Abstract
Background and Objectives:
The National Cancer Institute estimated 40,340 new cases of rectal cancer in the United States in 2013. The correct staging of rectal cancer is fundamental for appropriate treatment of this disease. Transrectal ultrasound is considered one of the best methods for locoregional staging of rectal tumors, both radial echoendoscope and rigid linear probes are used to perform these procedures. The objective of this study is to evaluate the correlation between radial echoendoscopy and rigid linear endosonography for staging rectal cancer.
Patients and Methods:
A prospective analysis of 48 patients who underwent both, radial echoendoscopy and rigid linear endosonography, between April 2009 and May 2011, was done. Patients were staged according to the degree of tumor invasion (T) and lymph node involvement (N), as classified by the American Joint Committee on Cancer. Anatomopathological staging of surgical specimen was the gold standard for discordant evaluations. The analysis of concordance was made using Kappa index.
Results:
The general Kappa index for T staging was 0.827, with general P < 0.001 (confidence interval [CI]: 95% 0.627-1). The general Kappa index for N staging was 0.423, with general P < 0.001 (CI: 95% 0.214-0.632).
Conclusion:
The agreement between methods for T staging was almost perfect, with a worse outcome for T2, but still with substantial agreement. The findings may indicate equivalence in the diagnostic value of both flexible and rigid devices. For lymph node staging, there was moderate agreement between the methods.
Keywords: Endorectal ultrasound, endoscopic ultrasound, rectal cancer, rectal cancer staging, transrectal ultrasound
INTRODUCTION
The National Cancer Institute estimated 40,340 new cases of rectal cancer in the United States in 2013.[1]
Colorectal cancer is one of the most prevalent malignant tumor in our country. According to data from Instituto Nacional do Cβncer, Brazilian National Cancer Institute, about 30,140 new cases of colorectal cancer were estimated in 2012. These values correspond to an estimated risk of 15 new cases per 100,000 men and women, respectively. This neoplasm has a better prognosis if the disease is diagnosed in the early stages. The average overall survival at 5 years is around 55% in developed countries and 40% for developing countries.[2]
Studies have shown that both the rate of recurrence and the tumor-free survival, in patients with locally advanced rectal cancer, showed significant improvement after the institution of preoperative adjuvancy.[3,4,5,6,7,8,9,10] Since neoajduvant therapy relays on correct staging of rectal cancer, this step is fundamental for appropriate treatment of this disease.
Endorectal ultrasound (ERUS) could be accomplished using rigid or flexible devices [Figures 1 and 2]. These transducers scanning may be radial or linear and for anorectal study, are preferably used in frequencies between 5 MHz and 12 MHz. Rigid linear probe may have limited insertion, but they are more practical and less expensive when compared with flexible devices. Therefore, in our institution, the rigid linear devices are being increasingly used for anorectal region study, especially for patients with intestinal endometriosis.[11,12,13,14,15,16,17]
Figure 1.
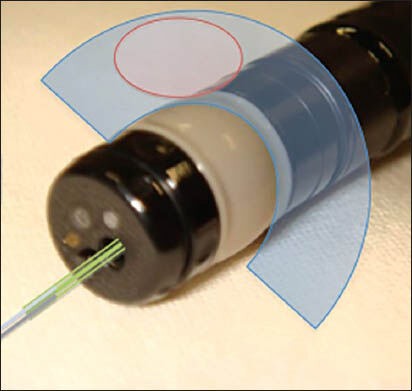
Radial flexible echoendoscope
Figure 2.
