Abstract
Individuals with Attention Deficit Hyperactivity Disorder (ADHD) smoke cigarettes at rates higher than the general population and questions have been raised about how stimulant drugs – the frontline pharmacological treatment for ADHD – influence smoking risk and behavior in those with ADHD. In the present study adult regular smokers with (n=16) and without (n=17) ADHD participated in 3 experimental sessions in which they completed a Progressive Ratio (PR) task to measure the relative reinforcing effects of cigarette smoking and money following oral administration of placebo and 2 active doses of methylphenidate (10 mg and 40 mg). We also measured attention and inhibitory control via a Continuous Performance Test (CPT). Methylphenidate had no effect on smoking-reinforced responding, attention, or inhibitory control in either group. Attention and inhibitory control were associated with smoking-reinforced responding, but unsystematically and only in the non-ADHD group. Several design features, such as the value of the monetary response option, the PR schedule, and the potential effects of smoking on attention and inhibitory control, could have contributed to the negative findings and are discussed as such. Although inconsistent with some previous human laboratory studies of stimulant drugs and smoking, results are consistent with recent trials of stimulant drugs as adjuncts for smoking cessation in adult smokers with ADHD. In general, methylphenidate at mild and moderate doses did not influence the relative reinforcing effects of cigarette smoking in adults with and without ADHD.
INTRODUCTION
Attention Deficit Hyperactivity Disorder (ADHD) is characterized by developmentally-inappropriate levels of inattention/distractibility, hyperactivity, and/or impulsivity that interfere with functioning, and affects nearly 8% of children and 4.4% of adults in the United States (Froehlich et al., 2007; Kessler et al., 2005). Although these rates of ADHD estimate true prevalence in the population, recent data from the CDC have indicated that a much higher proportion of children and adults are actually being diagnosed (http://www.cdc.gov/ncbddd/adhd/data.html; accessed April 11, 2013; see also, http://www.nytimes.com/2013/04/01/health/more-diagnoses-of-hyperactivity-causing-concern.html?pagewanted=all&_r=0; accessed April 11, 2013). These recent reports have been alarming, especially since much controversy has simultaneously centered around how the presentation of ADHD gives rise to increased smoking and other substance use, as well as the over-prescription of stimulant drugs, like methylphenidate.
ADHD is a significant independent risk factor for cigarette smoking and individuals with ADHD or high levels of ADHD symptoms start smoking at an earlier age, become more heavily nicotine-dependent, are more likely to progress from smoking experimentation to regular use, and have poorer cessation outcomes (Covey et al., 2008; Fuemmeler et al., 2007; Humfleet et al., 2005; Kessler et al., 2006; Kollins et al., 2005; Lambert & Hartsough, 1998; Milberger et al., 1997b; Milberger et al., 1997a; Molina & Pelham, 2003; Pomerleau et al., 1995; Wilens et al., 2008). In spite of the large number of studies documenting the association between ADHD and cigarette smoking, comparatively little experimental work has been conducted to elucidate the potential psychological mechanisms underlying this common comorbidity.
Nicotine – a principal component of cigarette smoke – has been found to improve cognitive processes that are commonly disrupted in individuals with ADHD, including sustained attention and behavioral inhibition (for reviews, see Heishman et al., 2010; Levin et al., 2006). Consistent with older theories of drug addiction and psychiatric comorbidity (Khantzian, 1985), it has been proposed that individuals with ADHD and related conditions may smoke more to help reduce their core attentional and other cognitive deficits (Conners et al., 1996; Glass & Flory, 2010; Heishman, 1998; Heishman et al., 2010; Levin et al., 2001). This so-called “self-medication hypothesis” is supported by several lines of research. First, at a neuropharmacological level, nicotine facilitates dopamine release in relevant pathways in a manner similar to psychostimulants that are commonly used in the clinical treatment of ADHD /(Rush et al., 2002; Brody et al., 2004; De Biasi & Dani, 2011; Grace, 2001; Volkow et al., 2012). Second the behavioral and cognitive effects of nicotine are similar to those of the psychostimulants in non-human species as well as in humans with and without ADHD (Heishman et al., 2010; Levin et al., 2006). For example, nicotine has been shown to improve performance on attention-related tasks in regular smokers and in non-smokers (Levin et al., 1998; Warburton & Mancuso, 1998), including fine motor skills, alerting and orienting response time, short term episodic memory, and working memory (Heishman et al., 2010). Nicotine-induced improvements in attention have been found in both ADHD and non-ADHD samples (Levin et al., 1996; Levin et al., 1998; Levin et al., 2001), though prior studies have not directly compared the cognitive effects of nicotine for these two populations. In addition to decreasing reaction time on cognitive tasks for individuals with ADHD (Levin et al., 1996; Poltavski & Petros, 2006; Potter & Newhouse, 2004; Potter & Newhouse, 2008), transdermal nicotine is also found to improve clinical global impressions of ADHD symptomatology and self-ratings of positive affect in ADHD patients (Levin et al., 1996). Three lines of evidence address the issue of how stimulant medication may influence smoking outcomes in individuals with ADHD: 1) clinical studies of how stimulant medication affects risk for smoking; 2) human laboratory studies of stimulant drug administration and its effect on smoking behavior; and 3) clinical trials examining the efficacy of stimulant drugs to promote smoking cessation in individuals with ADHD.
The effects of nicotine on cognitive performance and ADHD symptoms are similar to the effects of pharmacological treatments for ADHD – namely d-amphetamine and methylphenidate – and the substances operate similarly on dopaminergic pathways (Brody et al., 2004; De Biasi & Dani, 2011; Grace, 2001; Volkow et al., 2012). One might therefore expect individuals whose ADHD symptoms are already treated with stimulants to be less likely to smoke or derive less reinforcement from smoking. Clinical studies are mixed in terms of the long-term effects of stimulant treatment on smoking outcomes with some finding a protective effect of treatment (Huss et al., 2008; Milberger et al., 1997b; Milberger et al., 1997a; Monuteaux et al., 2007; Whalen et al., 2003), and others finding no effect (Biederman et al., 2008; Burke et al., 2001; Winters et al., 2011). One study even reported that stimulant therapy increased risk for smoking (Lambert, 1998).
On the other hand, stimulant drugs have been shown to increase smoking behavior in human laboratory studies (Rush et al., 2005; Stoops et al., 2011; Vansickel et al., 2007; Vansickel et al., 2011). Only one study of which we are aware has directly examined the effects of stimulant drugs on smoking in individuals with a diagnosis of ADHD, and reported that methylphenidate increased ad libidum smoking in ADHD-diagnosed smokers compared to placebo (Vansickel et al., 2011). This study did not include a non-ADHD comparison group, thus precluding examination of differential effects of psychostimulants on smoking-reinforced responding in individuals with and without ADHD.
