic mice, providing intriguing genetic evidence that neurofilament dysfunction alone could be adequate to cause ALS (Figlewicz et al., 1994; Lee et al., 1994). Aggregation of neurofilaments within motor neurons is an early sign of degeneration and a pathological hallmark of ALS. Immunoreactivity for nNOS and SOD1 are found in regions of neurofilament accumulation implicating their involvement in the nitration of neurofilaments in motor neurons (Chou et al., 1996a; Chou et al., 1996b). More specifically, neurofilament light subunit (NF-L) is selectively nitrated by SOD1 in vitro in the head and rod domains, which are essential in intersubunit contact and polymerization of neurofilaments (Crow et al., 1997b). As a consequence of nitration in these regions, nitrated NF-L monomers could inhibit proper assembly of NF-L, ultimately leading to aggregation of the protein. Nitration of less than 10% of total NF-L was sufficient to disrupt neurofilament assembly.
NF-L may also play a critical role in promoting SOD1-catalyzed protein nitration. Neurofilament proteins show a high affinity and binding capacity for zinc (Pierson and Evenson, 1988), and NF-L can specifically extract zinc from wild-type and mutant forms of SOD1. NF-L may form a high affinity and abundant site favoring the accumulation of zinc-deficient SOD1 (Crow et al., 1997a). Zinc-deficient SOD1 shows reduced scavenging activity for superoxide and greater efficiency of peroxynitrite-mediated protein nitration. This process can create a positive feedback loop whereby NF-L removes zinc from SOD, which promotes nitration of proteins, including NF-L itself and increased oxidant levels. Nitrated NF-L inhibits proper subunit assembly leading to increased levels of NF-L monomers, which may further chelate zinc from SOD1 or form protein aggregates. Ultimately, motor neuron death may occur as a result of detrimental effects caused by zinc-deficient SOD1 (Estevez et al., 1999). Therefore, initial nitration of NF-L may serve to propagate motor neuron injury.
Mitochondrial dysfunction associated with oxidative damage is commonly observed in nearly all cases of neurodegeneration. Mitochondrial-localized SOD (SOD2) is responsible for scavenging superoxide in the mitochondrial matrix. While motor neurons develop normally in SOD1-deficient mice (Reaume et al., 1996), SOD2 is essential for survival. SOD2-deficient mice exhibit neonatal lethality with severe neurological deficits and oxidative injury (Lebovitz et al., 1996). Partial knockdown of SOD2 accelerates motor neuron loss and disease progression in transgenic mice expressing mutant SOD1 (Andreassen et al., 2000). SOD2 is quite distinct from SOD1, using manganese to catalyze superoxide dismutation rather than copper in the active site and with a tyrosine at position 34 at the entrance to the active site.
SOD2 was among the first proteins to be identified as an endogenous and specific target of nitration (MacMillan-Crow et al., 1996). Nitration of a single tyrosine residue in close proximity to manganese in the active site leads to complete enzyme inactivation (MacMillan-Crow et al., 1998). Recent studies have demonstrated that peroxynitrite is the responsible agent for SOD2 nitration and subsequent inactivation in vivo (Surmeli et al., 2010). Impaired scavenging of superoxide upon inactivation of SOD2 has significant implications on the formation of peroxynitrite and resulting oxidative damage in mitochondria. Importantly, nitrated SOD2 has been detected PD, ALS, AD, and traumatic brain injury (Aoyama et al., 2000; Bayir et al., 2007) suggesting that peroxynitrite-induced mitochondrial damage is a commonly shared mechanism in various cases of neurodegeneration.
NO serves a physiological role in mitochondria by reversibly inhibiting cytochrome c oxidase (complex IV) of the respiratory chain (Palacios-Callender et al., 2004). This inhibition of electron flow increases electron leakage at upstream complexes, resulting in superoxide production in the matrix and formation of peroxynitrite. Subsequently, peroxynitrite can nitrate and inactivate SOD2, inhibiting scavenging of superoxide and thereby accelerating peroxynitrite formation. Hence, NO production can rapidly amplify mitochondrial dysfunction when mitochondria produce a significant amount of superoxide.
Peroxynitrite formed in the mitochondria can also nitrate other susceptible proteins including cytochrome c, respiratory chain complexes, and enzymes of the tricarboxylic acid cycle, all of which can significantly impact cellular metabolism and redox biology (Radi et al., 2002). For example, nitration of cytochrome c yields a more acidic protein species with peroxidatic activity, leading to increased production of hydrogen peroxide and oxidation of membrane phospholipids in the mitochondrial membrane (Cassina et al., 2000). Most components of the respiratory chain can also be inhibited by peroxynitrite through a variety of oxidative mechanisms including nitration. Complex I (NADH dehydrogenase) is particularly sensitive to nitration and has been implicated in the pathology of PD (Chinta and Andersen, 2011). Damage to the respiratory chain promotes electron leak and increases superoxide production in the mitochondria, amplifying the formation of peroxynitrite and creating a positive feedback loop that further escalates oxidant production and mitochondrial dysfunction.
Mitochondrial dysfunction, along with increased production of superoxide, may have significant implications on the survival of neurons and support by glial cells. Mitochondrial dysfunction in mutant SOD1-expressing astrocytes promotes a reactive phenotype associated with increased superoxide production and mitochondrial protein nitration, which in turn promotes motor neuron degeneration (Cassina et al., 2008). Furthermore, increased superoxide resulting from decreased scavenging by SOD2 could divert NO from stimulating cGMP synthesis and promote oxidative stress and apoptosis through formation of peroxynitrite.
PEROXYNITRITE PROMOTES TOXIC GLIAL PHENOTYPES
Neurodegenerative diseases, including ALS, have long been viewed as diseases mainly affecting vulnerable neuron populations. Increasing evidence suggests that interactions between motor neurons and glial cells, including astrocytes and microglia, play a crucial role in determining the selective vulnerability characteristic of ALS. The involvement of diffusible molecules such as NO and activation of receptor-mediated apoptosis via FasL and NGF suggests that neighboring cells and local environment strongly influence motor neuron survival (Raoul et al., 2000).
Although exuberant astrogliosis is not observed in the spinal cord of ALS patients, reactive astrocytes expressing either GFAP or S100β are hallmarks in ALS (Hirano, 1991; Schiffer et al., 1996; Migheli et al., 1999). The same glial populations have also been shown to increase expression of MnSOD, suggesting a compensatory response to increased oxidative stress (Blaauwgeers et al., 1996). Some degree of gliosis is also found in the lateral descending corticospinal tracts and in the entering points of the tracts into the gray matter (Schiffer et al., 1996), thus forming a continuum along the damaged regions. Microglia also proliferate and become activated in these regions, and invading T cells can be found around the capillaries (McGeer and McGeer, 2002).
Compared to ALS patients, astrocytosis and neuroinflammation is more dramatic in mice and rats carrying SOD1 mutations (Kawamata et al., 1992; Bruijn et al., 1997; Bruijn et al., 1998; Hall et al., 1998; Levine et al., 1999; Alexianu et al., 2001; Olsen et al., 2001; Howland et al., 2002; Henkel et al., 2004). This may be reflective of the extraordinarily rapid development of disease in the SOD transgenic animal models. Reactive astrocytes found in ALS show expression of inflammatory mediators such as iNOS and immunoreactivity for nitrotyrosine (Almer et al., 1999; Sasaki et al., 2000) and down-regulate glutamate transporters (Rothstein et al., 1992; Sasaki et al., 2001b; Howland et al., 2002), suggesting that astrocytes might play a pathogenic role in the disease by both peroxynitrite-mediated and excitotoxic mechanisms.
