Abstract
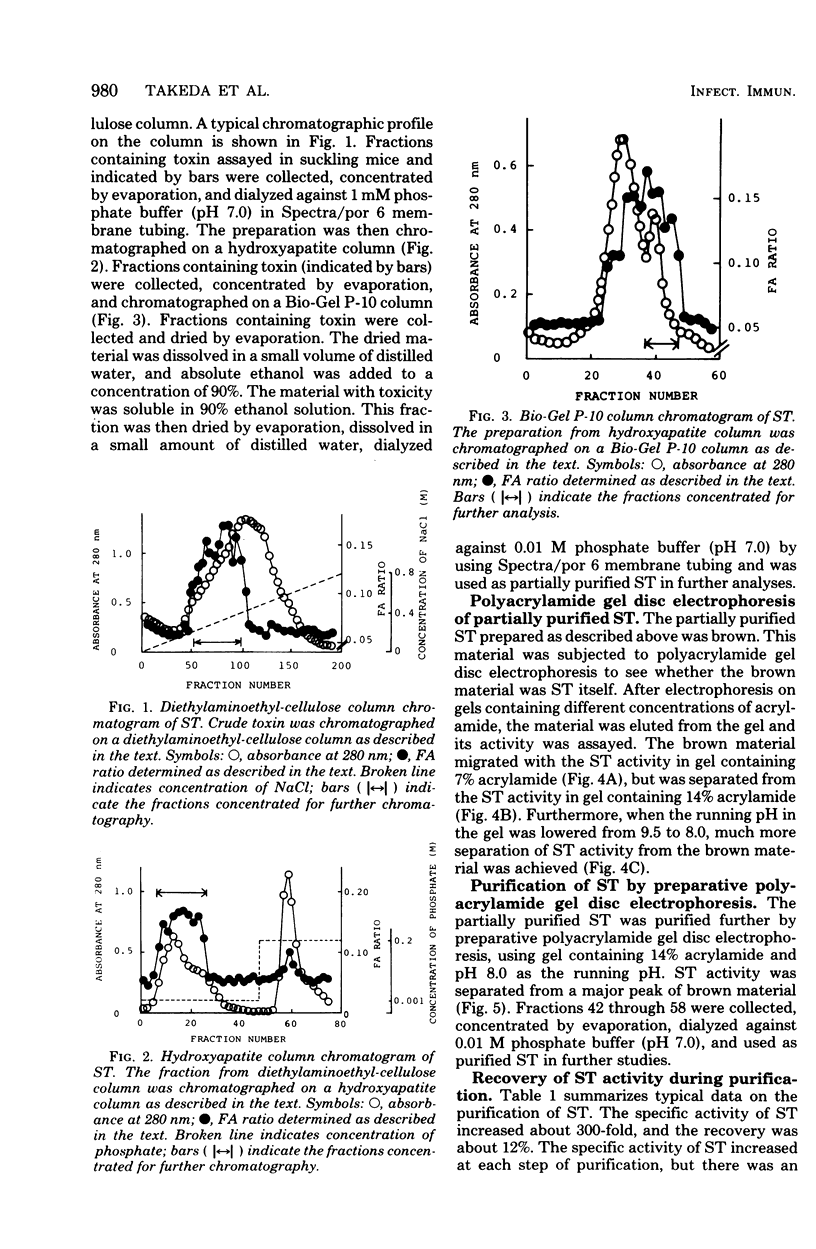
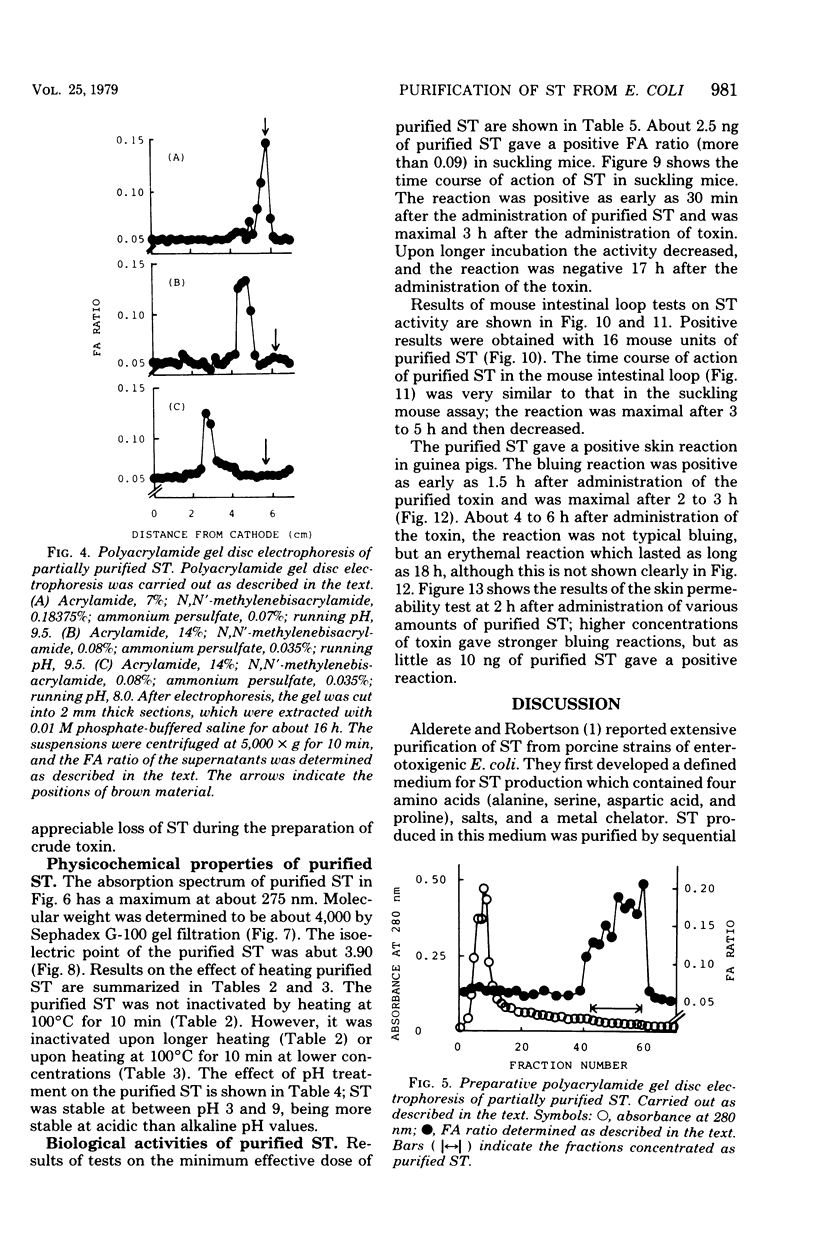
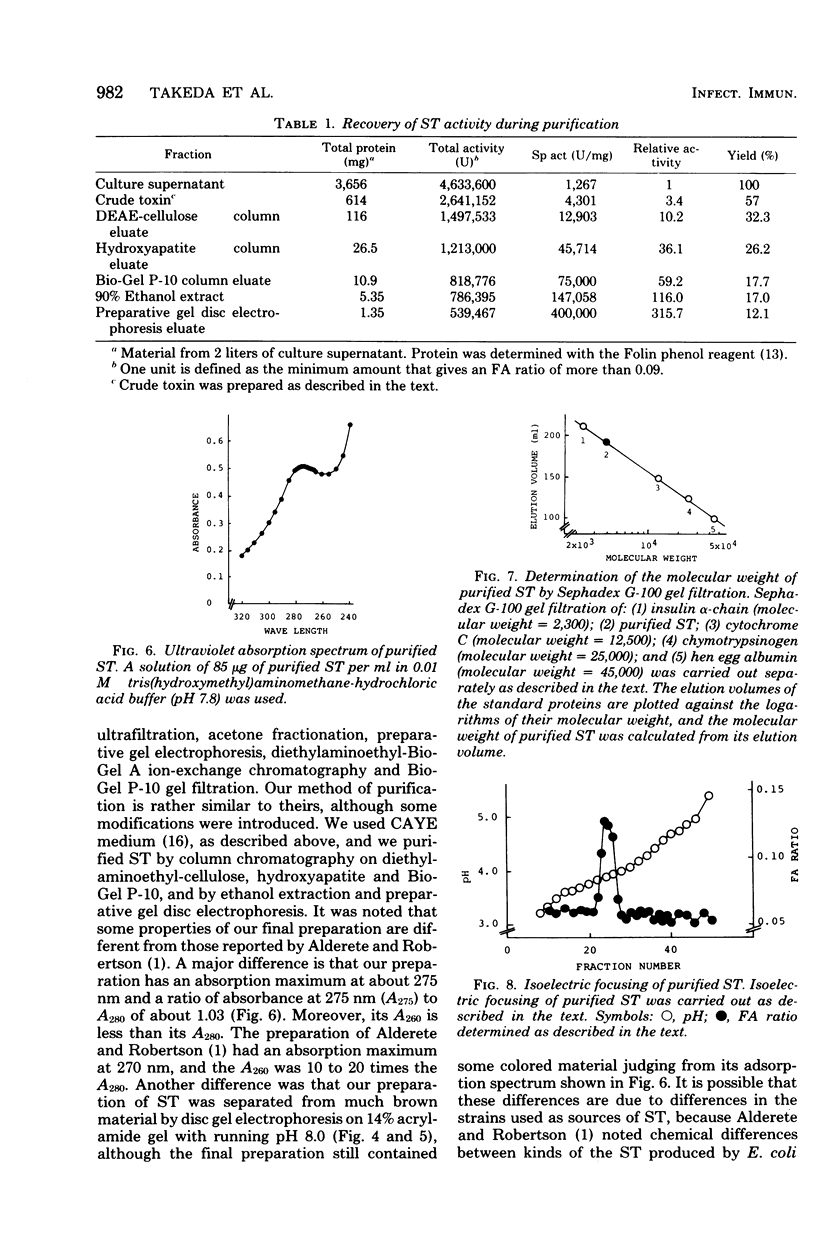
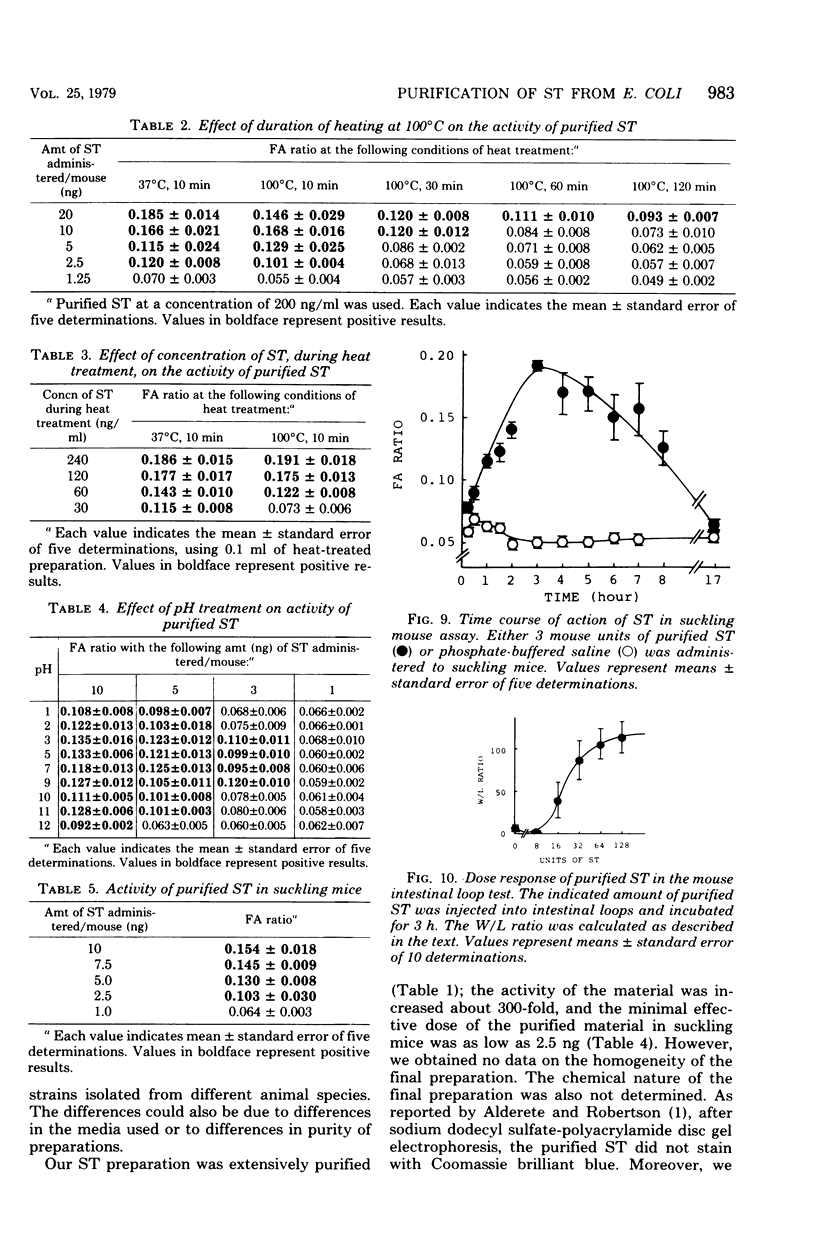
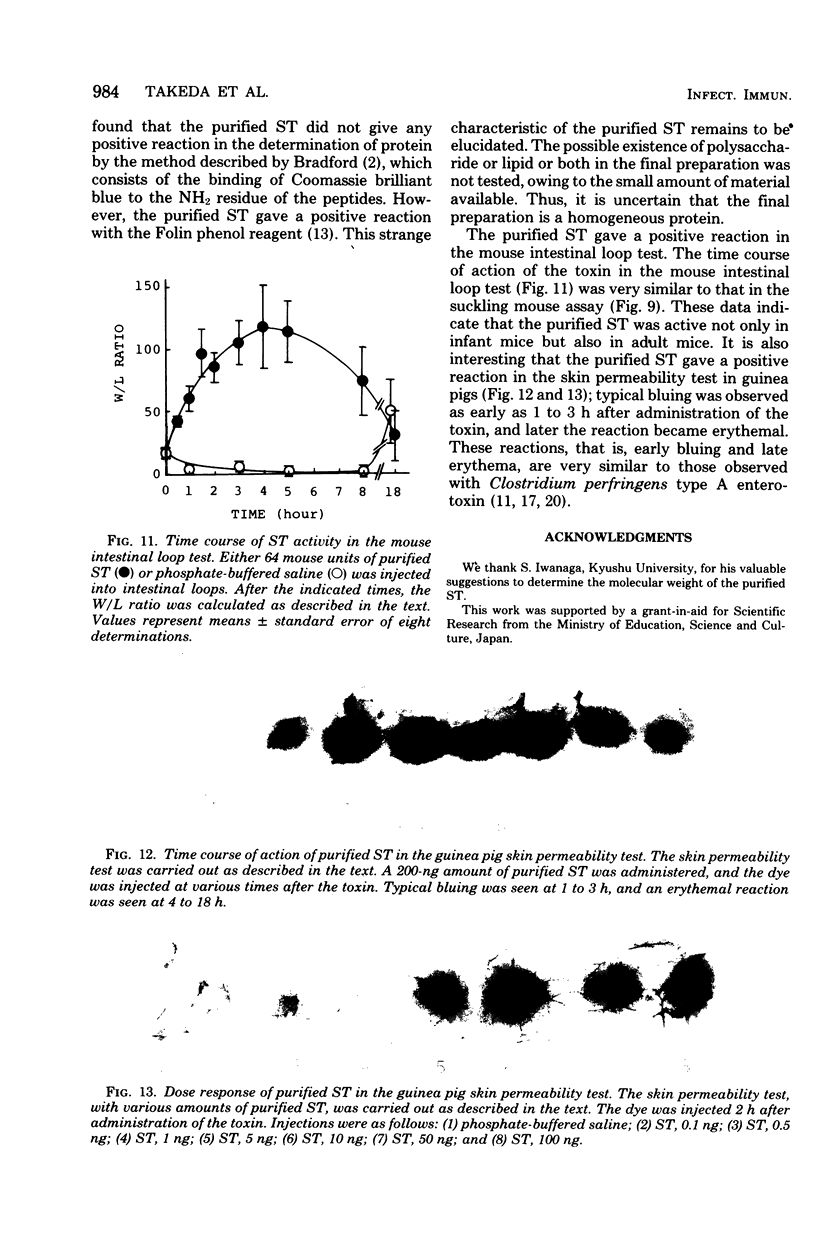
Heat-stable enterotoxin was purified from a strain of enterotoxigenic Escherichia coli 53402 A-1 from human intestine. The cells were cultured in Casamino Acids-yeast extract-salts medium, and the purification procedure consisted of protamine sulfate treatment of the culture supernatant, ultrafiltration with an Amicon PM-10 membrane, diethylaminoethyl-cellulose column chromatography, hydroxyapatite column chromatography, Bio-Gel P-10 gel filtration, 90% ethanol extraction, and preparative polyacrylamide gel disc electrophoresis. About 300-fold purification was achieved, with a yield of about 12%. However, the homogeneity of the purified heat-stable enterotoxin was not rigorously demonstrated. The purified heat-stable enterotoxin had an absorption maximum at about 275 nm, and its isoelectric point was about 3.90. The