Two placebo-controlled randomized trials have examined the efficacy of 2 different stimulant drugs (OROS-methylphenidate [Concerta] and lisdexamfetamine dimesylate [Vyvanse]), alone and in combination with nicotine patch, on smoking cessation outcomes in adult regular smokers with ADHD (Kollins et al., 2012a; Winhusen et al., 2009). While neither of these studies demonstrated efficacy of the stimulant drugs compared to placebo for promoting smoking cessation, both studies reported significant decreases in smoking behavior for stimulant treated individuals, a finding that stands in contrast to human laboratory studies reviewed above. Moreover, in both studies, stimulant treated patients exhibited significant reductions in ADHD symptoms compared to placebo-treated individuals.
Overall the literature is mixed with respect to the role that stimulant drugs may play in influencing smoking behavior in individuals with ADHD. The goal of the present study was to experimentally examine the effects of methylphenidate on attentional functioning, inhibitory control, and smoking-reinforced responding in adult regular smokers with and without ADHD. Given the reported differences in baseline dopamine functioning between ADHD and non-ADHD individuals (Grace, 2001; Volkow et al., 2009), we hypothesized that smoking reinforced responding would be decreased in regular smokers with ADHD following administration of methylphenidate compared to placebo, but that the opposite would be true for smokers without ADHD. Since previous work from our lab has demonstrated that smoking reinforced responding is mediated by changes in attention and inhibitory control (Kollins et al., 2012b), we further hypothesized that stimulant-induced changes in these processes would also predict smoking-reinforced responding.
METHODS
Participants
Participants for the study were 33 male and female adult regular smokers between the ages of 18-50 years recruited from the community. Sixteen of the participants were diagnosed with ADHD by a licensed clinician and the remaining 17 participants were free from any psychiatric diagnosis, except for nicotine dependence. Diagnostic status of all participants was determined using the Structured Clinical Interview Schedule for DSM (SCID; First et al., 1997), the Conners Adult ADHD Diagnostic Interview for ADHD (CAADID; Epstein et al., 2000), and the Conners Adult ADHD Rating Scale (CAARS; Conners et al., 1998). Other inclusion criteria included self-reported current smoking of at least 10 cigarettes/day and afternoon expired air CO levels of > 10 ppm. Exclusion criteria included estimated IQ scores of <80 measured using the Kaufman Brief Intelligence Test (Kaufman & Kaufman, 2004); positive urine drug screen for illicit drugs; breath alcohol level > 0.00; any significant medical condition (i.e., neurological or CNS-related disorder such as epilepsy, diabetes, coronary disease, renal function, or other conditions that in the judgment of the study physician would influence study participation or warrant more immediate medical care); positive serum pregnancy test (for females of childbearing potential); clinically significant blood chemistry results; and, for the Control group, T-scores on relevant CAARS scales > 55. Participants in both groups were also excluded if they had a clinically significant ECG reading at Screening or if they met the following criteria: if 40 years of age or younger, blood pressure readings exceeding 135/85 and/or resting heart rate > 90 beats per minute; if older than 40 years of age, blood pressure readings exceeding 130/80 and/or resting heart rate > 88 beats per minute. Individuals in the ADHD group who were currently treated pharmacologically for their ADHD (n = 2) were allowed to participate provided they washed out of their medication for a duration of at least 5 half lives prior to the Baseline and Experimental Sessions. Such a washout period typically represents only 1-2 days and is not inconsistent with how the medication is often taken in routine daily functioning. The washout period was also overseen by the study physician (RD) to monitor for adverse side effects and significant changes in functioning. Subjects in either group taking other kinds of psychotropic medication were excluded from participation. All participants provided informed consent and the study was reviewed and approved by the local Institutional Review Board.
Design & Procedures
Following Screening, all participants completed a Baseline visit and 3 Experimental visits. Participants were compensated $45 for the Screening visit, $40 for the Baseline visit, and $85 for each of the 3 Experimental sessions. A completion bonus of $100 was also provided for participants who completed all visits. In addition, participants could earn up to an additional $15 depending on their performance on the Progressive Ratio task (see below), for a total study compensation of $440-$455. Participants were also given $5 as reimbursement for one pack of preferred brand cigarettes that were used during the Baseline and Experimental sessions. During the Baseline visit, participants were familiarized with all procedures and measures to be used during the Experimental visits. The 3 Experimental visits were identical except for the dose of MPH administered (see below). Participants were randomly assigned to the order of Experimental visits during the Baseline visit and were scheduled for all 3 of their subsequent Experimental visits, which occurred no more than 7 days following Baseline. In general, participants were instructed to smoke as usual and to not use alcohol within 24 hours prior to their scheduled visits.
Experimental visits lasted approximately 5 ½ hours. Participants arrived at the lab and alcohol and drug use was assessed via breathalyzer and urine drug screen, respectively. Participants had to have a breath alcohol level of 0.0 and a negative drug screen to continue with the session. Vital signs were measured, a smoking diary was completed to record smoking since the last visit, and a standardized low-fat snack was provided. Approximately 45 minutes into each Experimental session, participants were allowed to smoke 1 cigarette to control for the time since last smoking. Immediately following the smoke break, drug was administered (see below). Following drug administration, subjective measures and vital signs were recorded over the next 60 minutes. Approximately 1 hour after drug administration, the Continuous Performance Test was administered, followed immediately by the Progressive Ratio (PR) task. Following the PR task, subjective effects and vital signs were recorded. Participants remained in the lab for an additional 90 minutes to provide a standardized delay from the end of the PR task to the next opportunity to smoke. Participants were then either debriefed (following the 3rd Experimental visit) or scheduled for the next session and dismissed from the laboratory.
Measures
Smoking-reinforced responding
The primary outcome measure for the study was the number of responses completed for cigarette puffs during a 90-minute Progressive Ratio (PR) task. During the task, subjects were positioned in front of a computer screen and keyboard and instructed that they would have the opportunity to press the buttons on the keyboard to earn either standardized puffs of a cigarette (2 puffs/opportunity), or a fixed amount of money ($0.50). Similar to other PR studies conducted with human subjects, participants were instructed that they could choose not to press the buttons, but that the duration of the session would be the same regardless of whether they completed none or all of the ratios for smoking opportunities or money (Kollins et al., 2012b; Sigmon et al., 2003; Tidey et al., 1999).
