Abstract
Gastrointestinal tract duplication cysts are rare congenital gastrointestinal malformation in young patients and adults. They consist of foregut duplication cysts, small bowel duplication cysts, and large bowel duplication cysts. Endoscopic ultrasound (EUS) has been widely used as a modality for the evaluation and diagnosis of duplication cysts. EUS is the diagnostic tool of choice to investigate duplication cysts since it can distinguish between solid and cystic lesions. The question of whether or not to perform EUS-fine needle aspiration (EUS-FNA) on a lesion suspected of being a duplication cyst is controversial as these lesions can become infected with significant consequences, although EUS-FNA is often required to obtain a definitive diagnosis and to rule out more ominous lesions. This manuscript will review the literature on duplication cysts throughout the body and will also focus on the role of EUS and FNA with regards to these lesions.
Keywords: Bronchogenic, duodenal, duplication cyst, esophageal, endoscopic ultrasound, fine needle aspiration, mediastinitis
INTRODUCTION
Gastrointestinal tract duplication cysts are rare congenital gastrointestinal malformation in young patients and adults. They consist of foregut duplication cysts, small bowel duplication cysts, and large bowel duplication cysts. Foregut duplication cysts are categorized on the basis of their embryonic origin into esophageal, bronchogenic, and neuroenteric cysts.[1] Bronchogenic and esophageal duplication cysts are thought to arise from abnormal budding of the embryonic foregut at 5-8 weeks gestation, although the exact embryonic origin of different types of duplication cysts remains a mystery.[2] Of note, 50-70% of foregut duplication cysts are enterogenous while 7-15% of them are bronchogenic.[3] Foregut duplication cysts constitute 6-15% of primary mediastinal masses.[4] Gastrointestinal tract duplication cysts most commonly occur in the ileum, esophagus, and colon. They may be contained within the gastrointestinal tract wall or extrinsic to it.[5] Duplication cysts can also be cystic (80%) or tubular (20%).[6]
Endoscopic ultrasound (EUS) has been widely used as a modality for the evaluation and diagnosis of duplication cysts. EUS is the diagnostic tool of choice to investigate duplication cysts since it can distinguish between solid and cystic lesions.[7] EUS can also establish cyst location relative to surrounding tissues.[8,9] EUS shows duplication cysts as anechoic, homogenous lesions with regular margins arising from the submucosal layer or extrinsic to the gut wall, although a hypoechoic echo pattern can also be seen with a duplication cyst.[5] On EUS, duplication cyst walls usually consists of 3-5 layers and the internal contents may be anechoic or hypoechoic.[3] Duplication cysts may contain thick mucinous material, septations, fluid levels, debris and they may also contain detached ciliary tufts which could be diagnostic.[5,9] In addition, duplication cysts can have peristalsis that appears as ring contractions with a concentric contraction of the cystic wall. Peristalsis in a juxta-enteric cyst is specific for a duplication cyst and can be a diagnostic feature.[3]
ESOPHAGEAL DUPLICATION CYSTS
Esophageal duplication cysts are the second most common duplication cysts following small bowel duplications cysts, accounting for approximately 10-15% of gastrointestinal duplication cysts. The prevalence of esophageal duplications cysts is 0.0122%.[3] As many as 80% of these lesions are diagnosed in childhood with the majority being symptomatic.[3,10] Most of the esophageal duplication cysts are located in the right posterior inferior mediastinum. Two-thirds of these lesions are found in the lower third of esophagus and 1/3 in the upper/middle third of esophagus.[5]
Esophageal duplication cysts have a double layer of surrounding smooth muscle, are lined by alimentary (squamous or enteric) epithelium, and are either attached to esophagus in a paraesophageal or intramural fasion.[3,11,12,13] Patients with esophageal duplication cysts are usually asymptomatic but can develop symptoms (such as dysphagia or chest pain) due to compression of surrounding structures.[3] One study showed that seven percent of esophageal duplication cysts can cause symptoms in adulthood, with 60% of these cysts located in the lower esophagus with no communication with esophageal lumen.[14] Upper esophageal duplication cysts can cause stridor and/or a nonproductive cough, while cysts in the middle and lower esophagus can cause dysphagia, epigastric discomfort, chest pain, and/or vomiting. Rare symptoms such as cardiac arrhythmia, retrosternal and thoracic back pain, cyst ulceration and bleeding, or cyst rupture with secondary mediastinitis have also been reported.[15,16]
On routine endoscopy, esophageal duplication cysts can be identified via extrinsic compression of the lumen with normal appearing overlying esophageal mucosa. Endoscopically, these lesions may be indistinguishable from a lipoma, leiomyoma, a gastrointestinal stromal tumor (GIST), or other submucosal lesions. On EUS, esophageal duplication cysts will often appear as a periesophageal homogenous hypoechoic mass with multi-layered wall and well-defined margins, although sometimes the lesion can manifest as an anechoic cyst if considerable central fluid is present[1] [Figure 1].
Figure 1a.
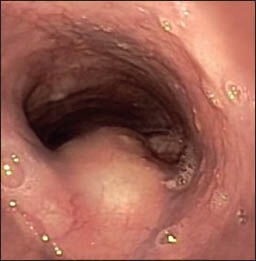
Endoscopic view of an esophageal duplication cyst in a 9-year-old male with dysphagia. Note the normal overlying mucosa and the narrowing of the esophageal lumen
Figure 1b.