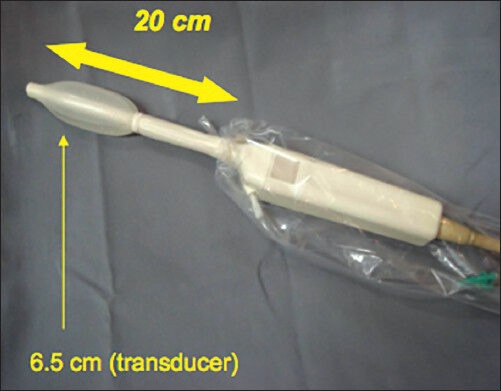
Rigid linear probe
The objective of this study is to evaluate the correlation between endosonography made with both radial echoendoscope and rigid lineal probe, for rectal cancer staging.
PATIENTS AND METHODS
This was a prospective case study, between April 2009 and May 2011, in a series of 91 consecutive patients with rectal cancer, who underwent both, radial echoendoscopy (EUG Radial, Fujinon System 7000) and rigid linear endosonography (EUP-U33, Hitachi. Probe: SU 11774004 7,5 Mhz). The Ethics Committees of the institution approved the study before it was started (study approved in 2009; protocol 293/2009).
All patients assented to participate by completing the informed consent form. We excluded 43 patients from this study; that had already undergone previous therapy such as surgery, radiotherapy, chemotherapy, or for lack of data tracking. Therefore, data from a total of 48 patients were analysed.
All procedures were performed with the assistance of the professional anesthesiologist, under conscious sedation, in hospital setting. The patients were either hospitalized or ambulatorial. The exams were performed by two senior endoscopists, with more than 500 exams each, blinded to each other. When the staging of the tumor was discordant, we used the results of anatomopathological staging (AP) of the surgical specimen as the gold standard.
The patients were initially submitted to the radial endoscopic ultrasound (EUS) and then, during the same anesthetic time, were re-examined by other blinded endoscopists using the rigid linear probe. At each stage of the examination, patients were staged on the degree of tumor invasion (T) and lymph node involvement (N), as classified by the American Joint Committee on Cancer, 7th edition, 2010 (AJCC).
Echographics findings were analysed as follows: T staging obtained using radial EUS were compared with T staging obtained using a rigid probe. Likewise, N staging obtained using radial EUS were compared with N staging obtained using a rigid probe [Figures 3 and 4].
Figure 3.
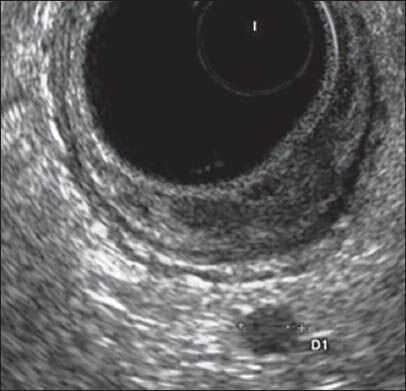
T1Sm3N1 with radial flexible echoendoscope
Figure 4.
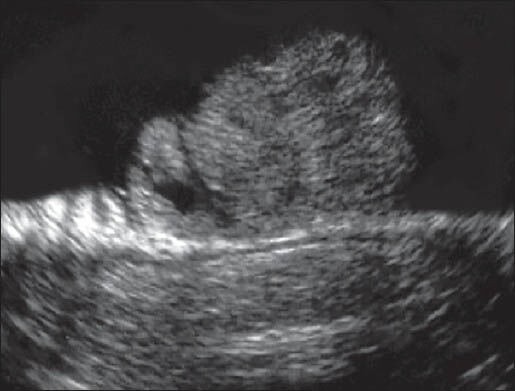
T1Sm3N1 with rigid linear probe
The analysis of concordance between the methods (both general and per classification) was made using the Kappa index.
RESULTS
Analysis of T stage showed the following results [Table 1]:
Table 1.
T staging results
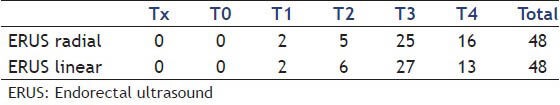
Among the 48 patients evaluated, T staging was similar between the methods in 43 (89.58%). In the five discordant cases, the histopathology study confirmed the results of the rigid probe in one of them; three patients did not undergo surgery and one had an AP distinct from both methods:
One patient was diagnosed as T2 (radial ERUS) and T3 (ERUS linear). Confirmed T3 in histopathology.