Ratios for cigarette puffs and money operated independently such that subjects could respond exclusively for one consequence or the other, alternate their responses for each of the consequences, or not respond at all. For each consequence, the first ratio required 75 responses and each successive ratio required 1.75 times the previous ratio such that the ratio requirements for each reinforcer were 75 responses, 131 responses, 229 responses, and so on (Kollins et al., 2009; Kollins et al., 2012b). Following the completion of each ratio, which was signaled by a message that appeared on the computer screen and an audible signal, there was a 2-minute time out period. During this period, participants received either 2 standardized puffs of their preferred brand of cigarette delivered via a controlled puff volume apparatus (Levin et al., 1989), or $0.50 in cash. The value of the cash alternative was selected on the basis of previous work conducted in our laboratory in which other manipulations (e.g., 24 hour abstinence) resulted in significant differences in ratios completed for puffs versus money (Kollins et al., 2012b). Puff volume was measured at 30 mL. Following the time-out period, subjects were allowed to resume responding on the PR task.
Attention and Inhibitory Control
The Conners’ Continuous Performance Task (CPT; Conners, 2000) was used to assess inhibitory and attentional control. During the task, participants were required to press a button whenever a letter appeared on the screen unless that letter was an ‘X’. Measures of inhibitory control (errors of commission—pressing the button on ‘X’ trials) and attentional control (errors of omission, reaction time standard error) served as primary measures from this task. These outcomes from the CPT have been shown in previous studies to be sensitive to the effects of smoking abstinence in smokers with and without ADHD (McClernon et al., 2008).
Drug Dose and Administration
The doses of MPH (10 mg, 40 mg) were selected for study for two primary reasons. First, the 40 mg dose has been shown to increase ad lib smoking in both ADHD and non-ADHD participants in previous studies (Rush et al., 2005; Vansickel et al., 2011). Second, the doses selected for study are clinically relevant and within the range of doses patients with ADHD are likely to receive in clinical practice.
All drug doses were administered under double-blind conditions. Sequences for the order of administration of the 3 doses were randomized across participants. MPH and placebo capsules were prepared by encapsulating commercially available MPH and lactose filler in a size 00 capsule, and dispensed by the local Investigational Pharmacy. Placebo capsules contained only lactose. Medical oversight for the protocol was the responsibility of the study physician (RD). During each experimental session, participants orally ingested one capsule with 150 ml water. Timing of drug administration was arranged so that the CPT and PR tasks occurred within a range that corresponded to peak effects of the drug (60-120 minutes post-ingestion).
Drug administration procedures were designed to ensure that participants swallow the capsules and did not open them in their mouths and taste the contents (Abreu & Griffiths, 1996). To accomplish this, the research assistant (a) watched the participant to ensure that he or she swallowed the capsules and did not remove them from his or her mouth, (b) conducted a brief oral examination to ensure that the participant was not hiding the capsules under her or his tongue, and (c) spoke with the participant to determine if he or she had anything in his or her mouth.
Data Analyses: Smoking Reinforced Responding and Attention
Initial General Linear Models examined the effects of group (ADHD, Control), dose (Placebo, 20 mg, 40 mg MPH) and the group x dose interaction independently for each of the primary dependent measures (smoking reinforced responding, CPT performance). The residuals from all tests were examined for violations of underlying assumptions that posed threats to stability and reliability, e.g., non-normality, heteroskedasticity, collinearity, and leverage. Violations of normality of residuals and leverage were identified, and data transformations did not correct these violations. Therefore all GLM tests were conducted using robust estimators, which are more resistant to distortions and provide better estimates under such conditions (Wilcox, 1997; Wilcox, 2010). For each of these initial models, sex and race were included as covariates; however, in no case was sex or race a significant predictor variable. Accordingly, for degrees of freedom appropriate to the initial hypotheses, sex and race were removed from the final models. Exclusion of these variables did not alter the outcomes (results from full models with sex and race available from the first author). The following dependent measures were examined for smoking reinforced responding: number of completed ratios for puffs; total number of responses for puffs during the 90-min PR task; and the proportion of ratios completed for puffs/ratios completed for cash. The following dependent measures were examined for attention and inhibitory control on the CPT: commission errors and reaction time standard error.
We were also interested in the relationships among attention and inhibitory control and smoking-reinforced responding, both across groups and under different doses of methylphenidate. To examine these associations, we first calculated Spearman’s rank correlation coefficients among CPT commission errors and hit reaction time standard error, with completed ratios for puffs. These correlations were examined separately for each group at each dose level. We also examined change in commission errors and hit rate standard error between the placebo condition and each of the active dose conditions. These correlations were examined separately for each group.
RESULTS
Participant Characteristics
A total of 179 participants were initially telephone screened for the study, resulting in 62 in-person screens. Of these, 37 passed all inclusion and exclusion criteria and enrolled in the study. A total of 33 participants (n=16 ADHD and n=17 Control) completed all sessions and provided useable data for the main outcome measures. Of the 4 participants who were enrolled but did not complete the study, 2 discontinued due to adverse events from the study medication, 1 discontinued due to repeated scheduling difficulties, and 1 was lost to follow-up. Table 1 shows demographic and smoking characteristics of the final, analyzed sample at screening. In general, the groups were comparable across demographic and baseline smoking variables except for race, which was statistically controlled as described above.
Table 1.
Subject Characteristics at Screening.