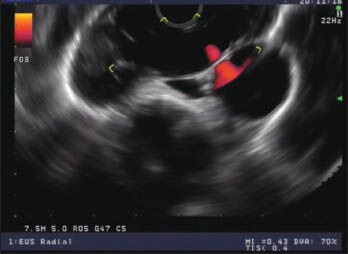
7.5 MHz radial endoscopic ultrasound (EUS) image of the cyst seen in Figure 1a. The lesion has a multi-layered wall structure and areas of anechoic fluid as well as some echogenic material (seen on the left side of the lesion). EUS-fine needle aspiration was deferred. The lesion was surgically resected
The role of EUS-guided fine needle aspiration (FNA) in the diagnosis of esophageal duplication cysts, and duplication cysts in general, has been controversial. While FNA can show squamous cells or other cyst contents consistent with a duplication cyst that can aid in the diagnosis, the risk of infecting the cyst is a significant concern, with some studies showing an infection rate as high as 14%.[7,10] EUS-FNA should be performed if there is a concern about more serious lesion or if the nature of the lesion is in doubt.
Endoscopic ultrasound-guided FNA is usually reserved for lesions of indeterminate appearance, lesions that are concerning for malignancy, and lesions that are atypical in appearance for duplication cysts. It should be noted that many duplication cysts can have an unusual appearance and can mimic other pathologic entities, including the malignant adenopathy. EUS-guided FNA may reveal a lesion felt to be a node to be, in fact, a duplication cyst. Even expert endosonographers can mistake a duplication cyst for a more ominous lesion, and aspirating a lesion of concern should not be considered a breach of the standard of care.
Concerns regarding causing an adverse event following EUS-guided FNA of esophageal duplication cysts are well founded. Cevasco et al. reported a case in which a patient underwent EUS-FNA of an esophageal hyperechoic duplication cyst that subsequently got infected. The infected esophageal duplication cyst led to acute development and infection of a thoracic aorta pseudoaneurysm.[10] Similarly, Trojan et al. reported a case of an infected hypoechoic esophageal duplication cyst after performing EUS-FNA on a paraesophageal mass concerning for persistent nonHodgkin's lymphoma (again showing how esophageal duplication cysts can mimic ominous lesions such as a malignant adenopathy).[17] Although some authors advocate the use of antibiotics to help prevent infection, Diehl et al. reported one patient with infected esophageal duplication cysts due to EUS-FNA despite having received pre and postprocedure prophylactic antibiotics prior to the procedure. This patient subsequently underwent thoracotomy with cyst resection.[1] Overall, EUS-guided FNA of duplication cysts should be avoided if the endosonographer has a high index of suspicion that the lesion in question really is a duplication cyst.[18] In some cases, EUS-FNA may be warranted in order to characterize the lesion in question, but if a duplication cyst is discovered appropriate antibiotic coverage should be utilized.
From a treatment perspective, surgical removal/enucleation is the treatment of choice in most symptomatic cases. In asymptomatic cases, surgery can be considered as the cyst could develop ulceration or perforation and the short-term postoperative outcome in these patients has been excellent.[19] Noguchi et al. reported a case of successful laparoscopic surgery of an asymptomatic esophageal cyst in a 26-year-old patient who remained asymptomatic at 3 year follow-up.[20] On the other hand, surgical intervention for asymptomatic cyst can also lead to long-term complications such as heartburn and reflux esophagitis and can carry a mortality as high as 1%.[19]
Another treatment strategy is observation in asymptomatic individuals. Versleijen et al. described a case in which a patient with asymptomatic esophageal duplication cyst (diameter 1.1-4.1 cm) was followed for 13 years and routine EUS did not show cyst growth. These authors advocated EUS surveillance over surgery in asymptomatic patients, although the cost implications of such an approach have not been formally studied to date.[21]
GASTRIC DUPLICATION CYSTS
Gastric duplication cysts makeup between 4% and 9% of all intestinal duplication cysts.[11] They are usually single and, in general, do not communicate with gastric lumen. Histologically, the cyst wall can consist of mucosa, subepithelial connective tissue, a layer of smooth muscle, and an outer fibrous capsule. The mucosa is typically lined by gastric foveolar epithelium, but most of the cystic wall is lined by a pseudostratified columnar ciliated epithelium. Sometimes small intestinal or colonic mucosa can also be found.[22] Gastric duplication cysts may also contain ciliated cells, proteinaceous debris, crystal formations, or engulfed histiocytes.[22]
The origin of gastric duplication cysts remains uncertain. Khoury and Rivera reported two cases where the gastric duplication cysts appear to originate from a respiratory diverticulum which arises from the ventral foregut.[23] Most gastric duplication cysts are located along the greater curvature of the stomach. Only 5.5% arise along the lesser curvature.[24] Gastric duplication cysts can also be found in the upper part of the stomach at the level of the cardia, near the gastroesophageal junction, along the greater curvature of the stomach, or in the anterior or posterior wall of the fundus.[3,22]
Patients with gastric duplication cysts can be asymptomatic but can also develop symptoms such as diffuse abdominal pain, epigastric pain, vomiting, weight loss, gastric outlet obstruction, ulcerated antral mass, or failure to thrive.[22,24,25]