molecular weight of the purified heat-stable enterotoxin was ca. 4,000 by Sephadex G-100 gel filtration. The minimum effective dose of purified heat-stable enterotoxin was about 2.5 ng in the suckling mouse assay. The purified heat-stable enterotoxin gave a positive reaction in not only the suckling mouse assay but also the mouse intestinal loop test and the guinea pig skin permeability test.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Robertson D. C. Purification and chemical characterization of the heat-stable enterotoxin produced by porcine strains of enterotoxigenic Escherichia coli. Infect Immun. 1978 Mar;19(3):1021–1030. doi: 10.1128/iai.19.3.1021-1030.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burgess M. N., Bywater R. J., Cowley C. M., Mullan N. A., Newsome P. M. Biological evaluation of a methanol-soluble, heat-stable Escherichia coli enterotoxin in infant mice, pigs, rabbits, and calves. Infect Immun. 1978 Aug;21(2):526–531. doi: 10.1128/iai.21.2.526-531.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Evans D. J., Evans D. G., Gorbach S. L. Polymyxin B-Induced Release of Low-Molecular-Weight, Heat-Labile Enterotoxin from Escherichia coli. Infect Immun. 1974 Nov;10(5):1010–1017. doi: 10.1128/iai.10.5.1010-1017.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Graf L. H., Jr, Laird W. J., Smith P. L. Heat-stable enterotoxin of Escherichia coli: in vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2800–2804. doi: 10.1073/pnas.75.6.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschild A. H. Erythemal activity of the cellular enteropathogenic factor of Clostridium perfringens type A. Can J Microbiol. 1970 Aug;16(8):651–654. doi: 10.1139/m70-112. [DOI] [PubMed] [Google Scholar]
- Hughes J. M., Murad F., Chang B., Guerrant R. L. Role of cyclic GMP in the action of heat-stable enterotoxin of Escherichia coli. Nature. 1978 Feb 23;271(5647):755–756. doi: 10.1038/271755a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mullan N. A., Burgess M. N., Newsome P. M. Characterization of a partially purified methanol-soluble heat-stable Escherichia coli enterotoxin in infant mice. Infect Immun. 