| ADHD | Control | p-value | |
|---|---|---|---|
| Sex | ns* | ||
| Female | 8 | 8 | |
| Male | 8 | 9 | |
| Race | 0.02* | ||
| White | 12 | 6 | |
| Non-White | 4 | 11 | |
| Age in Yrs (SD) | 29.8 (8.5) | 27.5 (8.8) | ns+ |
| Marital Status (%) | ns* | ||
| Single | 81% | 88% | |
| Married/Divorced/Separated | 19% | 12% | |
| Educational Level (%) | ns* | ||
| High School or less | 13% | 35% | |
| Some College/College Grad | 88% | 65% | |
| Estimated IQ | 110.9 (10.1) | 105.4 (9.6) | ns* |
| CAARS Subscale Scores (T-scores) | |||
| DSM-IV Inattentive (mean, SD) | 73.2 (5.5) | 42.2 (4.5) | <0.0001+ |
| DSM-IV Hyperactive-Impulsive (mean, SD) | 72.3 (7.4) | 42.4 (7.1) | <0.0001+ |
| DSM-IV Total Symptoms (mean, SD) | 76.0 (5.8) | 41.6 (5.6) | <0.0001+ |
| ADHD Subtype (%) | |||
| Predominantly Inattentive | 25% | ||
| Predominantly Hyperactive-Impulsive | 0% | ||
| Combined | 75% | ||
| Cigarettes/Day (SD) | 14.4 (4.5) | 14.1 (4.4) | ns+ |
| FTND total score (SD) | 4.9 (2.1) | 4.2 (2.1) | ns+ |
| CO Level (SD) | 20.1 (8.8) | 29.1 (15.4) | ns+ |
| Years Regular Smoking (SD) | 10.5 (6.4) | 8.5 (8.5) | ns+ |
Chi-Square test;
t-test
Smoking-Reinforced Responding under Differing Methylphenidate Doses
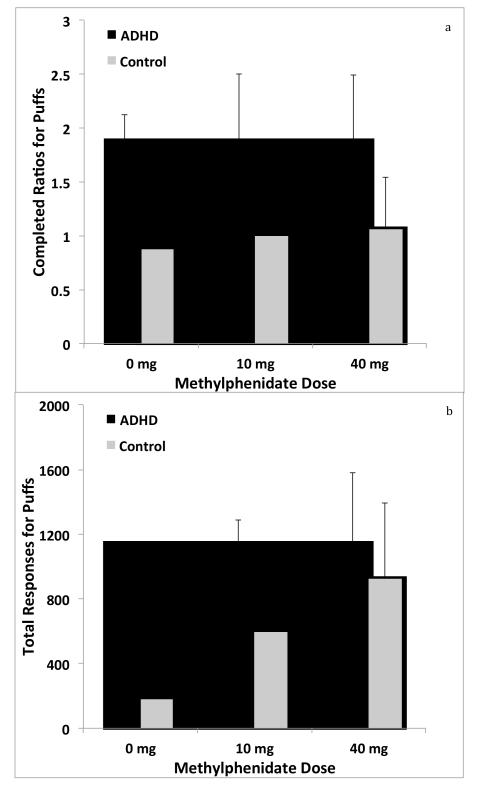
CO levels obtained at the beginning of each experimental session suggested no differences in smoking behavior across sessions as a function of group or sex (All F’s < 2.0; p’s >0.15). In the placebo condition, smokers with and without ADHD completed 1.5 (SD=2.3) and 0.9 (SD=1.6) ratios for cigarette puffs, respectively. In the 10 mg condition, ADHD and non-ADHD smokers completed 1.9 (SD=2.3) and 1.0 (SD=2.2) ratios for puffs, respectively. Finally, in the 40 mg condition, smokers with and without ADHD completed 1.9 (SD=2.8) and 1.1 (SD=2.2) ratios for cigarette puffs, respectively. Figure 1a illustrates completed ratios for cigarette puffs across groups under as a function of the 3 different doses of methylphenidate (0 mg, 10 mg, and 40 mg). GLM testing with robust estimators revealed no main effects of dose or group, and no dose x group interactions (all F scores < 1.5, all p values > 0.2). Figure 1b illustrates total responses made for puffs. Similarly, no significant main effects or interactions were obtained for the measure of total number of responses made for cigarette puffs during the 90 min PR sessions (all F scores < 1.5, all p values > 0.2).
Figure 1.
Completed ratios for cigarette puffs (Figure 1a) and total responses made for cigarette puffs (Figure 1b) across the 3 doses of MPH for ADHD and non-ADHD participants. Error bars indicate SEM. Values represent means for each group under each condition. The ranges varied across dose conditions. For completed ratios, the ranges were 0 -6, 0-8, and 0-8 for the 0 mg , 10 mg, and 40 mg conditions, respectively. For responses made for puffs, the ranges were 0-4060, 0-8676, and 0-13701 for the 0 mg , 10 mg, and 40 mg conditions, respectively.
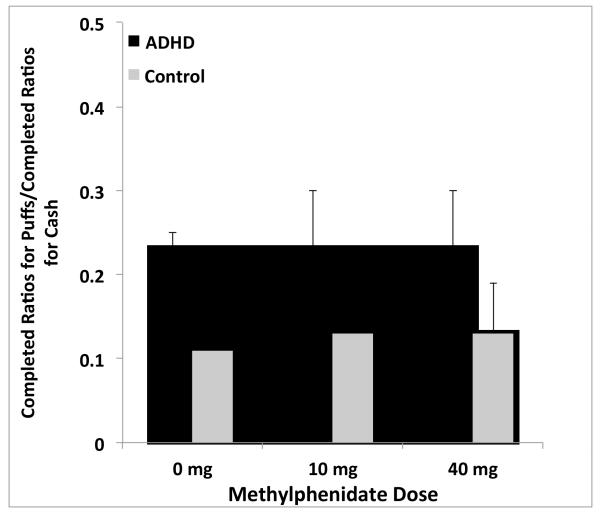
Since participants had continuous access to response options for both cigarette puffs and money, the final way in which the relative reinforcing effects of smoking were examined was by comparing the proportion of ratios completed for cigarette puffs to ratios completed for money, across the 3 dose conditions (Figure 2). There were no significant main effects of group, dose, or group x dose interactions (all F values < 1.5, p’s > 0.2).
Figure 2.
Ratio between completed ratios for cigarette puffs: completed ratios for cash across MPH dose conditions for ADHD and non-ADHD participants. Error bars indicate SEM. Bars represent mean values for each group. The range of values was 0-0.75, 0-1.0 and 0-1.0 for the 0 mg , 10 mg, and 40 mg conditions, respectively.
A substantial proportion of individuals did not complete any ratios for cigarette puffs: across groups, 61%, 55%, and 58% of individuals, respectively, in the 0 mg, 10 mg, and 40 mg conditions did not complete a ratio for cigarette puffs. We examined whether these proportions differed by group status at each of the dose levels. In the 0 mg condition, 61% of the ADHD group and 65% of the non-ADHD group failed to complete a single ratio for cigarette puffs (chi-square = 0.25, p = NS). For the 10 mg condition, these proportions were 38% and 71%, for the ADHD and the non-ADHD groups, respectively (chi-square = 3.64, p = 0.06). For the 40 mg condition, the proportions were 50% and 65%, respectively (chi-square = 0.73, p = NS).
Attentional Function and Inhibitory Control under Differing Methylphenidate Doses
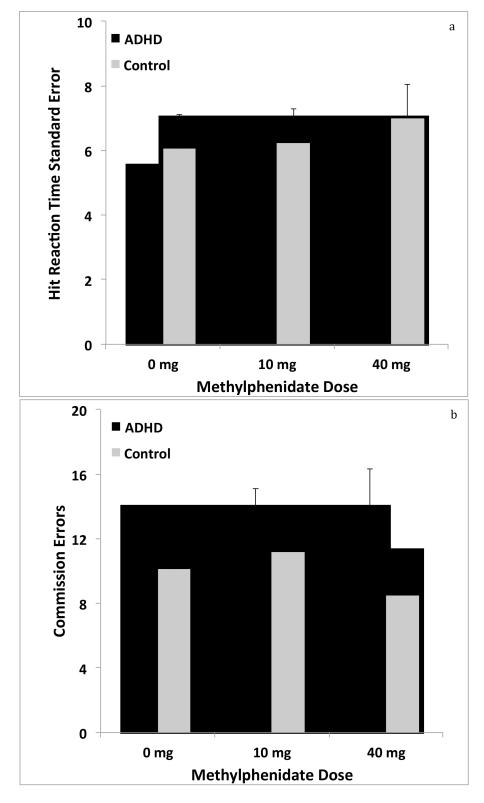
Figures 3a and 3b illustrate hit reaction time standard error and commission errors from the CPT as a function of differing MPH dose. No significant main effects of group or dose or significant group x dose interactions were observed. For hit reaction time standard error, there was a trend towards a main effect of dose: F(2, 32) = 3.23, p = .053. For number of commission errors, there was a trend toward a group x dose interaction: F(2, 32) = 3.07, p = .060. For all other outcomes, F scores were < 1.5 and p values were > .2.