On EUS, gastric duplication cyst can appear as a hypoechoic lesion with a heterogeneous internal echotexture and regular margins. Gastric duplication cysts can be contiguous with the muscularis propria of the gastric wall.[22] For example, Bhatia et al. described an anechoic cyst with four distinct wall layers adjacent to the distal gastric body and antrum in a patient with gastric duplication cyst; this cyst was found to be lined with gastric mucosa.[25] Seijo et al. also described an anechoic cyst arising from the muscularis propria that was ultimately found to be a gastric duplication cyst. This study did not describe the wall appearance of the cyst in detail.[26]
The role of FNA in establishing the diagnosis remains controversial. Some authors such as Seijo et al. argued that a cytological and histological examination of the cyst via EUS-FNA was necessary in order to rule out malignant cyst transformation.[26] Napolitano et al. also reported a case of a gastric duplication cyst that was initially misdiagnosed as a GIST with EUS. Later on a separate procedure it was diagnosed as a gastric duplication cyst with EUS-FNA.[22] Other authors argued that FNA aspiration of duplication cysts should not be routine as this could increase the risk of infection and medistinal abscess formation, although in this case the FNA itself allowed for a definitive diagnosis and ruled out a more ominous lesion.[18]
There have been <10 reported cases of gastric cancer arising from gastric duplication cyst to date.[27] Since it is still possible for cancer to arise from gastric-type lining epithelium or pseudostratified columnar ciliated epithelium of the gastric duplication cyst, some authors favor surgery for these lesions in asymptomatic patients.[28,29,30,31] On the other hand, others authors favor conservative treatment since malignant transformation of these lesions is anecdotal.[13,34] Ponder, and Collins suggested that surgery may not be necessary if respiratory epithelium, instead of the gastric epithelium, is recognized on EUS-FNA because gastric cancer is less likely to arise from the respiratory epithelium than from gastric epithelium.[33]
BRONCHOGENIC DUPLICATION CYSTS
Bronchogenic duplication cysts are lined by respiratory epithelium (which is usually ciliated pseudostratified columnar in nature) and may contain cartilage/bronchial glands in their wall.[3] Bronchogenic duplication cysts can also contain one or more layers of smooth muscle.[5] Most are located in the mediastinum around the tracheobronchial tree or within the pulmonary parenchyma.[5]
Symptomatically, patients with bronchogenic duplication cysts can present with dysphagia, chest pain, cough, shortness of breath, or abdominal pain. On EUS, these lesions often appear as round or oval lesion with a thin outer wall and located adjacent to the thoracic esophagus with well-defined endosonographic borders.[1] Their intracystic contents can be anechoic, hypoechoic, or contain dense hyperechoic debris, which could be potentially confused with a soft-tissue masses or a malignant adenopathy[34] [Figure 2].
Figure 2a.
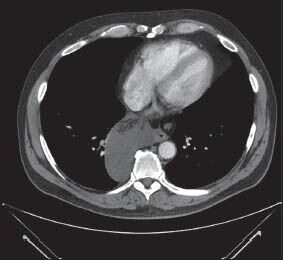
Computed tomography scan image of a large mediastinal cyst in a 57-year-old man with chest discomfort. Note that the cyst appears to contain a fluid as well as a suggestion of solid material that does not enhance with contrast
Figure 2b.
7.5 MHz linear endoscopic ultrasound (EUS) image of the lesion seen in Figure 2a. The lesion is seen to contain anechoic fluid, as well as a hyperechoic structure with finger-like projections. The lesion did undergo EUS-fine needle aspiration and revealed bronchogenic elements and mucus with a copious debris. The lesion was surgically resected
Figure 2c.
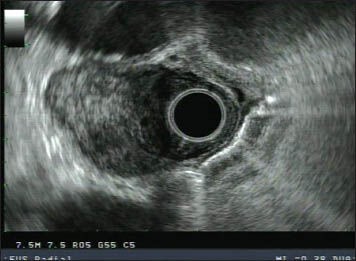
7.5 MHz radial endoscopic ultrasound (EUS) image of a hypoechoic, solid-appearing lesion felt to be a malignant lymph node on EUS. Fine needle aspiration revealed bronchogenic elements consistent with a benign bronchogenic duplication cyst
When aspirated by EUS-FNA, cytology can reveal cellular debris, hemosiderin laden macrophages, ciliated columnar cells, and goblet cells.[7] Their contents can range from thin, free-flowing fluid to solidified mucoid material that cannot be aspirated on FNA.[34]
With regards to EUS-FNA, some authors have favored this while others defer it. Fazel et al. reported a series of 22 patients in which the use of 22-gauge needles and prophylactic antibiotics in the diagnosis of bronchogenic duplication cysts resulted in no infectious complications during the 6 months follow-up.[34] On the other hand, the use of EUS-FNA to confirm the diagnosis of benign cysts has been associated with infections and mediastinitis.[7,35] Diehl et al. reported three cases of infected duplication cyst due to EUS-FNA. Two of them involved patients with bronchogenic cyst who received pre and postprocedure prophylactic antibiotics. These two patients went on to develop infected cysts. One was resected with video-assisted thoracoscopy and the other one with thoracotomy.[1]
With regards to treatment, surgical enucleation is the treatment of choice in symptomatic cases. In asymptomatic cases, surgical resection has been suggested due to the rare development of complications or malignancy, but many of these lesions can be safely observed.[36] Some experts also recommend close surveillance alone when the diagnosis has been confirmed by needle aspiration, but no standard regimen for surveillance exists.[37] Indeed, consensus on whether these lesions need to be followed at all does not exist at this time.