1978 Mar;19(3):779–784. doi: 10.1128/iai.19.3.779-784.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundell D. H., Anselmo C. R., Wishnow R. M. Factors influencing heat-labile Escherichia coli enterotoxin activity. Infect Immun. 1976 Aug;14(2):383–388. doi: 10.1128/iai.14.2.383-388.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niilo L. Mechanism of Action of the Enteropathogenic Factor of Clostridium perfringens Type A. Infect Immun. 1971 Jan;3(1):100–106. doi: 10.1128/iai.3.1.100-106.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oashi M., Shimada T., Fukumi H. In vitro production of enterotoxin and hemorrhagic principle by Vibrio cholerae, NAG. Jpn J Med Sci Biol. 1972 Jun;25(3):179–194. doi: 10.7883/yoken1952.25.179. [DOI] [PubMed] [Google Scholar]
- Schenkein I., Green R. F., Santos D. S., Maas W. K. Partial purification and characterization of a heat-labile enterotoxin of Escherichia coli. Infect Immun. 1976 Jun;13(6):1710–1720. doi: 10.1128/iai.13.6.1710-1720.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark R. L., Duncan C. L. Biological characteristics of Clostridium perfringens type A enterotoxin. Infect Immun. 1971 Aug;4(2):89–96. doi: 10.1128/iai.4.2.89-96.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda T., Takeda Y., Miwatani T., Ohtomo N. Detection of cholera enterotoxin activity in suckling hamsters. Infect Immun. 1978 Feb;19(2):752–754. doi: 10.1128/iai.19.2.752-754.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS D. E., REISFELD R. A. DISC ELECTROPHORESIS IN POLYACRYLAMIDE GELS: EXTENSION TO NEW CONDITIONS OF PH AND BUFFER. Ann N Y Acad Sci. 1964 Dec 28;121:373–381. doi: 10.1111/j.1749-6632.1964.tb14210.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Ohishi I., Sakaguchi G. Fluid accumulation in mouse ligated intestine inoculated with Clostridium perfringens enterotoxin. Appl Environ Microbiol. 1979 Feb;37(2):181–186. doi: 10.1128/aem.37.2.181-186.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]