Figure 3.
Attention and inhibitory control measured as hit reaction time standard error (Figure 3a) and commission errors (Figure 3b), respectively across the 3 MPH doses for ADHD and non-ADHD participants. Error bars indicate SEM. Values represent means for each group under each condition. The ranges varied across dose conditions. For hit reaction time standard error, the ranges were 2-17, 2-19, and 2-30 for the 0 mg , 10 mg, and 40 mg conditions, respectively. For commission errors, the ranges were 0 -36, 0-34, and 0-30 for the 0 mg , 10 mg, and 40 mg conditions, respectively.
Relationship Among Attention, Inhibitory Control, and Smoking Reinforced Responding
There was no relationship between hit reaction time standard error and commission errors on the CPT and completed ratios for puffs at any of the dose levels for the ADHD group. In the non-ADHD group, there were several significant or trend level associations observed. There was a significant association between hit reaction time standard error and completed ratios for puffs in the 0 mg condition (rho = -0.67; p < 0.01). There was also a trend level association between commission errors on the CPT and completed ratios for puffs in the 40 mg condition (rho = -0.46; p = 0.06). Finally, the change in commission errors between the 10 mg and 0 mg conditions was associated with completed ratios for puffs (rho = 0.43; p = 0.08). When the data were pooled across both groups there were no significant or trend level associations at any of the dose levels.
DISCUSSION
This study is the first, to our knowledge, to systematically assess whether methylphenidate influenced smoking-reinforced responding in individuals with and without ADHD using a well-validated PR task. The present study is also the first to examine associations among attention and inhibitory control, acute methylphenidate administration, and smoking-reinforced responding. Contrary to our initial hypotheses, we did not find relationships between moderate doses of methylphenidate (10 mg and 40 mg) and smoking-reinforced responding in regular smokers, either with or without ADHD. There were some modest associations between CPT parameters and smoking-reinforced behavior in the non-ADHD group; however, these findings were inconsistent and suggest that the links between attention and inhibitory control and smoking-reinforced responding were minimal. The present findings do not provide evidence for systematic changes in smoking reinforced responding following acute stimulant medication administration in individuals with ADHD. There are several design features that are important to consider in light of the negative findings.
Results from this study are not consistent with previous human laboratory studies of stimulant administration and smoking behavior in smokers with and without ADHD. For example, one of the first studies to examine the effects of methylphenidate on smoking reported dose dependent increases in ad libitum smoking in adults, presumably without a diagnosis of ADHD (Rush et al., 2005). This finding was similar to previous studies reporting that amphetamine also increases smoking behavior in non-ADHD adults (Cousins et al., 2001; Tidey et al., 2000). Another study reported that 5-40 mg methylphenidate increased smoking specifically in adult regular smokers diagnosed with ADHD (Vansickel et al., 2011).
Findings from the present experiment are more consistent with a previous study evaluating the effects of d-amphetamine on a PR task in which participants could respond for cigarette puffs or money. This study reported no significant effects of d-amphetamine on smoking-reinforced responding for the sample (n=18) as a whole, but did find a subset of individuals responded significantly more for cigarette puffs following drug administration (Sigmon et al., 2003). We found a trend for smokers with ADHD to be more likely to complete any ratios for cigarette puffs under the 10 mg compared to placebo. Given the trend level effect and lack of findings from the higher dose, this should be interpreted with caution.
There are a several reasons why our findings may be somewhat at odds with previous studies of stimulant effects on smoking. First, we used a concurrent PR task in which subjects could respond for both cigarette puffs and money. Previous studies reporting increases in smoking behavior following stimulant drug administration have examined ad libitium smoking (i.e., with no response requirements; Cousins et al., 2001; Rush et al., 2005), a PR task with only a single alternative available (Sigmon et al., 2003), or a choice procedure in which participants made discrete choices for cigarette puffs or money (Stoops et al., 2011; Tidey et al., 2000). It is important to note that in both of the studies using discrete choices for cigarettes versus money, the value of the cash option was lower than that used in the present study ($0.25 vs. $0.50; Stoops et al., 2011; Tidey et al., 2000), though neither of those studies examined smokers with ADHD. It may be that the use of a higher monetary reinforcer in the present study muted the effects of methylphenidate on smoking-reinforced responding.
The present findings are also similar to those recently reported in another study of smoking-reinforced responding in ADHD and non-ADHD smokers. Using a PR task identical to that used in the present study, that study reported that smokers with ADHD exhibited significantly higher levels of smoking reinforced responding, but this effect was more pronounced following 24-hour smoking abstinence (Kollins et al., 2012b). The lack of ADHD group differences in the 0 mg condition in the present study is similar to the findings from the Satiated condition from Kollins et al (2012) and suggests that smoking abstinence (versus methylphenidate administration) may be a more robust manipulation to occasion ADHD vs. non-ADHD group differences in smoking-reinforced responding, The previous study also demonstrated main effects of condition (abstinence versus satiated) across ADHD and non-ADHD groups, with higher levels of smoking-reinforced responding for both groups in the abstinent condition – again suggesting that abstinence is a more powerful manipulation for increasing smoking reinforced responding than methylphenidate administration, at least at the doses tested here. Future research should seek to more fully characterize the behavioral economic profile of monetary versus smoking-reinforced responding following stimulant administration and following other relevant experimental manipulations.
Finally, it is possible that our study was underpowered to detect effects of group or drug, although this may be less likely for at least 2 reasons. First, our sample size was nearly double the next largest study of stimulant drug effects on smoking behavior (N=33 vs. N=18; Sigmon et al., 2003). Although our study employed a mixed, between-within design, versus purely within subjects designs for the other studies, each of our 2 groups was still larger than any of the other studies except for Sigmon et al. (2003). Second, even considering our more complex design, we were more than adequately powered to detect effect sizes of the magnitude reported in previous studies (e.g., Vansickel et al., 2011). As such, our lack of statistically significant findings is more likely to be related to methodological differences than Type II error.