SMALL BOWEL DUPLICATION CYSTS
Small bowel duplication cysts can be associated with all three small bowel subtypes: Duodenal, jejunal, and ileal. Jejunal duplications are the most common, followed by ileal and duodenal duplications.[38] Duodenal duplication cysts makeup 2-12% of GI tract duplications.[39] Ileal duplication cysts makeup about 44% of GI tract duplications.[40] Jejunal duplication cysts makeup about 50% of GI tract duplications.[41]
In general, the wall of small bowel duplication cysts can contain two-mucosal layers sharing a common muscle layer.[42] More specifically, duodenal duplication cyst consists of submosa, muscularis propria, a duodenal epithelial lining, and intimate attachment to the GI tract[43] [Figure 3]. Jejunal duplication cyst consists of submosa, muscularis propria, and are lined with jejunal mucus glands. Similarly, ileal duplication cyst consists of submosa, muscularis propria, and are lined with ileal mucus glands and can contain heterotopic gastric mucosae.[44]
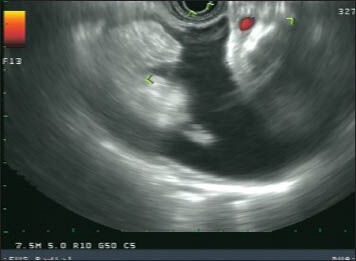
Figure 3.
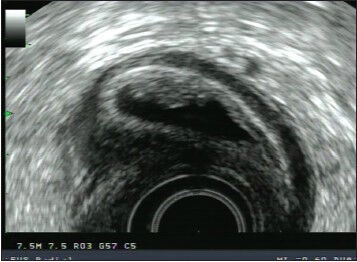
7.5 MHz radial endoscopic ultrasound (EUS) image of a duodenal duplication cyst in a 22-year-old female. The lesion was discovered incidentally on a computed tomography scan performed to evaluate for renal stones. The lesion manifests as a multi-layered cystic structure with the cyst muscularis propria communicating with the duodenal muscularis propria, as well as what appears to be a mucosal prominence. The patient declined surgical resection and has undergone serial EUS exams without interval change
Most duodenal duplication cysts are located in the second or third portion of the duodenum.[42] The most common type of duodenal cyst is the cystic and noncommunicating type, usually located at the medial border of the second part of the duodenum and extending anteriorly or posteriorly.[45] Jejunal duplication cysts can be found arising from the mesenteric aspect of the jejunum.[46] Ileal duplication cysts can arise anywhere in the native ileum.[44]
Patients with small bowel duplication cysts can present with a variety of symptoms including vomiting and abdominal pain.[47] Ko et al. reported a patient with a duplication cyst in the second and third portion of the duodenum with duodenoduodenal intussuception, melena, and abdominal pain. The patient underwent small bowel resection and duodenotomy with uneventful recovery. Her symptoms had resolved following surgical resection.[42]
Duodenal cysts can cause other complications such as pancreatitis, infection, weight loss, and GI bleeding from ulceration of the ectopic gastric mucosa within the cyst.[39,48] Jejunal duplication cyst can cause abdominal bloating, constipation, intussusception, vovulus, and partial small bowel obstruction.[46,49] Ileal duplication cysts may be asymptomatic or present with abdominal pain, small bowel obstruction, a palpable abdominal mass, or hematochezia. Rarely, malignant transformation can occur in the setting of gastric mucosa heterotopia within the duplication cyst.[50]
On EUS, duodenal duplication cysts can have a 3-5 layer wall consistent with cyst of intestinal origin, and the cyst's muscularis propria can be continuous with the muscularis propria of the duodenum.[47] Duodenal duplication cyst can have an echogenic inner mucosa surrounded by a hypoechoic outer muscular layer.[51] Until date, no data has been published on the utility of EUS-FNA in diagnosing small bowel duplication cysts.
