Abstract
Background:
Endoscopic ultrasonography (EUS) is a newly imagine procedure for assessment and therapeutic in option. The aims of this study are comparison two techniques about EUS-fine-needle aspiration (EUS-FNA), including successful tissue sampling, complication, procedure time, and safety.
Materials and Methods:
A total of 100 patients with pancreatic solid masses were in the study, 50 patients underwent EUS-FNA with negative pressure as Group 1 and 50 patients underwent EUS-FNA without negative pressure and stylet as Group 2 over a 36 months period.
Results:
The study period was from March 2011 to January 2014. In total case, the male-to-female ratio was 1.27 with a mean age of 61.7 ± 1.3 years. The involvement of different regions of the pancreas, pancreatic head had the most frequent (69%) after that uncinate (12%), body (11%) and tail (8%). In 100 pancreatic EUS-FNA samples, 48% were interpreted as malignant on pathology evaluation, 15% as suspicious for malignancy, 27% as benign processes and 10% inadequate specimen. There were no significant differences between the adequacy of sample cells in two techniques (P < 0.148).
Conclusion:
The EUS-FNA without negative pressure and stylet technique was related with less contamination by blood and raise the diagnostic yield. We recommend further studies for better evaluation of our study with higher the cases because clinically the low the inadequate samples (6% vs. 14%) and less contamination with blood (20% vs. 50%) in the second group (P < 0.002).
Keywords: Endoscopic ultrasonography-fine needle aspiration, solid pancreatic lesions, suction method, without suction and stylet
INTRODUCTION
Endoscopic ultrasonography (EUS) has developed a diagnostic imaging procedure to one that can also be used for invasive diagnostic and therapeutic procedures.[1,2] Since this device is a part of the linear scan tool and allows different interventions to physician has widely welcomed. EUS-fine needle aspiration (EUS-FNA) is capable takes samples from masses that are not saw by computed tomography (CT) or magnetic resonance imaging and lesions too well encased by adjacent vascular structures to allow percutaneous biopsy.[3,4,5]
Solid pancreatic masses (exocrine and endocrine) classify as benign and malignant neoplasm. Diagnostic biopsy of a pancreatic malignancy is performed for the treatment of systemic spread of disease, local evidence of unrepeatability, or if neoadjuvant treatment is being contemplated. Positive biopsy can confirm the diagnosis, but a benign sample does not exclude the malignancy.[6,7,8] EUS-guided FNA is the best modality for taking a tissue diagnosis, even if the mass is weakly saw by other imaging modalities.[9] Intraperitoneal spread of the tumor occurs less with this procedure.[10] It assumes that suctioning dilutes the specimen by blood, and stylet injures malignant cells and raises suspicions or atypical results.
Thus in the current study, we prospectively evaluated the cellular yield of the technique EUS-FNA without suctioning and stylet at time of insertioning needle inside the biopsy channel of echoendoscope in patients with pancreatic masses.
MATERIALS AND METHODS
This was a study conducted over 36 months (March 2011 to January 2014) at a Tertiary Care Center in Tehran, Iran. We prospectively evaluated two techniques EUS-FNA in 100 patients with solid pancreatic masses. Samples were selected randomized that these two methods used as an alternate.
Exclusion criteria were cystic masses, coagulopathy (international normalized ratio >1.5), thrombocytopenia (platelet count >50,000) advanced cardiovascular disease, stenosis or obstruction of esophagus, stomach or duodenum. This study conducted to observe all ethical and allow all patients.
A total of 100 patients with pancreatic masses were in the study, 50 patients underwent EUS-FNA with negative pressure was applied with a 10 mL syringe (Group 1) and 50 patients underwent EUS-FNA, the negative pressure was not applied and stylet was withdrawing before insertion. Indeed EUS-FNA was done without negative pressure and stylet (Group 2). When we saw the sheet of needle in contact with the mucosa of stomach or duodenum overlying the pancreatic mass. We were withdrawing the stylet 2-3 mm out of the needle and were inserting the needle inside the mass suddenly. Furthermore, we were pushed the stylet inside needle and were removed the stylet and begun the procedure.