It is somewhat surprising that there were no group or drug effects on CPT performance in the present study. In general, both of these measures have been shown to discriminate adults with ADHD from those without, with meta-analytically-derived effect sizes of 0.53 and 0.63 for hit reaction time standard error and commission errors, respectively (Hervey et al., 2004). However, these studies have compared ADHD and non-ADHD individuals without a drug manipulation. It has been shown that transdermal nicotine influences CPT performance in adults with and without ADHD (Conners et al., 1996; Levin et al., 1996; Levin et al., 1998; Levin et al., 2001). In this study, participants smoked a cigarette prior to CPT performance and drug administration to control for time since last cigarette. It is likely that this manipulation served to mitigate subsequent effects of drug administration or group differences. In other words, the design feature to control for time since last cigarette likely resulted in a ceiling effect that obscured group and methylphenidate effects on CPT performance. Of course this feature would also obscure the potential mediating effects of CPT performance on smoking reinforcement. It would be interesting to examine the effects of stimulant drugs on CPT performance in smokers with and without ADHD following a controlled abstinence period.
The study includes several important limitations to be considered. First, as noted above, our use of only a single, relatively large value for the cash alternative ($0.50) may have obscured our results and led to more exclusive responding for the cash option. Second, we observed differences in racial composition across our groups, though this was controlled statistically in all of the analyses. Third, we evaluated a relatively narrow dose range. Although the 10-40 mg range used is relevant clinically, it is not uncommon for much higher doses of methylphenidate to be used in clinical practice and it would be interesting to see if drug effects on smoking-reinforced behavior emerged under high doses. Fourth, we did not collect within-session data for any of the participants. It would have been useful to evaluate patterns of responding on the PR task within a given session (ie., how behavioral allocation changed or did not change over the course of the 90-minute task). In light of these limitations, there were several methodologically noteworthy features to the present study. It is the first to examine smoking-reinforced responding in both ADHD and non-ADHD individuals following stimulant drug administration. It is also among the largest studies to systematically evaluate smoking-reinforced responding in any population. The use of a concurrent PR task arguably provides a face valid approach to assessing how stimulant drugs affect smoking reinforced responding in “real-world” settings since smokers are continually faced with choices about whether to smoke or to seek other reinforcers.
It is important to consider the findings of the present study and of previous human laboratory studies of stimulant effects on smoking behavior in light of relevant clinical literature. There have been 2 published randomized controlled trials of stimulant medication (1 with osmotic release methylphenidate; 1 with lis-dexamfetamine) as an adjunct to nicotine patch for smoking cessation in adult regular smokers with ADHD (Kollins et al., 2012a; Winhusen et al., 2009). Neither of these studies found that stimulant drugs significantly increased rates of continuous smoking abstinence more than placebo. However, both reported significant reductions in cigarettes/day in the stimulant-treated groups, suggesting that in the context of a smoking cessation attempt among smokers interested in quitting, chronic stimulant administration does not increase rates of smoking in adults with ADHD. Importantly, both of these studies also found that the stimulants significantly improved ADHD symptoms across the trials compared to placebo (Kollins et al., 2012a; Winhusen et al., 2009). It is not known what the effects of stimulant drugs would be for smokers without ADHD undergoing a cessation attempt. Several important features of these clinical trials should be considered when interpreting the results vis-à-vis laboratory studies. First, these clinical trials used chronic, ongoing stimulant administration over the course of several weeks, versus acute administration in the laboratory studies. Second, the clinical trials used extended release formulations of methylphenidate and amphetamine versus immediate release formulations used in the laboratory studies. Finally, the clinical trials were examining smokers who were explicitly interested in quitting smoking. Although not reported in all of the laboratory studies, the present study enrolled only those smokers who were not interested in quitting. For ethical reasons, individuals for the present laboratory study who were interested in quitting were referred for treatment. This necessary sampling bias may have influenced the outcomes. Collectively these methodological differences make direct comparisons between laboratory studies and clinical trials somewhat tenuous. Still, the clinical relevance of the randomized controlled trials should be carefully considered in the context of understanding how stimulant drugs influence smoking behavior in individuals likely to receive those drugs.
The present study found that low to moderate doses of immediate release methylphenidate did not influence smoking-reinforced behavior, attentional functioning, or inhibitory control in adult smokers with and without ADHD. Although previous laboratory studies in smokers with and without ADHD have reported stimulant-induced increases in smoking behavior, our experimental preparation may have mitigated such effects in the present experiment. There are a number of important future directions that the present findings suggest, such as to examine the potential moderating effects of smoking abstinence on the relationship between stimulant administration and smoking behavior. Moreover, the effects of other pharmacological manipulations, such as pre-treatment with non-stimulant drugs approved for use with ADHD, would be interesting to examine vis-à-vis smoking behavior. Although the value of the monetary option in our PR task may have influenced results, findings from this study suggest that methylphenidate does not increase the relative reinforcing value of smoking in adult smokers with and without ADHD. These results are consistent with recently published RCTs of smoking cessation in adults with ADHD. Continued work should address the important question of how stimulant drugs influence smoking behavior in smokers with and without ADHD. This kind of work will be particularly important given the above noted increases in rates of ADHD diagnosis and concerns over how these rates may influence prescription stimulant use among individuals without ADHD.