Regarding the treatment, duodenal cysts are usually treated with surgical resection, although endoscopic treatment has been reported especially in cases where the duplication is in close proximity to the major duodenal papillae.[52]
Treatment of asymptomatic duodenal cysts remains controversial. Al-Harake et al. recommended complete surgical resection of duodenal cysts, which may require pancreaticoduodenectomy if the cyst is located near the biliary-pancreatic duct.[38] Surgery has been recommended by some authors due to possible malignant transformation based on case reports.[39] Johnson and Poole reported three out of 13-adult ileal duplication cyst patients who had ileal cancer arising from the cysts, including two patients with adenocarcinoma and one patient with squamous cell carcinoma. This has been seen as an argument for resection of ileal duplication cysts.[50] Wan et al. also advocated for surgical resection of asymptomatic jejunal duplication cysts due to the risk of malignant degeneration.[46]
LARGE BOWEL/RECTAL DUPLICATION CYSTS
Colonic duplication cysts represent 6.8% of gastrointestinal duplication cysts.[53] On histology, heterotopic gastric mucosa may be found in 33% of colonic duplication cysts.[53] Colonic cysts can also contain multiple layers of the bowel wall including mucosa, submucosa, and muscularis propria. They can contain at least one outer muscular layer with an inner gastrointestinal mucosal lining. Colonic duplication cysts can also contain well-organized layers of smooth muscle with intimate attachment of the common wall to the colon and fibrosis, inflammatory cells, lymphoid aggregates, necrosis, and calcification.[54]
Colonic duplication cysts can be located anywhere in the large intesting.[55,6,54] Colonic cysts can be asymptomatic or present as abdominal pain to the point of an acute abdomen, obstruction, and/or bleeding.[55] These lesions may be asymptomatic as well. Gastrointestinal bleeding can occur if ectopic gastric mucosa ulcerates and erodes into adjacent organs or vessels.[54] In addition, malignant degeneration is most often reported in the colon as up to 67% of malignancies diagnosed in duplication cysts occurred in the colon.[56]
On EUS, colonic duplication cysts can show a “gut signature” which will manifest as a multi-layered wall, with a relatively hyperechoic inner layer produced by the mucosa surrounded by a relatively hypoechoic outer layer caused by smooth muscle, or can have a more irregular and atypical appearance[27] [Figure 4]. It should be noted that some cysts lack a well-organized layered wall due to some degree of involution or degeneration. The submucosa may be difficult to see and has no universally agreed upon appearance. Peristalsis of the cyst wall has also been reported on EUS.[57] Computed tomography scanning is also a highly sensitive diagnostic modality and is often the initial test of choice.[55] Contrast enema can also establish the diagnosis if it can demonstrate a colonic filling defect or luminal communication with the bowel, but is much less commonly performed in the current era.[6] Colonoscopy will show the duplication cyst only if there is communication with the colon or extrinsic compression and may fail to detect a significant number of colonic duplication cysts.[6] To date, no data has been published on the utility of EUS-FNA in diagnosing colonic duplication cysts.
Figure 4a.
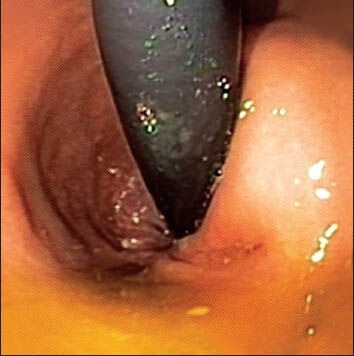
Endoscopic view of an extrinsically compressing cystic lesion adjacent to the rectum in a 72-year-old female. The lesion was discovered during a staging computed tomography scan for uterine cancer
Figure 4b.
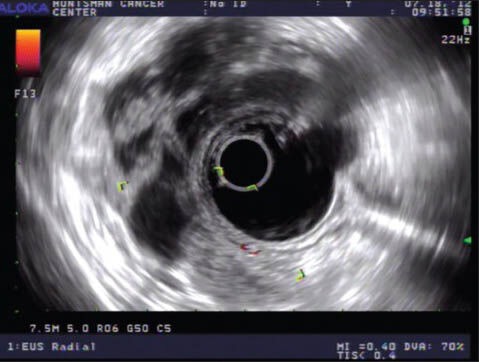
7.5 MHz radial image of the lesion shown in Figure 4a. The lesion manifests as a complex, multi-layered structure with a complex luminal contour. Endoscopic ultrasound-fine needle aspiration was deferred given the appearance, and the patient declined any intervention on the lesion given that she had a concurrent cancer diagnosis
Regarding the treatment, some authors recommend routine resection of asymptomatic colonic duplication cysts due to the potential for perforation, bleeding, obstruction, and malignant degeneration.[58] In patients with symptomatic colonic cysts, surgical resection is usually recommended in good operative candidates. Reiser-Erkan et al. reported a case of colonic duplication cyst manifesting as acute abdomen and intussusception necessitating a right-sided hemicolectomy.[55] Domajnko and Salloum, Puligandla et al. recommended en block resection of symptomatic colonic duplications.[6,53] In addition, Mourra et al. reported colonic duplication cysts in seven patients (four presenting with abdominal pain, three presenting with bowel obstruction) who subsequently underwent surgical partial colectomy with associated complete cyst excision.[54]
CONCLUSION
Gastrointestinal duplication cysts are rare congenital lesions in adults. Some patients are asymptomatic while others can present with abdominal pain, bleeding, and abdominal pain. EUS can offer an accurate diagnosis of duplication cysts. EUS-FNA allows high-resolution morphologic analysis as well as sampling and microscopic examination of cyst contents; it can also lead to duplication cyst infection with associated complications despite pre and postprocedural antibiotics in some patients. EUS-FNA of duplication cysts can carry an increased risk of complications, but may be warranted to obtain a definitive diagnosis and to rule out more serious pathology.