In co-cultures, astrocytes support the survival and growth of motor neurons. However, upon activation by LPS or cytokines, astrocytes can become neurotoxic by mechanisms involving iNOS expression (Dawson et al., 1994). Production of NO by astrocytes damages mitochondrial complexes in co-cultured neurons and enhances NMDA-induced excitotoxicity (Hewett et al., 1994; Bolanos et al., 1995; Stewart et al., 2000). We have shown that brief exposure to peroxynitrite, but not hydrogen peroxide, was sufficient to elicit an inflammatory-like phenotype in isolated spinal cord astrocytes, and would initiate apoptosis of subsequently co-cultured motor neurons (Cassina et al., 2002). Antioxidant scavengers of peroxynitrite and NOS inhibitors significantly reduced motor neuron loss, suggesting that the neurotoxic effect was dependent on NO production from astrocytes and mediated via peroxynitrite. Similar dependence of motor neuron death upon NO production via iNOS was also observed in activated microglia (Zhao et al., 2004). Both NO and superoxide are produced by motor neurons in vitro in response to trophic factor deprivation, Fas pathway activation, or zinc-deficient SOD1 (Estevez et al., 1998a; Estevez et al., 1999; Raoul et al., 2002). The resultant peroxynitrite formation constitutes a potential mechanism for astrocyte activation in ALS. Peroxynitrite can also hinder communications via gap junctions and inhibit glutamate transporters in astrocytes, preventing their regulatory role on neuronal excitability and neurotransmission (Bolanos and Medina, 1996; Trotti et al., 1996).
Following injury, motor neurons may also secrete inflammatory mediators and cytokines that can induce astrocytes to adopt a reactive phenotype. One such example is fibroblast growth factor 1 (FGF1). FGF1 is expressed at high concentrations in motor neurons (Elde et al., 1991; Kresse et al., 1995). Oxidation of critical sulfhydryl groups leads to the secretion of FGF1, which is mediated is mediated by a copper-dependent assembly of a multi-protein aggregate (Landriscina et al., 2001). Thus, oxidative stress or possibly copper from zinc-deficient SOD in ALS may facilitate the release of FGF1 from motor neurons. Transgenic mice expressing mutant SOD1 show more widely distributed expression of FGF1 than nontransgenic littermates, consistent with FGF1 being released from motor neurons following oxidative stress (Cassina et al., 2005). Although FGF1 shows neurotrophic activity in vitro and is protective in models of spinal cord injury (Walicke, 1988; Cuevas et al., 1995; Teng et al., 1998; Teng et al., 1999), FGF1 effectively transforms astrocytes into a reactive phenotype that induces motor neuron death in co-cultures via a NGF/NO-dependent pathway (Cassina et al., 2005).
Numerous studies have suggested a common link between ALS-associated SOD1 mutations and the involvement of non-neuronal cell populations in motor neuron death. Restricting expression of mutant SOD1 in neurons or astrocytes alone may not be sufficient to induce motor neuron disease (Gong et al., 2000; Pramatarova et al., 2001). Further evidence emerged in chimeric mice possessing mixtures of mutant SOD1-expressing and normal cells, which indicated that toxicity to motor neurons required damage from mutant SOD1 acting within non-neuronal cells. More intriguing, normal non-neuronal cells extended the survival of mutant SOD1-expressing motor neurons (Clement et al., 2003). Reduced expression of mutant SOD1 in astrocytes or microglia in transgenic mice did not affect disease onset but significantly slowed disease progression (Boillee et al., 2006; Yamanaka et al., 2008). These animal models demonstrate that mutant SOD1 expression within non-neuronal cells can be a determinant of disease progression, while the mutant enzyme’s presence in motor neurons contributes to disease onset. However, a recent study showed that such models utilizing conditional transgene expression based on a Cre-loxP strategy was associated with microencephaly, which may cause the loss of SOD1-expressing cells during development leading to observed phenotype (Qiu et al., 2011)
Astrocytes expressing mutant SOD1 are also more prone to adopting an activated, inflammatory phenotype (Hensley et al., 2006), display mitochondrial dysfunction, and increased NO plus superoxide production (Cassina et al., 2008). Interestingly, astrocytes expressing mutant SOD1 decrease the survival of motor neurons in vitro through secretion of soluble neurotoxic factors (Vargas et al., 2006; Nagai et al., 2007). Recently, astrocytes from postmortem tissue of human sporadic and familial ALS cases showed similar toxicity to motor neurons (Haidet-Phillips et al., 2011). Most importantly, knockdown of SOD1 in sporadic ALS cases, with no mutations to the protein, significantly attenuated astrocyte-linked motor neuron death. These results suggest a common link for SOD1 and astrocyte-mediated toxicity leading to motor neuron death in both sporadic and familial ALS.
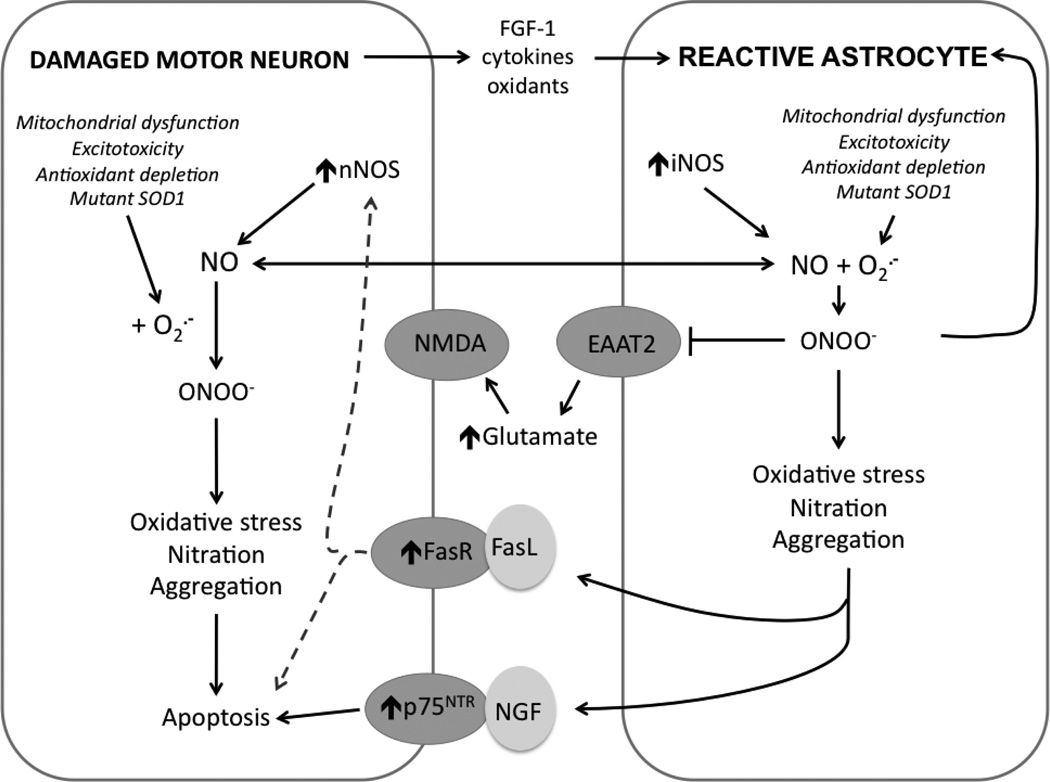
In addition to NO produced from NOS up-regulation and enhanced mitochondrial superoxide production, reactive glial cells also release NGF and various cytokines that promote inflammatory response and motor neuron death (Vargas et al., 2006; Di Giorgio et al., 2007; Nagai et al., 2007; Di Giorgio et al., 2008; Marchetto et al., 2008). Elevated levels of NGF have been reported in multiple sclerosis, AD, and muscle from ALS patients (Crutcher et al., 1993; Fahnestock et al., 1996; Stuerenburg and Kunze, 1998). As described previously, NGF plays an essential role for survival and differentiation of neuronal populations, but can also stimulate death through activation of p75NTR. Recent evidence shows that reactive spinal cord astrocytes secrete an oxidized form of immature precursor NGF, suggesting a novel mechanism for astrocytes to contribute to loss of p75NTR-expressing motor neurons in ALS (Pehar et al., 2004; Pehar et al., 2006a). The interplay between motor neurons and astrocytes is summarized in Figure 2.