To perform the procedure, the patient lied to the left lateral decubitus position and by using intravenous propofol for deep sedation. EUS for guided puncture of the lesion, carried by using Olympus equipment (UC 24OP-AL5) and Aloka Prosound color Doppler. Also, all samples carried out by a specialist. All FNA performed with a 22-gauge needle (Echotip; Wilson-Cook, Winston Salem, NC). A median of four needle passes performed. After the aspiration needle withdrawn from the endoscope, the endoscopist immediately washed the aspiration needle in 70% ethanol inside an appropriately labeled screw capped sterile plastic test tube. After transferring to laboratory, the tubes centrifuged lightly to concentrate the content at the bottom of the tube and processed for cytology evaluation. Then smears and sections of the cell block evaluated by an expert pathologist for determining the adequacy of specimen (presence of pancreatic cells) and other cytology findings such as the cellularity, necrosis, evidence of fibrosis and inflammation.
The cytology results classified as negative for malignancy, suspicious for malignancy, positive for malignancy, and inadequate cells for histological examination. Gold standard of diagnosis.
Statistical analysis
Patients were first analyzed as one group, then as two separate groups. Continuous variables reported as means and standard deviations (SD) or as median based on their distribution. The Pearson Chi-square used for comparison of two groups. Statistical analyses performed using SPSS 17 software (SPSS, Inc., Chicago, IL).
RESULTS
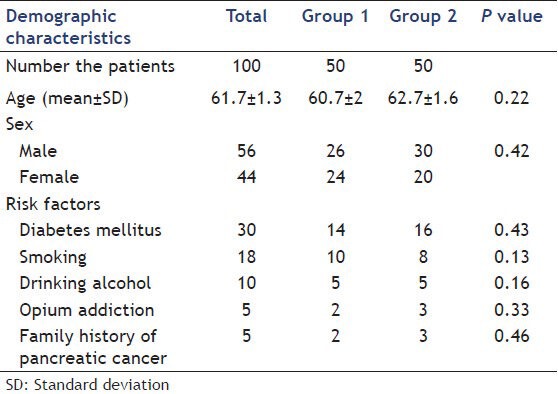
One hundred patients with a mean age (±SD) of 61.7 ± 1.3 years, including 56 male and 44 female, studied. 18 (18%) patients had a history of smoking, 10% taking alcohol, 5% opium addiction, 30% diabetes mellitus and 5% family history of pancreatic cancer. Table 1 presents demographic characteristics and risk factors of each group.
Table 1.
Demographic characteristics and risk factors of study groups
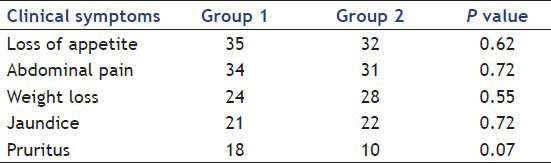
Most of the patients 67% presented with a complaint of loss of appetite, 52% had Weight loss, nausea in 17%, 43% presented with jaundice, 28% with pruritus, 65% presented with abdominal pain and 21% had anemia. Patients’ clinical symptoms of Groups 1 and 2 are summarized in Table 2.
Table 2.
Clinical symptoms of study groups
Totally, 69% of the lesions were in the head of the pancreas, 12% were in the uncinate, 11% were in the body, and 8% were in the tail. The locations of the mass in the two groups are shown in Table 3.
Table 3.
Location mass in study groups
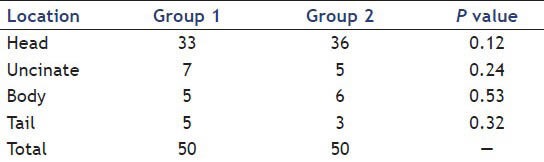
Table 4 presents the time of the procedure, inadequate sampling and contamination of samples with blood. During sampling, two patient of the first group and three persons of the second group were hypotension, after 10 min comeback to normal that seem to be caused by the side effects of anesthesia drugs.
Table 4.
Time of procedure, inadequate sampling and contamination of samples with blood
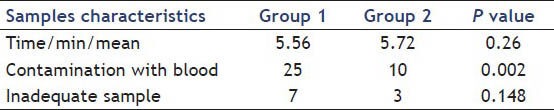
There were no significant differences between in cellularity of the two techniques (P < 0.148).
In 100 pancreatic EUS-FNA samples, 48% were interpreted as malignant on pathology evaluation, 15% as suspicious for malignancy, 27% as benign processes and 10% inadequate cells (7 samples in Groups 1 and 3 samples in Group 2). The cytology results in the two groups are shown in Table 5.
Table 5.