Once the diagnosis of duplication cyst is established, treatment can vary depending on the presence of symptoms. In symptomatic patients, surgical resection is often the choice for symptom relief. In asymptomatic patients, surgical resection is controversial. While some authors advocate for resection due to possible malignant degeneration of the duplication cyst, others have advocated for observation. Since there have been case reports of stable duplication cysts on EUS surveillance, this may be a suitable method of outpatient follow-up and surgical resection can be considered if patient develops symptoms. In any case, surgical versus nonsurgical management of asymptomatic duplication cysts is likely to remain controversial until we understand more about the time course and risk factors associated with their malignant degeneration.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Diehl DL, Cheruvattath R, Facktor MA, et al. Infection after endoscopic ultrasound-guided aspiration of mediastinal cysts. Interact Cardiovasc Thorac Surg. 2010;10:338–40. doi: 10.1510/icvts.2009.217067. [DOI] [PubMed] [Google Scholar]
- 2.Nobuhara KK, Gorski YC, La Quaglia MP, et al. Bronchogenic cysts and esophageal duplications: Common origins and treatment. J Pediatr Surg. 1997;32:1408–13. doi: 10.1016/s0022-3468(97)90550-9. [DOI] [PubMed] [Google Scholar]
- 3.Whitaker JA, Deffenbaugh LD, Cooke AR. Esophageal duplication cyst. Case report. Am J Gastroenterol. 1980;73:329–32. [PubMed] [Google Scholar]
- 4.Snyder ME, Luck SR, Hernandez R, et al. Diagnostic dilemmas of mediastinal cysts. J Pediatr Surg. 1985;20:810–5. doi: 10.1016/s0022-3468(85)80048-8. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia V, Tajika M, Rastogi A. Upper gastrointestinal submucosal lesions — clinical and endosonographic evaluation and management. Trop Gastroenterol. 2010;31:5–29. [PubMed] [Google Scholar]
- 6.Domajnko B, Salloum RM. Duplication cyst of the sigmoid colon. Gastroenterol Res Pract 2009. 2009:918401. doi: 10.1155/2009/918401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wildi SM, Hoda RS, Fickling W, et al. Diagnosis of benign cysts of the mediastinum: The role and risks of EUS and FNA. Gastrointest Endosc. 2003;58:362–8. [PubMed] [Google Scholar]
- 8.Faigel DO, Burke A, Ginsberg GG, et al. The role of endoscopic ultrasound in the evaluation and management of foregut duplications. Gastrointest Endosc. 1997;45:99–103. doi: 10.1016/s0016-5107(97)70315-8. [DOI] [PubMed] [Google Scholar]
- 9.Eloubeidi MA, Cohn M, Cerfolio RJ, et al. Endoscopic ultrasound-guided fine-needle aspiration in the diagnosis of foregut duplication cysts: The value of demonstrating detached ciliary tufts in cyst fluid. Cancer. 2004;102:253–8. doi: 10.1002/cncr.20369. [DOI] [PubMed] [Google Scholar]
- 10.Cevasco M, Menard MT, Bafford R, et al. Acute infectious pseudoaneurysm of the descending thoracic aorta and review of infectious aortitis. Vasc Endovascular Surg. 2010;44:697–700. doi: 10.1177/1538574410376449. [DOI] [PubMed] [Google Scholar]
- 11.Wang B, Hunter WJ, Bin-Sagheer S, et al. Rare potential pitfall in endoscopic ultrasound-guided fine needle aspiration biopsy in gastric duplication cyst: A case report. Acta Cytol. 2009;53:219–22. doi: 10.1159/000325129. [DOI] [PubMed] [Google Scholar]
- 12.Arbona JL, Fazzi JG, Mayoral J. Congenital esophageal cysts: Case report and review of literature. Am J Gastroenterol. 1984;79:177–82. [PubMed] [Google Scholar]
- 13.Geller A, Wang KK, DiMagno EP. Diagnosis of foregut duplication cysts by endoscopic ultrasonography. Gastroenterology. 1995;109:838–42. doi: 10.1016/0016-5085(95)90392-5. [DOI] [PubMed] [Google Scholar]
- 14.Pisello F, Geraci G, Arnone E, et al. Acute onset of esophageal duplication cyst in adult. Case report. G Chir. 2009;30:17–20. [PubMed] [Google Scholar]
- 15.Bowton DL, Katz PO. Esophageal cyst as a cause of chronic cough. Chest. 1984;86:150–2. doi: 10.1378/chest.86.1.150. [DOI] [PubMed] [Google Scholar]
- 16.Neo EL, Watson DI, Bessell JR. Acute ruptured esophageal duplication cyst. Dis Esophagus. 2004;17:109–11. doi: 10.1111/j.1442-2050.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 17.Trojan J, Mousset S, Caspary WF, et al. An infected esophageal duplication cyst in a patient with non-Hodgkin's lymphoma mimicking persistent disease. Dis Esophagus. 2005;18:287–9. doi: 10.1111/j.1442-2050.2005.00490.x. [DOI] [PubMed] [Google Scholar]