Figure 2. Interplay between motor neurons and astrocytes in ALS.
In response to injury or chronic oxidative stress, damaged motor neurons upregulate expression of critical genes involved in their survival/death including nNOS, Fas, and p75NTR, increase superoxide production via numerous sources, and release inflammatory mediators such as FGF-1, cytokines, NO, and other oxidants. These soluble factors promote astrocytes to adopt a reactive phenotype associated with induction of iNOS and mitochondrial dysfunction. Increased production of NO and superoxide favors the formation of peroxynitrite (ONOO−). Peroxynitrite has multiple effects: 1) further promoting reactive astrocyte phenotype, 2) inhibiting glutamate transporters (EAAT2), leading to increased extracellular glutamate concentrations and NMDA-induced excitotoxic injury in motor neurons, and 3) causing oxidative damage to cellular macromolecules leading to increase in general markers of pathology including protein nitration and aggregation. Reactive astrocytes release excessive NO and soluble proapoptotic factors (FasL and NGF) that activate upregulated receptors to amplify damage in affected motor neurons, ultimately leading to apoptosis.
MODEL FOR THE PROGRESSIVE DEATH OF MOTOR NEURONS IN ALS
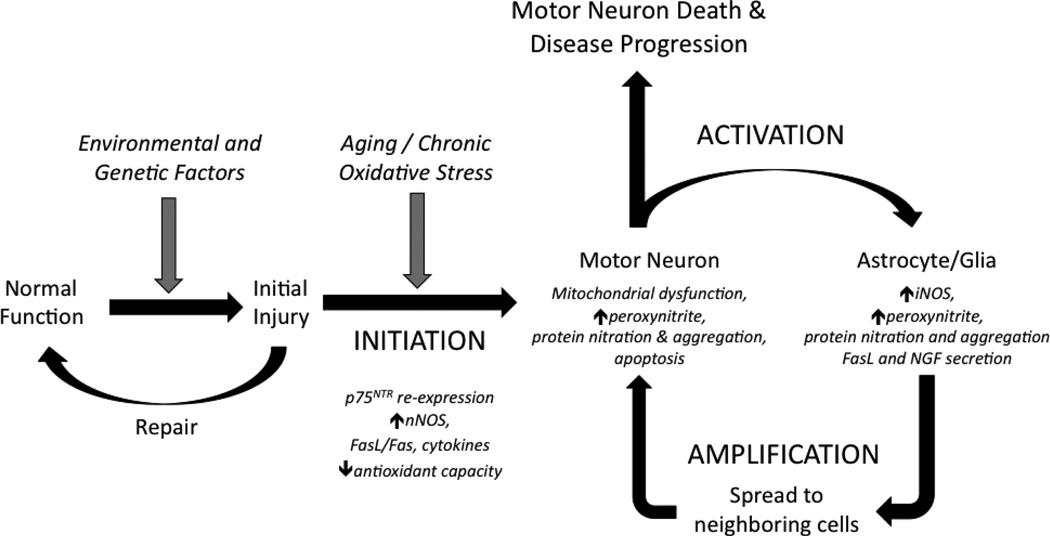
Given the results reviewed here, we hypothesize that NO-mediated oxidative stress serves key roles in the initiation, amplification, and spread of neurodegeneration in ALS (Figure 3). Various environmental insults or genetic defects are capable of inducing damage in motor neurons and astrocytes. In most cases, effective repair mechanisms exist to restore cells to their normal function. However, physiological responses associated with aging or chronic stressors can lead to adaptations in motor neurons including upregulation of nNOS, p75NTR, FasL/Fas, and cytokines, decreased antioxidant capacities and mitochondrial dysfunction. Under normal circumstances, these changes may serve to determine which neurons survive or undergo apoptosis following damage.
Figure 3. Model of progressive neurodegeneration in ALS.
The proposed mechanism leading to motor neuron death in ALS includes three key steps: initiation, activation, and amplification. INITIATION: Cells and tissue possess effective means to repair injury, but aging or chronic stressors including oxidative stress can lead to physiological adaptations aimed to determine cellular survival. Changes including upregulation of nNOS, p75NTR, Fas, and cytokines coupled with decreased anti-oxidant capacity leave damaged motor neurons susceptible to oxidative stress resulting from increased ROS/RNS production. ACTIVATION: Neighboring astrocytes and other glial cells become hyperactive in response to mediators (FGF1, cytokines) released by motor neurons and enact an inflammatory response with induction of iNOS. Pathological changes cause motor neurons to become further damaged as the result of oxidative stress mediated by enhanced production of NO and peroxynitrite. Astrocytes become further activated, adopt a reactive phenotype, and suffer non-lethal peroxynitrite-mediated oxidative damage. AMPLIFICATION: Astrocytes amplify motor neuron damage through production of NO, cytokines, and proapoptotic factors such as NGF and FasL. Both motor neurons and astrocytes display increasing markers of pathology including protein nitration and aggregation and mitochondrial dysfunction. Feed-forward reinforcement of the activation/amplification loop via NO-mediated oxidative damage spreads damage to neighboring cells within tissue via diffusion of NO and secretion of soluble inflammatory and pro-apoptotic factors. Increasing numbers of motor neurons and glia become affected which leads to disease progression.
Neighboring astrocytes adopt a hypertrophic morphology in response to mediators, including peroxynitrite and FGF-1, in an effort to isolate the site of injury, promote the growth and survival of healthy motor neurons, as well as initiate the death of those that are critically damaged. However, physiological adaptations or genetic susceptibilities such as mutant SOD1 expression may cause further damage to neuronal cells through a combination of factors including mitochondrial dysfunction, increased excitatory inputs, enhanced NO production, and nitrative and oxidative stress. Astrocytes become further activated, adopting a reactive phenotype with upregulated NOS activity, and themselves suffer non-lethal peroxynitrite-mediated oxidative stress. Astrocytes further amplify damage to motor neurons through excessive NO production and release of proapoptotic factors such as NGF and FasL. During this process, both motor neurons and astrocytes accumulate general markers of pathology including increased protein nitration, oxidation and aggregation. Functional and structural changes to proteins such as MnSOD and NF-L resulting from nitration may be pathogenic by further propagating oxidative damage and cellular dysfunction. These cumulative changes are sufficient to initiate apoptosis in the initially damaged cells, but diffusion of NO and secretion of soluble inflammatory and proapoptotic factors spreads damage to surrounding cells. Susceptible motor neurons in the vicinity can be damaged directly or activation of neighboring glial cells can render additional motor neurons vulnerable to damage, thus propagating the disease as increasing number of motor neurons are affected.