One patient was diagnosed as T3 (radial ERUS) and T2 (ERUS linear). Did not undergo surgery.
One patient was diagnosed as T4 (radial ERUS) and T3 (ERUS linear). Confirmed T2 in histopathology.
One patient was diagnosed as T4 (radial ERUS) and T3 (ERUS linear). Did not undergo surgery.
One patient was diagnosed as T4 (radial ERUS) and T2 (ERUS linear). Did not undergo surgery.
Regarding the N staging [Table 2], 32 patients (66.66%) showed agreement between the methods. In the 16 discordant cases, the histopathology study confirmed the findings of a rigid probe in seven of them; in one patient the histopathology study was different from both methods and the remaining eight did not undergo surgery:
Table 2.
N staging results
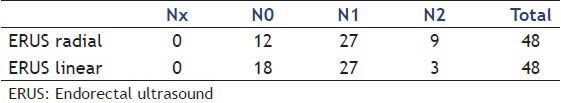
One patient was diagnosed as N0 (radial ERUS) and N1 (ERUS linear). Confirmed N1 in histopathology.
Five patients were diagnosed as N1 (radial ERUS) and N0 (ERUS linear). Two cases confirmed as N0 in histopathology and three cases did not undergo surgery.
Two patients were diagnosed as N1 (radial ERUS) and N2 (ERUS linear). Two cases confirmed N2 in histopathology.
Two patients were diagnosed as N2 (radial ERUS) and N0 (ERUS linear). One case confirmed as N0 in histopathology and one case did not undergo surgery.
Six patients were diagnosed as N2 (radial ERUS) and N1 (ERUS linear). One case confirmed as N0 and one case as N1 in histopathology. Four cases did not undergo surgery.
The general Kappa index for T and N stages are shown in Tables 3 and 4.
Table 3.
General Kappa index for T staging

Table 4.
General Kappa index for N staging

The agreement between the methods for individual T classification are shown in Table 5.
Table 5.
Kappa index per category for T staging

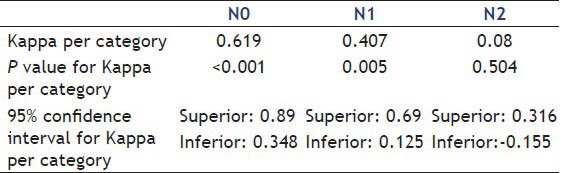
The agreement between the methods for individual N classification are shown in Table 6.
Table 6.
Kappa index per category for N staging
DISCUSSION
The initial clinical evaluation and staging procedures may include the following: Anorectal examination, colonoscopy, computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography, measurement of the serum carcinoembryonic antigen level and ERUS. ERUS is considered one of the best method for locoregional staging (T staging) of rectal tumors.[12]
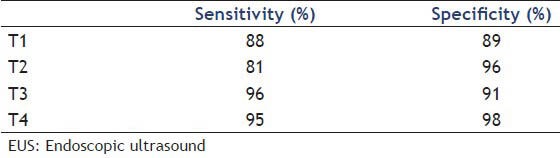
Endoscopic ultrasound presents average accuracy of 85.2%, with average sensitivity and specificity of 87.5% and 83.5%, respectively. Meanwhile, CT showed accuracy of 65-75% and the MRI of 75-85%. A recent meta-analysis estimated the sensibility and specificity of EUS in determining separately the different stages of tumor invasion [Table 7].
Table 7.