Acknowledgements
Funding for this project was from grants 1R01DA025653 (Kollins) & K24DA023464 (Kollins)
REFERENCES
- Abreu ME, Griffiths RR. Drug tasting may confound human drug discrimination studies. Psychopharmacology (Berl) 1996;125(3):255–257. doi: 10.1007/BF02247336. [DOI] [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Spencer T, Wilens TE, Macpherson HA, Faraone SV. Stimulant therapy and risk for subsequent substance use disorders in male adults with ADHD: a naturalistic controlled 10-year follow-up study. Am J Psychiatry. 2008;165(5):597–603. doi: 10.1176/appi.ajp.2007.07091486. [DOI] [PubMed] [Google Scholar]
- Brody AL, Olmstead RE, London ED, Farahi J, Meyer JH, Grossman P, Lee GS, Huang J, Hahn EL, Mandelkern MA. Smoking-induced ventral striatum dopamine release. Am J Psychiatry. 2004;161(7):1211–1218. doi: 10.1176/appi.ajp.161.7.1211. [DOI] [PubMed] [Google Scholar]
- Burke JD, Loeber R, Lahey BB. Which aspects of ADHD are associated with tobacco use in early adolescence? J Child Psychol Psychiatry. 2001;42(4):493–502. [PubMed] [Google Scholar]
- Conners CK. The Conners Continuous Performance Test. Second Edition 2000. [Google Scholar]
- Conners CK, Levin ED, Sparrow E, Hinton SC, Erhardt D, Meck WH, Rose JE, March J. Nicotine and attention in adult attention deficit hyperactivity disorder (ADHD) Psychopharmacol Bull. 1996;32(1):67–73. [PubMed] [Google Scholar]
- Conners CK, Erhardt D, Sparrow E, staff MHS. The Conners Adult ADHD Rating Scale (CAARS) Multi-Health Systems, Inc.; Toronto, Canada: 1998. [Google Scholar]
- Cousins MS, Stamat HM, de Wit H. Acute doses of d-amphetamine and bupropion increase cigarette smoking. Psychopharmacology (Berl) 2001;157(3):243–253. doi: 10.1007/s002130100802. [DOI] [PubMed] [Google Scholar]
- Covey LS, Manubay J, Jiang H, Nortick M, Palumbo D. Smoking cessation and inattention or hyperactivity/impulsivity: a post hoc analysis. Nicotine Tob Res. 2008;10(12):1717–1725. doi: 10.1080/14622200802443536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biasi M, Dani JA. Reward, addiction, withdrawal to nicotine. Annu Rev Neurosci. 2011;34:105–130. doi: 10.1146/annurev-neuro-061010-113734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JN, Johnson D, Conners CK. Conners’ Adult ADHD Diagnostic Interview for DSM-IV. Multi-Health Systems, Inc.; North Tonawanda, NY: 2000. [Google Scholar]
- First MB, Gibbon M, Williams JBW, Spitzer RL. SCID Screen Patient Questionnaire - Extended Version. Multi-Health Systems, Inc.; North Tonawanda, NY: 1997. [Google Scholar]
- Froehlich TE, Lanphear BP, Epstein JN, Barbaresi WJ, Katusic SK, Kahn RS. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch Pediatr Adolesc Med. 2007;161(9):857–864. doi: 10.1001/archpedi.161.9.857. [DOI] [PubMed] [Google Scholar]
- Fuemmeler BF, Kollins SH, McClernon FJ. Attention deficit hyperactivity disorder symptoms predict nicotine dependence and progression to regular smoking from adolescence to young adulthood. J Pediatr Psychol. 2007;32(10):1203–1213. doi: 10.1093/jpepsy/jsm051. [DOI] [PubMed] [Google Scholar]
- Glass K, Flory K. Why does ADHD confer risk for cigarette smoking? A review of psychosocial mechanisms. Clin Child Fam Psychol Rev. 2010;13(3):291–313. doi: 10.1007/s10567-010-0070-3. [DOI] [PubMed] [Google Scholar]
- Grace AA. Psychostimulant action on dopamine and limbic system function: Relevance to the pathophysiology and treatment of ADHD. In: Solanto MV, Arnsten AFT, Castellanos FX, editors. Stimulant Drugs and ADHD: Basic and Clinical Neuroscience. Oxford University Press; Oxford: 2001. pp. 134–157. [Google Scholar]
- Heishman SJ. What aspects of human performance are truly enhanced by nicotine? Addiction. 1998;93(3):317–320. doi: 10.1080/09652149835864. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl) 2010;210(4):453–469. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervey AS, Epstein JN, Curry JF. Neuropsychology of adults with attention-deficit/hyperactivity disorder: a meta-analytic review. Neuropsychology. 2004;18(3):485–503. doi: 10.1037/0894-4105.18.3.485. [DOI] [PubMed] [Google Scholar]
- Humfleet GL, Prochaska JJ, Mengis M, Cullen J, Munoz R, Reus V, Hall SM. Preliminary evidence of the association between the history of childhood attention-deficit/hyperactivity disorder and smoking treatment failure. Nicotine Tob Res. 2005;7(3):453–460. doi: 10.1080/14622200500125310. [DOI] [PubMed] [Google Scholar]
- Huss M, Poustka F, Lehmkuhl G, Lehmkuhl U. No increase in long-term risk for nicotine use disorders after treatment with methylphenidate in children with attention-deficit/hyperactivity disorder (ADHD): evidence from a non-randomised retrospective study. J Neural Transm. 2008;115(2):335–339. doi: 10.1007/s00702-008-0872-3. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test, Second Edition (KBIT-II) AGS Publishing; Circle Pines, MN: 2004. [Google Scholar]
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, Faraone SV, Greenhill LL, Howes MJ, Secnik K, Spencer T, Ustun TB, Walters EE, Zaslavsky AM. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Adler LA, Barkley R, Biederman J, Conners CK, Faraone SV, Greenhill LL, Jaeger S, Secnik K, Spencer T, Ustun TB, Zaslavsky AM. Patterns and predictors of attention-deficit/hyperactivity disorder persistence into adulthood: results from the national comorbidity survey replication. Biol Psychiatry. 2005;57(11):1442–1451. doi: 10.1016/j.biopsych.2005.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am J Psychiatry. 1985;142(11):1259–1264. doi: 10.1176/ajp.142.11.1259. [DOI] [PubMed] [Google Scholar]
- Kollins SH, McClernon FJ, Fuemmeler BF. Association between smoking and attention-deficit/hyperactivity disorder symptoms in a population-based sample of young adults. Arch Gen Psychiatry. 2005;62(10):1142–1147. doi: 10.1001/archpsyc.62.10.1142. [DOI] [PubMed] [Google Scholar]
- Kollins SH, English J, Robinson R, Hallyburton M, Chrisman AK. Reinforcing and subjective effects of methylphenidate in adults with and without attention deficit hyperactivity disorder (ADHD) Psychopharmacology (Berl) 2009;204(1):73–83. doi: 10.1007/s00213-008-1439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins SH, English JS, Itchon-Ramos N, Chrisman AK, Dew R, O’Brien B, McClernon FJ. A Pilot Study of Lis-Dexamfetamine Dimesylate (LDX/SPD489) to Facilitate Smoking Cessation in Nicotine-Dependent Adults With ADHD. J Atten Disord. 2012a doi: 10.1177/1087054712440320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins SH, English JS, Roley ME, O’Brien B, Blair J, Lane SD, McClernon FJ. Effects of smoking abstinence on smoking-reinforced responding, withdrawal, and cognition in adults with and without attention deficit hyperactivity disorder. Psychopharmacology (Berl) 2012b doi: 10.1007/s00213-012-2937-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert NM. NIH Consensus Development Conference Diagnosis and Treatment of Attention Deficit Hyperactivity Disorder. NIH; Bethesda, MD: 1998. Stimulant treatment as a risk factor for nicotine use and substance abuse; pp. 191–198. [Google Scholar]
- Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil. 1998;31(6):533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Silva D, Canu W, March J. Effects of chronic nicotine and methylphenidate in adults with attention deficit/hyperactivity disorder. Exp Clin Psychopharmacol. 2001;9(1):83–90. doi: 10.1037/1064-1297.9.1.83. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Silva D, Hinton SC, Meck WH, March J, Rose JE. Transdermal nicotine effects on attention. Psychopharmacology (Berl) 1998;140(2):135–141. doi: 10.1007/s002130050750. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Sparrow E, Hinton SC, Erhardt D, Meck WH, Rose JE, March J. Nicotine effects on adults with attention-deficit/hyperactivity disorder. Psychopharmacology (Berl) 1996;123(1):55–63. doi: 10.1007/BF02246281. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rose JE, Behm F. Controlling puff volume without disrupting smoking topography. Behav Res Meth Instrum Comput. 1989;21:383–386. [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 2006;184(3-4):523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Kollins SH, Lutz AM, Fitzgerald DP, Murray DW, Redman C, Rose JE. Effects of smoking abstinence on adult smokers with and without attention deficit hyperactivity disorder: results of a preliminary study. Psychopharmacology (Berl) 2008;197(1):95–105. doi: 10.1007/s00213-007-1009-3. [DOI] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Chen L, Jones J. ADHD is associated with early initiation of cigarette smoking in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1997a;36(1):37–44. doi: 10.1097/00004583-199701000-00015. [DOI] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Wilens T, Chu MP. Associations between ADHD and psychoactive substance use disorders. Findings from a longitudinal study of high-risk siblings of ADHD children. Am J Addict. 1997b;6(4):318–329. [PubMed] [Google Scholar]
- Molina BS, Pelham WE., Jr. Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. J Abnorm Psychol. 2003;112(3):497–507. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- Monuteaux MC, Spencer TJ, Faraone SV, Wilson AM, Biederman J. A randomized, placebo-controlled clinical trial of bupropion for the prevention of smoking in children and adolescents with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2007;68(7):1094–1101. doi: 10.4088/jcp.v68n0718. [DOI] [PubMed] [Google Scholar]
- Poltavski DV, Petros T. Effects of transdermal nicotine on attention in adult non-smokers with and without attentional deficits. Physiol Behav. 2006;87(3):614–624. doi: 10.1016/j.physbeh.2005.12.011. R. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Downey KK, Stelson FW, Pomerleau CS. Cigarette smoking in adult patients diagnosed with attention deficit hyperactivity disorder. J Subst Abuse. 1995;7(3):373–378. doi: 10.1016/0899-3289(95)90030-6. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA. Effects of acute nicotine administration on behavioral inhibition in adolescents with attention-deficit/hyperactivity disorder. Psychopharmacology (Berl) 2004;176(2):182–194. doi: 10.1007/s00213-004-1874-y. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA. Acute nicotine improves cognitive deficits in young adults with attention-deficit/hyperactivity disorder. Pharmacol Biochem Behav. 2008;88(4):407–417. doi: 10.1016/j.pbb.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Rush CR, Higgins ST, Vansickel AR, Stoops WW, Lile JA, Glaser PE. Methylphenidate increases cigarette smoking. Psychopharmacology (Berl) 2005;181(4):781–789. doi: 10.1007/s00213-005-0021-8. [DOI] [PubMed] [Google Scholar]
- Rush CR, Kelly TH, Hays LR, Baker RW, Wooten AF. Acute behavioral and physiological effects of modafinil in drug abusers. Behav Pharmacol. 2002;13(2):105–115. doi: 10.1097/00008877-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Tidey JW, Badger GJ, Higgins ST. Acute effects of D-amphetamine on progressive-ratio performance maintained by cigarette smoking and money. Psychopharmacology (Berl) 2003;167(4):393–402. doi: 10.1007/s00213-003-1416-z. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Poole MM, Vansickel AR, Hays KA, Glaser PE, Rush CR. Methylphenidate increases choice of cigarettes over money. Nicotine Tob Res. 2011;13(1):29–33. doi: 10.1093/ntr/ntq198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, O’Neill SC, Higgins ST. Effects of abstinence on cigarette smoking among outpatients with schizophrenia. Exp Clin Psychopharmacol. 1999;7(4):347–353. doi: 10.1037//1064-1297.7.4.347. [DOI] [PubMed] [Google Scholar]
- Tidey JW, O’Neill SC, Higgins ST. d-amphetamine increases choice of cigarette smoking over monetary reinforcement. Psychopharmacology (Berl) 2000;153(1):85–92. doi: 10.1007/s002130000600. [DOI] [PubMed] [Google Scholar]
- Vansickel AR, Stoops WW, Glaser PE, Rush CR. A pharmacological analysis of stimulant-induced increases in smoking. Psychopharmacology (Berl) 2007;193(3):305–313. doi: 10.1007/s00213-007-0786-z. [DOI] [PubMed] [Google Scholar]
- Vansickel AR, Stoops WW, Glaser PE, Poole MM, Rush CR. Methylphenidate increases cigarette smoking in participants with ADHD. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, Fowler JS, Zhu W, Logan J, Ma Y, Pradhan K, Wong C, Swanson JM. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA. 2009;302(10):1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Kollins SH, Wigal TL, Newcorn JH, Telang FW, Fowler JS, Logan J, Wong CT, Swanson JM. Methylphenidate-elicited dopamine increases in ventral striatum are associated with long-term symptom improvement in adults with attention deficit hyperactivity disorder. J Neurosci. 2012;32(3):841–849. doi: 10.1523/JNEUROSCI.4461-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton DM, Mancuso G. Evaluation of the information processing and mood effects of a transdermal nicotine patch. Psychopharmacology (Berl) 1998;135(3):305–310. doi: 10.1007/s002130050514. [DOI] [PubMed] [Google Scholar]
- Whalen CK, Jamner LD, Henker B, Gehricke JG, King PS. Is there a link between adolescent cigarette smoking and pharmacotherapy for ADHD? Psychol Addict Behav. 2003;17(4):332–335. doi: 10.1037/0893-164X.17.4.332. [DOI] [PubMed] [Google Scholar]
- Wilcox RR. Measuring and detecting associations: methods based on robust regression estimators or smoothers that allow curvature. Br J Math Stat Psychol. 2010;63(Pt 2):379–393. doi: 10.1348/000711009X467618. [DOI] [PubMed] [Google Scholar]
- Wilcox RR. Introduction to robust estimation and hypothesis testing. Academic Press; San Diego, CA: 1997. [Google Scholar]
- Wilens TE, Vitulano M, Upadhyaya H, Adamson J, Sawtelle R, Utzinger L, Biederman J. Cigarette smoking associated with attention deficit hyperactivity disorder. J Pediatr. 2008;153(3):414–419. doi: 10.1016/j.jpeds.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen T, Somoza E, al. e. Impact of attention-deficit/hyperactivity disorder (ADHD) treatment on smoking cessation intervention in ADHD smokers: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2010;71(12):16801688. doi: 10.4088/JCP.09m05089gry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters KC, Lee S, Botzet A, Fahnhorst T, Realmuto GM, August GJ. A Prospective Examination of the Association of Stimulant Medication History and Drug Use Outcomes among Community Samples of ADHD Youths. J Child Adolesc Subst Abuse. 2011;20(4):314–329. doi: 10.1080/1067828X.2011.598834. [DOI] [PMC free article] [PubMed] [Google Scholar]