CONCLUSIONS
Among Dr. Mark Smith’s most significant contributions were his novel views towards origin of neurological disease including the recognition that physiological responses and ageing are fundamental aspects of degenerative processes. In particular, Dr. Smith and colleagues recently proposed an “age-induced cascade of neurodegeneration” whereby chronic and self-propagating oxidative stress plays a key role in the age-related progression of disease (Bonda et al., 2010). A major contribution to this cascade is likely to involve NO because 1) NO promotes oxidative damage through its reaction with superoxide to form peroxynitrite and 2) the production of NO is a key step in many feed-forward loops driving injury and activating death cascades.
The implication of NO in neurodegeneration provides an intriguing case whereby a molecule involved in numerous beneficial physiological processes in the CNS can also be transformed to promote tissue damage and disease progression. Evidence for peroxynitrite-mediated oxidative stress extends beyond ALS to include nearly all neurodegenerative diseases including PD, AD, multiple sclerosis, Huntington’s disease, cerebral ischemia and even the genesis of central chronic pain (Pacher et al., 2007; Calabrese et al., 2009; Salvemini et al., 2011).
ACKNOWLEDGEMENTS
This work was financially supported in part by funding from the National Institute of Health grants NIEHS T32ES07060 & P30ES000210, NINDS R01NS058628A, and NCCAM P01AT002034, as well as support from the Amyotrophic Lateral Sclerosis Association (JSB).
REFERENCES
- Alexianu ME, Kozovska M, Appel SH. Immune reactivity in a mouse model of familial ALS correlates with disease progression. Neurology. 2001;57:1282–1289. doi: 10.1212/wnl.57.7.1282. [DOI] [PubMed] [Google Scholar]
- Almer G, Vukosavic S, Romero N, Przedborski S. Inducible nitric oxide synthase up-regulation in a transgenic mouse model of familial amyotrophic lateral sclerosis. J Neurochem. 1999;72:2415–2425. doi: 10.1046/j.1471-4159.1999.0722415.x. [DOI] [PubMed] [Google Scholar]
- Andreassen OA, Ferrante RJ, Klivenyi P, Klein AM, Shinobu LA, Epstein CJ, Beal MF. Partial deficiency of manganese superoxide dismutase exacerbates a transgenic mouse model of amyotrophic lateral sclerosis. Ann Neurol. 2000;47:447–455. [PubMed] [Google Scholar]
- Aoyama K, Matsubara K, Fujikawa Y, Nagahiro Y, Shimizu K, Umegae N, Hayase N, Shiono H, Kobayashi S. Nitration of manganese superoxide dismutase in cerebrospinal fluids is a marker for peroxynitrite-mediated oxidative stress in neurodegenerative diseases. Ann Neurol. 2000;47:524–527. [PubMed] [Google Scholar]
- Barbeito LH, Pehar M, Cassina P, Vargas MR, Peluffo H, Viera L, Estevez AG, Beckman JS. A role for astrocytes in motor neuron loss in amyotrophic lateral sclerosis. Brain Res Brain Res Rev. 2004;47:263–274. doi: 10.1016/j.brainresrev.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Barker PA. p75NTR is positively promiscuous: novel partners and new insights. Neuron. 2004;42:529–533. doi: 10.1016/j.neuron.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Bayir H, Kagan VE, Clark RS, Janesko-Feldman K, Rafikov R, Huang Z, Zhang X, Vagni V, Billiar TR, Kochanek PM. Neuronal NOS-mediated nitration and inactivation of manganese superoxide dismutase in brain after experimental and human brain injury. J Neurochem. 2007;101:168–181. doi: 10.1111/j.1471-4159.2006.04353.x. [DOI] [PubMed] [Google Scholar]
- Beal MF, Ferrante RJ, Browne SE, Matthews RT, Kowall NW, Brown RH., Jr Increased 3-nitrotyrosine in both sporadic and familial amyotrophic lateral sclerosis. Ann Neurol. 1997;42:644–654. doi: 10.1002/ana.410420416. [DOI] [PubMed] [Google Scholar]
- Beckman JS. Oxidative damage and tyrosine nitration from peroxynitrite. Chem Res Toxicol. 1996;9:836–844. doi: 10.1021/tx9501445. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman JS, Carson M, Smith CD, Koppenol WH. ALS, SOD and peroxynitrite. Nature. 1993;364:584. doi: 10.1038/364584a0. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Ye YZ, Anderson PG, Chen J, Accavitti MA, Tarpey MM, White CR. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe Seyler. 1994;375:81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- Bendotti C, Atzori C, Piva R, Tortarolo M, Strong MJ, DeBiasi S, Migheli A. Activated p38MAPK is a novel component of the intracellular inclusions found in human amyotrophic lateral sclerosis and mutant SOD1 transgenic mice. J Neuropathol Exp Neurol. 2004;63:113–119. doi: 10.1093/jnen/63.2.113. [DOI] [PubMed] [Google Scholar]
- Blaauwgeers HG, Vianney de Jong JM, Verspaget HW, van den Berg FM, Troost D. Enhanced superoxide dismutase-2 immunoreactivity of astrocytes and occasional neurons in amyotrophic lateral sclerosis. J Neurol Sci. 1996;140:21–29. doi: 10.1016/0022-510x(96)00110-4. [DOI] [PubMed] [Google Scholar]
- Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- Bolanos JP, Heales SJ, Land JM, Clark JB. Effect of peroxynitrite on the mitochondrial respiratory chain: differential susceptibility of neurones and astrocytes in primary culture. J Neurochem. 1995;64:1965–1972. doi: 10.1046/j.1471-4159.1995.64051965.x. [DOI] [PubMed] [Google Scholar]
- Bolanos JP, Medina JM. Induction of nitric oxide synthase inhibits gap junction permeability in cultured rat astrocytes. J Neurochem. 1996;66:2091–2099. doi: 10.1046/j.1471-4159.1996.66052091.x. [DOI] [PubMed] [Google Scholar]
- Bonda DJ, Wang X, Perry G, Nunomura A, Tabaton M, Zhu X, Smith MA. Oxidative stress in Alzheimer disease: a possibility for prevention. Neuropharmacology. 2010;59:290–294. doi: 10.1016/j.neuropharm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton SA. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci U S A. 1995;92:7162–7166. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, Cleveland DW. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- Bruijn LI, Houseweart MK, Kato S, Anderson KL, Anderson SD, Ohama E, Reaume AG, Scott RW, Cleveland DW. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science. 1998;281:1851–1854. doi: 10.1126/science.281.5384.1851. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Rizzarelli E, Owen JB, Dinkova-Kostova AT, Butterfield DA. Nitric oxide in cell survival: a janus molecule. Antioxid Redox Signal. 2009;11:2717–2739. doi: 10.1089/ars.2009.2721. [DOI] [PubMed] [Google Scholar]
- Calcerrada P, Peluffo G, Radi R. Nitric Oxide-derived Oxidants with a Focus on Peroxynitrite: Molecular Targets, Cellular Responses and Therapeutic Implications. Curr Pharm Des. 2011 doi: 10.2174/138161211798357719. [DOI] [PubMed] [Google Scholar]