EUS sensitivity and specificity in different locorregional staging of rectal tumor
Therefore, the combination of CT (to exclude distant metastases) and ERUS (for local staging) proved to be the best diagnostic approach for staging proximal rectal tumors without metastatic disease.[18,19]
Regarding lymph nodes staging (N staging), there is no evidence of superiority of ERUS over CT or MRI.[19] However, the association of fine-needle aspiration (FNA) to the ERUS procedure is a promising development in N staging of tumors of the gastrointestinal tract.[20,21,22,23,24,25,26,27] Similarly, for rectal tumors, echographic staging of perirectal regional lymph nodes by evaluating the size, shape, contour and echogenicity of nodes is feasible. If there are suspicious lymph nodes, histological evaluation can be achieved with FNA. The incorporation of FNA in staging strategy of patients with a rectal tumor is, therefore, related to improvement of method accuracy.[28]
A well-defined strategy of echoendoscopic staging is the use of radial flexible devices. If suspicious lymph nodes are detected, the linear echoendoscope is used to perform FNA.
Rigid endosonography probes are devices that provide an adequate assessment for local invasion of rectal tumor. Furthermore, they are more financially affordable, have lower maintenance costs than the flexible echoendoscopes and allow FNA [Figure 5]. However, consistent studies to confirm the equivalence of results in rectal tumors local staging using radial echoendoscopes and rigid endosonography probes are still lacking.
Figure 5.

Rigid linear probe with fine needle aspiration needle
According to the literature, when analyzed separately the different stages, it is observed that the main failures of ERUS T staging occur in overstaging T2 as T3 (attributed to peritumoral inflammatory process) and understaging T3 as T2 (related to a nonvisualization of tumor microinvasions in parietal layers or adjacent organ). Overstaging is more frequent than the understaging occurring respectively in about 4% to 25% and 5% to 12%.[29,30,31,32] No single diagnostic method is sufficiently accurate to reliably predict the presence or absence of lymph node involvement. Definitive diagnosis can be confirmed by obtaining histological material by FNA. In rectal cancers, the mere viewing of perirectal lymph nodes is indicative of possible neoplastic involvement, since normal perirectal fat tissue lymph nodes are usually not identified.[33]
As stated previously, patients with T3 or T4 stages, or who have compromised lymph nodes (N1), the benefits of preoperative chemotherapy were demonstrated.[7,34] In this context, it is expected that the use of FNA may help significantly the N staging in T1 or T2 tumors, since the presence of lymph node modifies the therapeutic approach.
In this study, the results show that for tumor invasion (T staging), we obtained a Kappa general index which indicates almost perfect agreement between the methods.[35] Individually, T1 staging had the highest concordance index between the methods of ultrasound evaluation, however, all degrees of T staging were substantially concordant.[33]
Analyzing the degree of lymph node involvement (N staging), the Kappa general index shows moderate agreement between the methods, but lower than observed for T staging. Individually, the agreement is higher in N0 staging.
Therefore, we can conclude that the findings indicate good agreement between the methods studied, especially when evaluating T staging.
As limitations of this study, we could state the fact that it is a single-centered, study and also the lack of follow-up and pathological results of all patients. We recommend future prospective studies that add to the evaluation of both methods data from MRI, distance between the anal verge and rectal lesion, degrees of anal sphincter and adjacent tissues impairment.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.American Cancer Society. Atlanta, Ga: American Cancer Society; 2013. [Last accessed on 2013 May 02]. Cancer Facts and Figures 2013. Available online. [Google Scholar]
- 2.Ministério da Saúde-INCA-Instituto Nacional do Câncer. [Last accessed on 2013 Jun 27]. Available from: http://www.inca.gov.br .