- 18.Béchade D, Desramé J, Algayres JP. Gastritis cystica profunda in a patient with no history of gastric surgery. Endoscopy. 2007;39(Suppl 1):E80–1. doi: 10.1055/s-2006-945070. [DOI] [PubMed] [Google Scholar]
- 19.Salo JA, Ala-Kulju KV. Congenital esophageal cysts in adults. Ann Thorac Surg. 1987;44:135–8. doi: 10.1016/s0003-4975(10)62023-1. [DOI] [PubMed] [Google Scholar]
- 20.Noguchi T, Hashimoto T, Takeno S, et al. Laparoscopic resection of esophageal duplication cyst in an adult. Dis Esophagus. 2003;16:148–50. doi: 10.1046/j.1442-2050.2003.00314.x. [DOI] [PubMed] [Google Scholar]
- 21.Versleijen MW, Drenth JP, Nagengast FM. A case of esophageal duplication cyst with a 13-year follow-up period. Endoscopy. 2005;37:870–2. doi: 10.1055/s-2005-870219. [DOI] [PubMed] [Google Scholar]
- 22.Napolitano V, Pezzullo AM, Zeppa P, et al. Foregut duplication of the stomach diagnosed by endoscopic ultrasound guided fine-needle aspiration cytology: Case report and literature review. World J Surg Oncol. 2013;11:33. doi: 10.1186/1477-7819-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khoury T, Rivera L. Foregut duplication cysts: A report of two cases with emphasis on embryogenesis. World J Gastroenterol. 2011;17:130–4. doi: 10.3748/wjg.v17.i1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim DH, Kim JS, Nam ES, et al. Foregut duplication cyst of the stomach. Pathol Int. 2000;50:142–5. doi: 10.1046/j.1440-1827.2000.01008.x. [DOI] [PubMed] [Google Scholar]
- 25.Bhatia V, Garg PK, Gupta SD, et al. Demonstration of peristalsis in gastric duplication cyst by EUS: Implications for diagnosis and symptomatology (with videos) Gastrointest Endosc. 2008;68:183–5. doi: 10.1016/j.gie.2007.10.057. [DOI] [PubMed] [Google Scholar]
- 26.Seijo Ríos S, Lariño Noia J, Abdulkader Nallib I, et al. Adult gastric duplication cyst: Diagnosis by endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) Rev Esp Enferm Dig. 2008;100:586–90. doi: 10.4321/s1130-01082008000900011. [DOI] [PubMed] [Google Scholar]
- 27.Barr LL, Hayden CK, Jr, Stansberry SD, et al. Enteric duplication cysts in children: Are their ultrasonographic wall characteristics diagnostic? Pediatr Radiol. 1990;20:326–8. doi: 10.1007/BF02013165. [DOI] [PubMed] [Google Scholar]
- 28.Coit DG, Mies C. Adenocarcinoma arising within a gastric duplication cyst. J Surg Oncol. 1992;50:274–7. doi: 10.1002/jso.2930500417. [DOI] [PubMed] [Google Scholar]
- 29.Murakami S, Isozaki H, Shou T, et al. Foregut duplication cyst of the stomach with pseudostratified columnar ciliated epithelium. Pathol Int. 2008;58:187–90. doi: 10.1111/j.1440-1827.2007.02209.x. [DOI] [PubMed] [Google Scholar]
- 30.Kuraoka K, Nakayama H, Kagawa T, et al. Adenocarcinoma arising from a gastric duplication cyst with invasion to the stomach: A case report with literature review. J Clin Pathol. 2004;57:428–31. doi: 10.1136/jcp.2003.013946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horie H, Iwasaki I, Takahashi H. Carcinoid in a gastrointestinal duplication. J Pediatr Surg. 1986;21:902–4. doi: 10.1016/s0022-3468(86)80021-5. [DOI] [PubMed] [Google Scholar]
- 32.Horne G, Ming-Lum C, Kirkpatrick AW, et al. High-grade neuroendocrine carcinoma arising in a gastric duplication cyst: A case report with literature review. Int J Surg Pathol. 2007;15:187–91. doi: 10.1177/1066896906295777. [DOI] [PubMed] [Google Scholar]
- 33.Ponder TB, Collins BT. Fine needle aspiration biopsy of gastric duplication cysts with endoscopic ultrasound guidance. Acta Cytol. 2003;47:571–4. doi: 10.1159/000326570. [DOI] [PubMed] [Google Scholar]
- 34.Fazel A, Moezardalan K, Varadarajulu S, et al. The utility and the safety of EUS-guided FNA in the evaluation of duplication cysts. Gastrointest Endosc. 2005;62:575–80. doi: 10.1016/j.gie.2005.06.014. Erratum in: Gastrointest Endosc 2005;62:996. [DOI] [PubMed] [Google Scholar]
- 35.Ryan AG, Zamvar V, Roberts SA. Iatrogenic candidal infection of a mediastinal foregut cyst following endoscopic ultrasound-guided fine-needle aspiration. Endoscopy. 2002;34:838–9. doi: 10.1055/s-2002-34262. [DOI] [PubMed] [Google Scholar]
- 36.Sirivella S, Ford WB, Zikria EA, et al. Foregut cysts of the mediastinum. Results in 20 consecutive surgically treated cases. J Thorac Cardiovasc Surg. 1985;90:776–82. [PubMed] [Google Scholar]
- 37.Kuhlman JE, Fishman EK, Wang KP, et al. Esophageal duplication cyst: CT and transesophageal needle aspiration. AJR Am J Roentgenol. 1985;145:531–2. doi: 10.2214/ajr.145.3.531. [DOI] [PubMed] [Google Scholar]