- Casoni F, Basso M, Massignan T, Gianazza E, Cheroni C, Salmona M, Bendotti C, Bonetto V. Protein nitration in a mouse model of familial amyotrophic lateral sclerosis: possible multifunctional role in the pathogenesis. J Biol Chem. 2005;280:16295–16304. doi: 10.1074/jbc.M413111200. [DOI] [PubMed] [Google Scholar]
- Cassina AM, Hodara R, Souza JM, Thomson L, Castro L, Ischiropoulos H, Freeman BA, Radi R. Cytochrome c nitration by peroxynitrite. J Biol Chem. 2000;275:21409–21415. doi: 10.1074/jbc.M909978199. [DOI] [PubMed] [Google Scholar]
- Cassina P, Cassina A, Pehar M, Castellanos R, Gandelman M, de Leon A, Robinson KM, Mason RP, Beckman JS, Barbeito L, Radi R. Mitochondrial dysfunction in SOD1G93A-bearing astrocytes promotes motor neuron degeneration: prevention by mitochondrial-targeted antioxidants. J Neurosci. 2008;28:4115–4122. doi: 10.1523/JNEUROSCI.5308-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassina P, Pehar M, Vargas MR, Castellanos R, Barbeito AG, Estevez AG, Thompson JA, Beckman JS, Barbeito L. Astrocyte activation by fibroblast growth factor-1 and motor neuron apoptosis: implications for amyotrophic lateral sclerosis. J Neurochem. 2005;93:38–46. doi: 10.1111/j.1471-4159.2004.02984.x. [DOI] [PubMed] [Google Scholar]
- Cassina P, Peluffo H, Pehar M, Martinez-Palma L, Ressia A, Beckman JS, Estevez AG, Barbeito L. Peroxynitrite triggers a phenotypic transformation in spinal cord astrocytes that induces motor neuron apoptosis. J Neurosci Res. 2002;67:21–29. doi: 10.1002/jnr.10107. [DOI] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Chen K, Northington FJ, Martin LJ. Inducible nitric oxide synthase is present in motor neuron mitochondria and Schwann cells and contributes to disease mechanisms in ALS mice. Brain Struct Funct. 2010;214:219–234. doi: 10.1007/s00429-009-0226-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Chinta SJ, Andersen JK. Nitrosylation and nitration of mitochondrial complex I in Parkinson's disease. Free Radic Res. 2011;45:53–58. doi: 10.3109/10715762.2010.509398. [DOI] [PubMed] [Google Scholar]
- Chou SM, Wang HS, Komai K. Colocalization of NOS and SOD1 in neurofilament accumulation within motor neurons of amyotrophic lateral sclerosis: an immunohistochemical study. J Chem Neuroanat. 1996a;10:249–258. doi: 10.1016/0891-0618(96)00137-8. [DOI] [PubMed] [Google Scholar]
- Chou SM, Wang HS, Taniguchi A. Role of SOD-1 and nitric oxide/cyclic GMP cascade on neurofilament aggregation in ALS/MND. J Neurol Sci. 1996b;139(Suppl):16–26. doi: 10.1016/0022-510x(96)00090-1. [DOI] [PubMed] [Google Scholar]
- Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillee S, Rule M, McMahon AP, Doucette W, Siwek D, Ferrante RJ, Brown RH, Jr, Julien JP, Goldstein LS, Cleveland DW. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 2003;302:113–117. doi: 10.1126/science.1086071. [DOI] [PubMed] [Google Scholar]
- Crow JP, Sampson JB, Zhuang Y, Thompson JA, Beckman JS. Decreased zinc affinity of amyotrophic lateral sclerosis-associated superoxide dismutase mutants leads to enhanced catalysis of tyrosine nitration by peroxynitrite. J Neurochem. 1997a;69:1936–1944. doi: 10.1046/j.1471-4159.1997.69051936.x. [DOI] [PubMed] [Google Scholar]
- Crow JP, Ye YZ, Strong M, Kirk M, Barnes S, Beckman JS. Superoxide dismutase catalyzes nitration of tyrosines by peroxynitrite in the rod and head domains of neurofilament-L. J Neurochem. 1997b;69:1945–1953. doi: 10.1046/j.1471-4159.1997.69051945.x. [DOI] [PubMed] [Google Scholar]
- Crutcher KA, Scott SA, Liang S, Everson WV, Weingartner J. Detection of NGF-like activity in human brain tissue: increased levels in Alzheimer's disease. J Neurosci. 1993;13:2540–2550. doi: 10.1523/JNEUROSCI.13-06-02540.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas P, Carceller F, Gimenez-Gallego G. Acidic fibroblast growth factor prevents death of spinal cord motoneurons in newborn rats after nerve section. Neurol Res. 1995;17:396–399. [PubMed] [Google Scholar]
- Dawson VL, Brahmbhatt HP, Mong JA, Dawson TM. Expression of inducible nitric oxide synthase causes delayed neurotoxicity in primary mixed neuronal-glial cortical cultures. Neuropharmacology. 1994;33:1425–1430. doi: 10.1016/0028-3908(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell. 2008;3:637–648. doi: 10.1016/j.stem.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Noncell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10:608–614. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elde R, Cao YH, Cintra A, Brelje TC, Pelto-Huikko M, Junttila T, Fuxe K, Pettersson RF, Hokfelt T. Prominent expression of acidic fibroblast growth factor in motor and sensory neurons. Neuron. 1991;7:349–364. doi: 10.1016/0896-6273(91)90288-b. [DOI] [PubMed] [Google Scholar]
- Estevez AG, Crow JP, Sampson JB, Reiter C, Zhuang Y, Richardson GJ, Tarpey MM, Barbeito L, Beckman JS. Induction of nitric oxide-dependent apoptosis in motor neurons by zinc-deficient superoxide dismutase. Science. 1999;286:2498–2500. doi: 10.1126/science.286.5449.2498. [DOI] [PubMed] [Google Scholar]
- Estevez AG, Kamaid A, Thompson JA, Cornwell TL, Radi R, Barbeito L, Beckman JS. Cyclic guanosine 5' monophosphate (GMP) prevents expression of neuronal nitric oxide synthase and apoptosis in motor neurons deprived of trophic factors in rats. Neurosci Lett. 2002;326:201–205. doi: 10.1016/s0304-3940(02)00341-5. [DOI] [PubMed] [Google Scholar]
- Estevez AG, Radi R, Barbeito L, Shin JT, Thompson JA, Beckman JS. Peroxynitrite-induced cytotoxicity in PC12 cells: evidence for an apoptotic mechanism differentially modulated by neurotrophic factors. J Neurochem. 1995;65:1543–1550. doi: 10.1046/j.1471-4159.1995.65041543.x. [DOI] [PubMed] [Google Scholar]
- Estevez AG, Spear N, Manuel SM, Radi R, Henderson CE, Barbeito L, Beckman JS. Nitric oxide and superoxide contribute to motor neuron apoptosis induced by trophic factor deprivation. J Neurosci. 1998a;18:923–931. doi: 10.1523/JNEUROSCI.18-03-00923.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez AG, Spear N, Thompson JA, Cornwell TL, Radi R, Barbeito L, Beckman JS. Nitric oxide-dependent production of cGMP supports the survival of rat embryonic motor neurons cultured with brain-derived neurotrophic factor. J Neurosci. 1998b;18:3708–3714. doi: 10.1523/JNEUROSCI.18-10-03708.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahnestock M, Scott SA, Jette N, Weingartner JA, Crutcher KA. Nerve growth factor mRNA and protein levels measured in the same tissue from normal and Alzheimer's disease parietal cortex. Brain Res Mol Brain Res. 1996;42:175–178. doi: 10.1016/s0169-328x(96)00193-3. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Shinobu LA, Schulz JB, Matthews RT, Thomas CE, Kowall NW, Gurney ME, Beal MF. Increased 3-nitrotyrosine and oxidative damage in mice with a human copper/zinc superoxide dismutase mutation. Ann Neurol. 1997;42:326–334. doi: 10.1002/ana.410420309. [DOI] [PubMed] [Google Scholar]