- 3.Påhlman L, Glimelius B. Pre-or postoperative radiotherapy in rectal and rectosigmoid carcinoma. Report from a randomized multicenter trial. Ann Surg. 1990;211:187–95. doi: 10.1097/00000658-199002000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frykholm GJ, Glimelius B, Påhlman L, et al. Preoperative or postoperative irradiation in adenocarcinoma of the rectum: Final treatment results of a randomized trial and an evaluation of late secondary effects. Dis Colon Rectum. 1993;36:564–72. doi: 10.1007/BF02049863. [DOI] [PubMed] [Google Scholar]
- 5.Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish rectal cancer trial. N Engl J Med. 1997;336:980–7. doi: 10.1056/NEJM199704033361402. [DOI] [PubMed] [Google Scholar]
- 6.Randomised trial of surgery alone versus radiotherapy followed by surgery for potentially operable locally advanced rectal cancer. Medical research council rectal cancer working party. Lancet. 1996;348:1605–10. [PubMed] [Google Scholar]
- 7.Minsky BD. Adjuvant therapy for rectal cancer — A good first step. N Engl J Med. 1997;336:1016–7. doi: 10.1056/NEJM199704033361410. [DOI] [PubMed] [Google Scholar]
- 8.Grann A, Feng C, Wong D, et al. Preoperative combined modality therapy for clinically resectable uT3 rectal adenocarcinoma. Int J Radiat Oncol Biol Phys. 2001;49:987–95. doi: 10.1016/s0360-3016(00)01529-7. [DOI] [PubMed] [Google Scholar]
- 9.Hyams DM, Mamounas EP, Petrelli N, et al. A clinical trial to evaluate the worth of preoperative multimodality therapy in patients with operable carcinoma of the rectum: A progress report of National Surgical Breast and Bowel Project Protocol R-03. Dis Colon Rectum. 1997;40:131–9. doi: 10.1007/BF02054976. [DOI] [PubMed] [Google Scholar]
- 10.Hawes R, Fockens P. 2nd ed. Piladelphia: Saunders Elsevier; 2011. Endosonography; p. 297. [Google Scholar]
- 11.Ribeiro HS, Ribeiro PA, Rossini L, et al. Double-contrast barium enema and transrectal endoscopic ultrasonography in the diagnosis of intestinal deeply infiltrating endometriosis. J Minim Invasive Gynecol. 2008;15:315–20. doi: 10.1016/j.jmig.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Averbach M, Rossini L. Endometriose intestinal. In: Averbach M, Correa P, editors. Colonoscopia. São Paulo: Santos; 2010. pp. 299–309. [Google Scholar]
- 13.Ribeiro PA, Rossini L, Almeida-Prado RA, et al. The echo-logic for deep pelvic endometriosis. J Am Assoc Gynecol Laparosc. 2002;9:S74. [Google Scholar]
- 14.Rossini LG, Assef MS, Schneider NC, et al. EUS/Trus-FNA for preoperative histological diagnosis of deep intestinal endometriosis. Gastrointest Endosc. 2011;73:AB170–1. [Google Scholar]
- 15.Rossini LGB. Sensibility and technical application of fine needle aspiration (FNA) guided by intestinal endosonography for histological diagnosis of deep endometriosis located in the rectum and distal sigmoid. Thesis. 2010 [Google Scholar]
- 16.Rossini LG, Ribeiro PA, Rodrigues FC, et al. Transrectal ultrasound — Techniques and outcomes in the management of intestinal endometriosis. Endosc Ultrasound. 2012;1:23–35. doi: 10.7178/eus.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwok H, Bissett IP, Hill GL. Preoperative staging of rectal cancer. Int J Colorectal Dis. 2000;15:9–20. doi: 10.1007/s003840050002. [DOI] [PubMed] [Google Scholar]
- 18.Harewood GC, Wiersema MJ. Cost-effectiveness of endoscopic ultrasonography in the evaluation of proximal rectal cancer. Am J Gastroenterol. 2002;97:874–82. doi: 10.1111/j.1572-0241.2002.05603.x. [DOI] [PubMed] [Google Scholar]