- 38.Al-Harake A, Bassal A, Ramadan M, et al. Duodenal duplication cyst in a 52-year-old man: A challenging diagnosis and management. Int J Surg Case Rep. 2013;4:296–8. doi: 10.1016/j.ijscr.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen JJ, Lee HC, Yeung CY, et al. Meta-analysis: The clinical features of the duodenal duplication cyst. J Pediatr Surg. 2010;45:1598–606. doi: 10.1016/j.jpedsurg.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 40.Al-Sarem SA, Al-Shawi JS. Ileal duplication in adults. Saudi Med J. 2007;28:1734–6. [PubMed] [Google Scholar]
- 41.Tamvakopoulos GS, Sams V, Preston P, et al. Iron-deficiency anaemia caused by an enterolith-filled jejunal duplication cyst. Ann R Coll Surg Engl. 2004;86:W49–51. doi: 10.1308/147870804119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ko SY, Ko SH, Ha S, et al. A case of a duodenal duplication cyst presenting as melena. World J Gastroenterol. 2013;19:6490–3. doi: 10.3748/wjg.v19.i38.6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gross RE, Holcomb GW, Jr, Farber S. Duplications of the alimentary tract. Pediatrics. 1952;9:448–68. [PubMed] [Google Scholar]
- 44.Li BL, Huang X, Zheng CJ, et al. Ileal duplication mimicking intestinal intussusception: A congenital condition rarely reported in adult. World J Gastroenterol. 2013;19:6500–4. doi: 10.3748/wjg.v19.i38.6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merrot T, Anastasescu R, Pankevych T, et al. Duodenal duplications. Clinical characteristics, embryological hypotheses, histological findings, treatment. Eur J Pediatr Surg. 2006;16:18–23. doi: 10.1055/s-2006-923798. [DOI] [PubMed] [Google Scholar]
- 46.Wan XY, Deng T, Luo HS. Partial intestinal obstruction secondary to multiple lipomas within jejunal duplication cyst: A case report. World J Gastroenterol. 2010;16:2190–2. doi: 10.3748/wjg.v16.i17.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guibaud L, Fouque P, Genin G, et al. Case report. CT and ultrasound of gastric and duodenal duplications. J Comput Assist Tomogr. 1996;20:382–5. doi: 10.1097/00004728-199605000-00010. [DOI] [PubMed] [Google Scholar]
- 48.Lad RJ, Fitzgerald P, Jacobson K. An unusual cause of recurrent pancreatitis: Duodenal duplication cyst. Can J Gastroenterol. 2000;14:341–5. doi: 10.1155/2000/152809. [DOI] [PubMed] [Google Scholar]
- 49.Otter MI, Marks CG, Cook MG. An unusual presentation of intestinal duplication with a literature review. Dig Dis Sci. 1996;41:627–9. doi: 10.1007/BF02282353. [DOI] [PubMed] [Google Scholar]
- 50.Johnson JA, 3rd, Poole GV. Ileal duplications in adults. Presentation and treatment. Arch Surg. 1994;129:659–61. doi: 10.1001/archsurg.1994.01420300103018. [DOI] [PubMed] [Google Scholar]
- 51.Lee NK, Kim S, Jeon TY, et al. Complications of congenital and developmental abnormalities of the gastrointestinal tract in adolescents and adults: Evaluation with multimodality imaging. Radiographics. 2010;30:1489–507. doi: 10.1148/rg.306105504. [DOI] [PubMed] [Google Scholar]
- 52.Antaki F, Tringali A, Deprez P, et al. A case series of symptomatic intraluminal duodenal duplication cysts: Presentation, endoscopic therapy, and long-term outcome (with video) Gastrointest Endosc. 2008;67:163–8. doi: 10.1016/j.gie.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 53.Puligandla PS, Nguyen LT, St-Vil D, et al. Gastrointestinal duplications. J Pediatr Surg. 2003;38:740–4. doi: 10.1016/jpsu.2003.50197. [DOI] [PubMed] [Google Scholar]
- 54.Mourra N, Chafai N, Bessoud B, et al. Colorectal duplication in adults: Report of seven cases and review of the literature. J Clin Pathol. 2010;63:1080–3. doi: 10.1136/jcp.2010.083238. [DOI] [PubMed] [Google Scholar]
- 55.Reiser-Erkan C, Erkan M, Ulbrich E, et al. Cystic colon duplication causing intussusception in a 25-year-old man: Report of a case and review of the literature. BMC Surg. 2010;23(10):19. doi: 10.1186/1471-2482-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inoue Y, Nakamura H. Adenocarcinoma arising in colonic duplication cysts with calcification: CT findings of two cases. Abdom Imaging. 1998;23:135–7. doi: 10.1007/s002619900305. [DOI] [PubMed] [Google Scholar]
- 57.Spottswood SE. Peristalsis in duplication cyst: A new diagnostic sonographic finding. Pediatr Radiol. 1994;24:344–5. doi: 10.1007/BF02012124. [DOI] [PubMed] [Google Scholar]
- 58.Holcomb GW, 3rd, Gheissari A, O’Neill JA, Jr, et al. Surgical management of alimentary tract duplications. Ann Surg. 1989;209:167–74. doi: 10.1097/00000658-198902000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]