- Figlewicz DA, Krizus A, Martinoli MG, Meininger V, Dib M, Rouleau GA, Julien JP. Variants of the heavy neurofilament subunit are associated with the development of amyotrophic lateral sclerosis. Hum Mol Genet. 1994;3:1757–1761. doi: 10.1093/hmg/3.10.1757. [DOI] [PubMed] [Google Scholar]
- Gong YH, Parsadanian AS, Andreeva A, Snider WD, Elliott JL. Restricted expression of G86R Cu/Zn superoxide dismutase in astrocytes results in astrocytosis but does not cause motoneuron degeneration. J Neurosci. 2000;20:660–665. doi: 10.1523/JNEUROSCI.20-02-00660.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenacre SA, Ischiropoulos H. Tyrosine nitration: localisation, quantification, consequences for protein function and signal transduction. Free Radic Res. 2001;34:541–581. doi: 10.1080/10715760100300471. [DOI] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Haidet-Phillips AM, Hester ME, Miranda CJ, Meyer K, Braun L, Frakes A, Song S, Likhite S, Murtha MJ, Foust KD, Rao M, Eagle A, Kammesheidt A, Christensen A, Mendell JR, Burghes AH, Kaspar BK. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat Biotechnol. 2011;29:824–828. doi: 10.1038/nbt.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED, Oostveen JA, Gurney ME. Relationship of microglial and astrocytic activation to disease onset and progression in a transgenic model of familial ALS. Glia. 1998;23:249–256. doi: 10.1002/(sici)1098-1136(199807)23:3<249::aid-glia7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Henkel JS, Engelhardt JI, Siklos L, Simpson EP, Kim SH, Pan T, Goodman JC, Siddique T, Beers DR, Appel SH. Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Ann Neurol. 2004;55:221–235. doi: 10.1002/ana.10805. [DOI] [PubMed] [Google Scholar]
- Hensley K, Abdel-Moaty H, Hunter J, Mhatre M, Mou S, Nguyen K, Potapova T, Pye QN, Qi M, Rice H, Stewart C, Stroukoff K, West M. Primary glia expressing the G93A-SOD1 mutation present a neuroinflammatory phenotype and provide a cellular system for studies of glial inflammation. J Neuroinflammation. 2006;3:2. doi: 10.1186/1742-2094-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett SJ, Csernansky CA, Choi DW. Selective potentiation of NMDA-induced neuronal injury following induction of astrocytic iNOS. Neuron. 1994;13:487–494. doi: 10.1016/0896-6273(94)90362-x. [DOI] [PubMed] [Google Scholar]
- Hirano A. Cytopathology of amyotrophic lateral sclerosis. Adv Neurol. 1991;56:91–101. [PubMed] [Google Scholar]
- Hoffman PN, Cleveland DW, Griffin JW, Landes PW, Cowan NJ, Price DL. Neurofilament gene expression: a major determinant of axonal caliber. Proc Natl Acad Sci U S A. 1987;84:3472–3476. doi: 10.1073/pnas.84.10.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B, Erickson J, Kulik J, DeVito L, Psaltis G, DeGennaro LJ, Cleveland DW, Rothstein JD. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS) Proc Natl Acad Sci U S A. 2002;99:1604–1609. doi: 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RA, Sendtner M, Thoenen H. Members of several gene families influence survival of rat motoneurons in vitro and in vivo. J Neurosci Res. 1993;36:663–671. doi: 10.1002/jnr.490360607. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H. Biological selectivity and functional aspects of protein tyrosine nitration. Biochem Biophys Res Commun. 2003;305:776–783. doi: 10.1016/s0006-291x(03)00814-3. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H, Zhu L, Chen J, Tsai M, Martin JC, Smith CD, Beckman JS. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys. 1992;298:431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- Katzman R. Alzheimer's disease. N Engl J Med. 1986;314:964–973. doi: 10.1056/NEJM198604103141506. [DOI] [PubMed] [Google Scholar]
- Kawamata T, Akiyama H, Yamada T, McGeer PL. Immunologic reactions in amyotrophic lateral sclerosis brain and spinal cord tissue. Am J Pathol. 1992;140:691–707. [PMC free article] [PubMed] [Google Scholar]
- Koppenol WH, Moreno JJ, Pryor WA, Ischiropoulos H, Beckman JS. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem Res Toxicol. 1992;5:834–842. doi: 10.1021/tx00030a017. [DOI] [PubMed] [Google Scholar]
- Kresse A, Pettersson R, Hokfelt T. Distribution of acidic fibroblast growth factor mRNA-expressing neurons in the adult mouse central nervous system. J Comp Neurol. 1995;359:323–339. doi: 10.1002/cne.903590210. [DOI] [PubMed] [Google Scholar]
- Landriscina M, Bagala C, Mandinova A, Soldi R, Micucci I, Bellum S, Prudovsky I, Maciag T. Copper induces the assembly of a multiprotein aggregate implicated in the release of fibroblast growth factor 1 in response to stress. J Biol Chem. 2001;276:25549–25557. doi: 10.1074/jbc.M102925200. [DOI] [PubMed] [Google Scholar]
- Lebovitz RM, Zhang H, Vogel H, Cartwright J, Jr, Dionne L, Lu N, Huang S, Matzuk MM. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci U S A. 1996;93:9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MK, Marszalek JR, Cleveland DW. A mutant neurofilament subunit causes massive, selective motor neuron death: implications for the pathogenesis of human motor neuron disease. Neuron. 1994;13:975–988. doi: 10.1016/0896-6273(94)90263-1. [DOI] [PubMed] [Google Scholar]
- Levine JB, Kong J, Nadler M, Xu Z. Astrocytes interact intimately with degenerating motor neurons in mouse amyotrophic lateral sclerosis (ALS) Glia. 1999;28:215–224. [PubMed] [Google Scholar]
- Lowry KS, Murray SS, McLean CA, Talman P, Mathers S, Lopes EC, Cheema SS. A potential role for the p75 low-affinity neurotrophin receptor in spinal motor neuron degeneration in murine and human amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2001;2:127–134. doi: 10.1080/146608201753275463. [DOI] [PubMed] [Google Scholar]
- MacMillan-Crow LA, Crow JP, Kerby JD, Beckman JS, Thompson JA. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci U S A. 1996;93:11853–11858. doi: 10.1073/pnas.93.21.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan-Crow LA, Crow JP, Thompson JA. Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochemistry. 1998;37:1613–1622. doi: 10.1021/bi971894b. [DOI] [PubMed] [Google Scholar]
- Marchetto MC, Muotri AR, Mu Y, Smith AM, Cezar GG, Gage FH. Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell. 2008;3:649–657. doi: 10.1016/j.stem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Chen K, Liu Z. Adult motor neuron apoptosis is mediated by nitric oxide and Fas death receptor linked by DNA damage and p53 activation. J Neurosci. 2005;25:6449–6459. doi: 10.1523/JNEUROSCI.0911-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Kaiser A, Price AC. Motor neuron degeneration after sciatic nerve avulsion in adult rat evolves with oxidative stress and is apoptosis. J Neurobiol. 1999;40:185–201. [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammatory processes in amyotrophic lateral sclerosis. Muscle Nerve. 2002;26:459–470. doi: 10.1002/mus.10191. [DOI] [PubMed] [Google Scholar]