- 19.Puli SR, Reddy JB, Bechtold ML, et al. Accuracy of endoscopic ultrasound to diagnose nodal invasion by rectal cancers: A meta-analysis and systematic review. Ann Surg Oncol. 2009;16:1255–65. doi: 10.1245/s10434-009-0337-4. [DOI] [PubMed] [Google Scholar]
- 20.Wiersema MJ, Kochman ML, Cramer HM, et al. Endosonography-guided real-time fine-needle aspiration biopsy. Gastrointest Endosc. 1994;40:700–7. [PubMed] [Google Scholar]
- 21.Rodriguez J, Kasberg C, Nipper M, et al. CT-guided needle biopsy of the pancreas: A retrospective analysis of diagnostic accuracy. Am J Gastroenterol. 1992;87:1610–3. [PubMed] [Google Scholar]
- 22.Gress FG, Savides TJ, Sandler A, et al. Endoscopic ultrasonography, fine-needle aspiration biopsy guided by endoscopic ultrasonography, and computed tomography in the preoperative staging of non-small-cell lung cancer: A comparison study. Ann Intern Med. 1997;127:604–12. doi: 10.7326/0003-4819-127-8_part_1-199710150-00004. [DOI] [PubMed] [Google Scholar]
- 23.Wiersema MJ, Vilmann P, Giovannini M, et al. Endosonography-guided fine-needle aspiration biopsy: Diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087–95. doi: 10.1016/s0016-5085(97)70164-1. [DOI] [PubMed] [Google Scholar]
- 24.Giovannini M, Seitz JF, Monges G, et al. Fine-needle aspiration cytology guided by endoscopic ultrasonography: Results in 141 patients. Endoscopy. 1995;27:171–7. doi: 10.1055/s-2007-1005657. [DOI] [PubMed] [Google Scholar]
- 25.Williams DB, Sahai AV, Aabakken L, et al. Endoscopic ultrasound guided fine needle aspiration biopsy: A large single centre experience. Gut. 1999;44:720–6. doi: 10.1136/gut.44.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Catalano MF, Alcocer E, Chak A, et al. Evaluation of metastatic celiac axis lymph nodes in patients with esophageal carcinoma: A ccuracy of EUS. Gastrointest Endosc. 1999;50:352–6. doi: 10.1053/ge.1999.v50.98154. [DOI] [PubMed] [Google Scholar]
- 27.Giovannini M, Monges G, Seitz JF, et al. Distant lymph node metastases in esophageal cancer: Impact of endoscopic ultrasound-guided biopsy. Endoscopy. 1999;31:536–40. doi: 10.1055/s-1999-60. [DOI] [PubMed] [Google Scholar]
- 28.Harewood GC. Assessment of clinical impact of endoscopic ultrasound on rectal cancer. Am J Gastroenterol. 2004;99:623–7. doi: 10.1111/j.1572-0241.2004.04116.x. [DOI] [PubMed] [Google Scholar]
- 29.Hulsmans FJ, Tio TL, Fockens P, et al. Assessment of tumor infiltration depth in rectal cancer with transrectal sonography: Caution is necessary. Radiology. 1994;190:715–20. doi: 10.1148/radiology.190.3.8115617. [DOI] [PubMed] [Google Scholar]
- 30.Kim JC, Yu CS, Jung HY, et al. Source of errors in the evaluation of early rectal cancer by endoluminal ultrasonography. Dis Colon Rectum. 2001;44:1302–9. doi: 10.1007/BF02234788. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Aguilar J, Pollack J, Lee SH, et al. Accuracy of endorectal ultrasonography in preoperative staging of rectal tumors. Dis Colon Rectum. 2002;45:10–5. doi: 10.1007/s10350-004-6106-3. [DOI] [PubMed] [Google Scholar]
- 32.Massari M, De Simone M, Cioffi U, et al. Value and limits of endorectal ultrasonography for preoperative staging of rectal carcinoma. Surg Laparosc Endosc. 1998;8:438–44. [PubMed] [Google Scholar]
- 33.Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc. 1997;45:474–9. doi: 10.1016/s0016-5107(97)70176-7. [DOI] [PubMed] [Google Scholar]
- 34.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 35.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]