- Migheli A, Cordera S, Bendotti C, Atzori C, Piva R, Schiffer D. S-100beta protein is upregulated in astrocytes and motor neurons in the spinal cord of patients with amyotrophic lateral sclerosis. Neurosci Lett. 1999;261:25–28. doi: 10.1016/s0304-3940(98)01001-5. [DOI] [PubMed] [Google Scholar]
- Moreno-Lopez B, Sunico CR, Gonzalez-Forero D. NO orchestrates the loss of synaptic boutons from adult "sick" motoneurons: modeling a molecular mechanism. Mol Neurobiol. 2011;43:41–66. doi: 10.1007/s12035-010-8159-8. [DOI] [PubMed] [Google Scholar]
- Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, Mann M, Krammer PH, Peter ME, Dixit VM. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death--inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Pappolla MA, Friedland RP, Hirai K, Chiba S, Smith MA. Neuronal oxidative stress precedes amyloid-beta deposition in Down syndrome. J Neuropathol Exp Neurol. 2000;59:1011–1017. doi: 10.1093/jnen/59.11.1011. [DOI] [PubMed] [Google Scholar]
- Nykjaer A, Willnow TE, Petersen CM. p75NTR--live or let die. Curr Opin Neurobiol. 2005;15:49–57. doi: 10.1016/j.conb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Olsen MK, Roberds SL, Ellerbrock BR, Fleck TJ, McKinley DK, Gurney ME. Disease mechanisms revealed by transcription profiling in SOD1-G93A transgenic mouse spinal cord. Ann Neurol. 2001;50:730–740. doi: 10.1002/ana.1252. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW. Neurotrophic survival molecules for motoneurons: an embarrassment of riches. Neuron. 1996;17:195–197. doi: 10.1016/s0896-6273(00)80151-8. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios-Callender M, Quintero M, Hollis VS, Springett RJ, Moncada S. Endogenous NO regulates superoxide production at low oxygen concentrations by modifying the redox state of cytochrome c oxidase. Proc Natl Acad Sci U S A. 2004;101:7630–7635. doi: 10.1073/pnas.0401723101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehar M, Cassina P, Vargas MR, Castellanos R, Viera L, Beckman JS, Estevez AG, Barbeito L. Astrocytic production of nerve growth factor in motor neuron apoptosis: implications for amyotrophic lateral sclerosis. J Neurochem. 2004;89:464–473. doi: 10.1111/j.1471-4159.2004.02357.x. [DOI] [PubMed] [Google Scholar]
- Pehar M, Cassina P, Vargas MR, Xie Y, Beckman JS, Massa SM, Longo FM, Barbeito L. Modulation of p75-dependent motor neuron death by a small non-peptidyl mimetic of the neurotrophin loop 1 domain. Eur J Neurosci. 2006a;24:1575–1580. doi: 10.1111/j.1460-9568.2006.05040.x. [DOI] [PubMed] [Google Scholar]
- Pehar M, Vargas MR, Robinson KM, Cassina P, Diaz-Amarilla PJ, Hagen TM, Radi R, Barbeito L, Beckman JS. Mitochondrial superoxide production and nuclear factor erythroid 2-related factor 2 activation in p75 neurotrophin receptor-induced motor neuron apoptosis. J Neurosci. 2007;27:7777–7785. doi: 10.1523/JNEUROSCI.0823-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehar M, Vargas MR, Robinson KM, Cassina P, England P, Beckman JS, Alzari PM, Barbeito L. Peroxynitrite transforms nerve growth factor into an apoptotic factor for motor neurons. Free Radic Biol Med. 2006b;41:1632–1644. doi: 10.1016/j.freeradbiomed.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Pierson KB, Evenson MA. 200 Kd neurofilament protein binds Al, Cu and Zn. Biochem Biophys Res Commun. 1988;152:598–604. doi: 10.1016/s0006-291x(88)80080-9. [DOI] [PubMed] [Google Scholar]
- Pramatarova A, Laganiere J, Roussel J, Brisebois K, Rouleau GA. Neuron-specific expression of mutant superoxide dismutase 1 in transgenic mice does not lead to motor impairment. J Neurosci. 2001;21:3369–3374. doi: 10.1523/JNEUROSCI.21-10-03369.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratico D, Uryu K, Leight S, Trojanoswki JQ, Lee VM. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci. 2001;21:4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu L, Rivera-Perez JA, Xu Z. A non-specific effect associated with conditional transgene expression based on Cre-loxP strategy in mice. PLoS One. 2011;6:e18778. doi: 10.1371/journal.pone.0018778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radi R. Peroxynitrite reactions and diffusion in biology. Chem Res Toxicol. 1998;11:720–721. doi: 10.1021/tx980096z. [DOI] [PubMed] [Google Scholar]
- Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med. 2002;33:1451–1464. doi: 10.1016/s0891-5849(02)01111-5. [DOI] [PubMed] [Google Scholar]
- Raoul C, Buhler E, Sadeghi C, Jacquier A, Aebischer P, Pettmann B, Henderson CE, Haase G. Chronic activation in presymptomatic amyotrophic lateral sclerosis (ALS) mice of a feedback loop involving Fas, Daxx, and FasL. Proc Natl Acad Sci U S A. 2006;103:6007–6012. doi: 10.1073/pnas.0508774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoul C, Estevez AG, Nishimune H, Cleveland DW, deLapeyriere O, Henderson CE, Haase G, Pettmann B. Motoneuron death triggered by a specific pathway downstream of Fas. potentiation by ALS-linked SOD1 mutations. Neuron. 2002;35:1067–1083. doi: 10.1016/s0896-6273(02)00905-4. [DOI] [PubMed] [Google Scholar]
- Raoul C, Henderson CE, Pettmann B. Programmed cell death of embryonic motoneurons triggered through the Fas death receptor. J Cell Biol. 1999;147:1049–1062. doi: 10.1083/jcb.147.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoul C, Pettmann B, Henderson CE. Active killing of neurons during development and following stress: a role for p75(NTR) and Fas? Curr Opin Neurobiol. 2000;10:111–117. doi: 10.1016/s0959-4388(99)00055-0. [DOI] [PubMed] [Google Scholar]
- Reaume AG, Elliott JL, Hoffman EK, Kowall NW, Ferrante RJ, Siwek DF, Wilcox HM, Flood DG, Beal MF, Brown RH, Jr, Scott RW, Snider WD. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat Genet. 1996;13:43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- Reynolds MR, Berry RW, Binder LI. Nitration in neurodegeneration: deciphering the "Hows" "nYs". Biochemistry. 2007;46:7325–7336. doi: 10.1021/bi700430y. [DOI] [PubMed] [Google Scholar]
- Rhoads TW, Lopez NI, Zollinger DR, Morre JT, Arbogast BL, Maier CS, DeNoyer L, Beckman JS. Measuring copper and zinc superoxide dismutase from spinal cord tissue using electrospray mass spectrometry. Anal Biochem. 2011;415:52–58. doi: 10.1016/j.ab.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin LJ, Kuncl RW. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N Engl J Med. 1992;326:1464–1468. doi: 10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- Sahawneh MA, Ricart KC, Roberts BR, Bomben VC, Basso M, Ye Y, Sahawneh J, Franco MC, Beckman JS, Estevez AG. Cu,Znsuperoxide dismutase increases toxicity of mutant and zinc-deficient superoxide dismutase by enhancing protein stability. J Biol Chem. 2010;285:33885–33897. doi: 10.1074/jbc.M110.118901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini D, Little JW, Doyle T, Neumann WL. Roles of reactive oxygen and nitrogen species in pain. Free Radic Biol Med. 2011;51:951–966. doi: 10.1016/j.freeradbiomed.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S, Shibata N, Iwata M. Neuronal nitric oxide synthase immunoreactivity in the spinal cord in amyotrophic lateral sclerosis. Acta Neuropathol. 2001a;101:351–357. doi: 10.1007/s004010000282. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Shibata N, Komori T, Iwata M. iNOS and nitrotyrosine immunoreactivity in amyotrophic lateral sclerosis. Neurosci Lett. 2000;291:44–48. doi: 10.1016/s0304-3940(00)01370-7. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Warita H, Abe K, Komori T, Iwata M. EAAT1 and EAAT2 immunoreactivity in transgenic mice with a G93A mutant SOD1 gene. Neuroreport. 2001b;12:1359–1362. doi: 10.1097/00001756-200105250-00014. [DOI] [PubMed] [Google Scholar]
- Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer D, Cordera S, Cavalla P, Migheli A. Reactive astrogliosis of the spinal cord in amyotrophic lateral sclerosis. J Neurol Sci. 1996;139(Suppl):27–33. doi: 10.1016/0022-510x(96)00073-1. [DOI] [PubMed] [Google Scholar]
- Seeburger JL, Tarras S, Natter H, Springer JE. Spinal cord motoneurons express p75NGFR and p145trkB mRNA in amyotrophic lateral sclerosis. Brain Res. 1993;621:111–115. doi: 10.1016/0006-8993(93)90304-6. [DOI] [PubMed] [Google Scholar]
- Smith MA, Richey Harris PL, Sayre LM, Beckman JS, Perry G. Widespread peroxynitrite-mediated damage in Alzheimer's disease. J Neurosci. 1997;17:2653–2657. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza JM, Daikhin E, Yudkoff M, Raman CS, Ischiropoulos H. Factors determining the selectivity of protein tyrosine nitration. Arch Biochem Biophys. 1999;371:169–178. doi: 10.1006/abbi.1999.1480. [DOI] [PubMed] [Google Scholar]
- Stewart VC, Sharpe MA, Clark JB, Heales SJ. Astrocyte-derived nitric oxide causes both reversible and irreversible damage to the neuronal mitochondrial respiratory chain. J Neurochem. 2000;75:694–700. doi: 10.1046/j.1471-4159.2000.0750694.x. [DOI] [PubMed] [Google Scholar]
- Stuerenburg HJ, Kunze K. Tissue concentrations of nerve growth factor in aging rat heart and skeletal muscle. Muscle Nerve. 1998;21:404–406. doi: 10.1002/(sici)1097-4598(199803)21:3<404::aid-mus17>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Surmeli NB, Litterman NK, Miller AF, Groves JT. Peroxynitrite Mediates Active Site Tyrosine Nitration in Manganese Superoxide Dismutase. Evidence of a Role for the Carbonate Radical Anion. J Am Chem Soc. 2010 doi: 10.1021/ja105684w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng YD, Mocchetti I, Taveira-DaSilva AM, Gillis RA, Wrathall JR. Basic fibroblast growth factor increases long-term survival of spinal motor neurons and improves respiratory function after experimental spinal cord injury. J Neurosci. 1999;19:7037–7047. doi: 10.1523/JNEUROSCI.19-16-07037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng YD, Mocchetti I, Wrathall JR. Basic and acidic fibroblast growth factors protect spinal motor neurones in vivo after experimental spinal cord injury. Eur J Neurosci. 1998;10:798–802. doi: 10.1046/j.1460-9568.1998.00100.x. [DOI] [PubMed] [Google Scholar]
- Trotti D, Rossi D, Gjesdal O, Levy LM, Racagni G, Danbolt NC, Volterra A. Peroxynitrite inhibits glutamate transporter subtypes. J Biol Chem. 1996;271:5976–5979. doi: 10.1074/jbc.271.11.5976. [DOI] [PubMed] [Google Scholar]
- Trumbull KA, Beckman JS. A role for copper in the toxicity of zinc-deficient superoxide dismutase to motor neurons in amyotrophic lateral sclerosis. Antioxid Redox Signal. 2009;11:1627–1639. doi: 10.1089/ars.2009.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugolini G, Raoul C, Ferri A, Haenggeli C, Yamamoto Y, Salaun D, Henderson CE, Kato AC, Pettmann B, Hueber AO. Fas/tumor necrosis factor receptor death signaling is required for axotomy-induced death of motoneurons in vivo. J Neurosci. 2003;23:8526–8531. doi: 10.1523/JNEUROSCI.23-24-08526.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas MR, Pehar M, Cassina P, Beckman JS, Barbeito L. Increased glutathione biosynthesis by Nrf2 activation in astrocytes prevents p75NTR-dependent motor neuron apoptosis. J Neurochem. 2006;97:687–696. doi: 10.1111/j.1471-4159.2006.03742.x. [DOI] [PubMed] [Google Scholar]
- Vargas MR, Pehar M, Cassina P, Estevez AG, Beckman JS, Barbeito L. Stimulation of nerve growth factor expression in astrocytes by peroxynitrite. In Vivo. 2004;18:269–274. [PubMed] [Google Scholar]
- Walicke PA. Basic and acidic fibroblast growth factors have trophic effects on neurons from multiple CNS regions. J Neurosci. 1988;8:2618–2627. doi: 10.1523/JNEUROSCI.08-07-02618.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W. Expression of nitric-oxide synthase (NOS) in injured CNS neurons as shown by NADPH diaphorase histochemistry. Exp Neurol. 1993;120:153–159. doi: 10.1006/exnr.1993.1050. [DOI] [PubMed] [Google Scholar]
- Wu W, Li L. Inhibition of nitric oxide synthase reduces motoneuron death due to spinal root avulsion. Neurosci Lett. 1993;153:121–124. doi: 10.1016/0304-3940(93)90303-3. [DOI] [PubMed] [Google Scholar]
- Wu W, Li Y, Schinco FP. Expression of c-jun and neuronal nitric oxide synthase in rat spinal motoneurons following axonal injury. Neurosci Lett. 1994a;179:157–161. doi: 10.1016/0304-3940(94)90958-x. [DOI] [PubMed] [Google Scholar]
- Wu W, Liuzzi FJ, Schinco FP, Depto AS, Li Y, Mong JA, Dawson TM, Snyder SH. Neuronal nitric oxide synthase is induced in spinal neurons by traumatic injury. Neuroscience. 1994b;61:719–726. doi: 10.1016/0306-4522(94)90394-8. [DOI] [PubMed] [Google Scholar]
- Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11:251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Quijano C, Robinson KM, Ricart KC, Strayer AL, Sahawneh MA, Shacka JJ, Kirk M, Barnes S, Accavitti-Loper MA, Radi R, Beckman JS, Estevez AG. Prevention of peroxynitrite-induced apoptosis of motor neurons and PC12 cells by tyrosine-containing peptides. J Biol Chem. 2007;282:6324–6337. doi: 10.1074/jbc.M610800200. [DOI] [PubMed] [Google Scholar]
- Zhao W, Xie W, Le W, Beers DR, He Y, Henkel JS, Simpson EP, Yen AA, Xiao Q, Appel SH. Activated microglia initiate motor neuron injury by a nitric oxide and glutamate-mediated mechanism. J Neuropathol Exp Neurol. 2004;63:964–977. doi: 10.1093/jnen/63.9.964. [DOI] [PubMed] [Google Scholar]