Cytology results in two groups
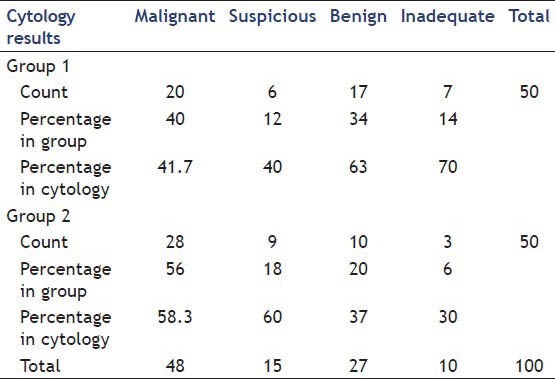
Of the 48 malignant lesions, 41 were adenocarcinoma, 5 were neuroendocrine tumors and 2 was lymphoma. CT guided biopsy confirmed that 12 suspicious and 3 benign smears were adenocarcinoma. Of the 20 benign smears was later found to have autoimmune pancreatitis on clinical follow-ups.
The median follow-up was 261 ± 71 days (120-360 days). The mean tumor size was 3.1 cm (range, 1.7-7.5 cm). Patients with malignant masses were older than benign masses (64 ± 10.3 compared with 51 ± 15, P < 0.005). The frequency of nondiagnostic results was significantly more in masses smaller than 3.5 cm (9 vs. 3, P < 0.05). Most of the nondiagnostic results were in the head of the pancreas (9 vs. 3, P < 0.05).
DISCUSSION
Endoscopic ultrasonography is progressively more uses in the diagnosis and staging of pancreatic carcinoma.[11] The indications for EUS-FNA in patients with pancreatic cancer are controversial. It is agreed that histological and cytological confirmation helps for planning chemotherapy or radiotherapy in patients who have distant metastases, are poorly surgical candidates, or have advanced locoregional disease. EUS-FNA is technically successful in 90-95% of procedures, with a sensitivity of 80-95% and a specificity of 100% for diagnosing pancreatic cancer.[12,13] The accuracy is lower in the chronic pancreatitis (74 compared with 91% in one report),[14,15] and in patients with obstructive jaundice.[16]
The benefits of EUS-guided FNA are illustrated by a study of 559 samples undergoing EUS-FNA for assessment of pancreatic masses.[16] In that study, when using strict cytologic criteria, the sensitivity for EUS-FNA diagnosing pancreatic carcinoma was 77%, with a specificity of 99%. When patients with atypical or suspicious cytology were reclassified as positive for malignancy, the sensitivity raised to 93%, without a change in the specificity.
The accuracy of EUS-FNA can improve with more FNA passes and onsite histologic interpretation.[17]
In one report a cytopathologist in attendance for all aspiration procedures accuracy was raised (95%) in diagnosing pancreatic cancer.[18] The absence of a cytopathologist required an average of at least five to six passes from the pancreatic mass to make sure enough cellularity. In another study, there was a high yield from only two FNA passes when the samples were looked at by histology and cytology.[19] Other approaches such as rising needle size or needle passing may harbors higher complications.
In another study was publishing in 2014 by Nakai et al., the slow-pull technique result in lower scores for cellularity (≥2 for 37.5% vs. 76.7%) but scores for contamination with blood were lower (≥2 for 25.0% vs. 66.7%) and sensitivity of diagnosis of malignancy were higher (90.0% vs. 67.9%) when a 25-gauge FNA needle were used. There were no significant differences between the two techniques when a 22-gauge needle was used. In multivariate analysis of 82 cases with malignancy, the slow-pull technique (odds ratio (OR): 1.92, P = 0.028), tumor size ≥25 mm (OR: 4.64, P < 0.001), and tumor location in the body or tail (OR: 2.82, P < 0.001) were associated with greater sensitivity.[20]
In all above studies, the EUS-FNA technique was accompanied by suctioning and stylet in place to gain most specimens. Suctioning, by diluting the specimen with blood (especially at the end of suctioning) and stylet traumatizes cells at the insertion cause diagnostic problems for cytologist and lowers the diagnostic yield.
In the current study, we reviewed our experience without suctioning and use of the stylet in EUS-FNA in patients with solid pancreatic masses. Although statistically significant differences were not found between the two methods (P < 0.148), but clinically the low the inadequate samples in the second group (6% vs. 14%) and less contamination with blood (20% vs. 50%) in the second group(P < 0.002). we recommend the second method. With this assumption, we performed this procedure without suctioning and stylet and found that false negative results were decreased. Larger studies are needed to confirm these benefits.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Artifon EL, Giovannini M, Sun S, et al. Therapeutic endoscopic ultrasonography. Gastroenterol Res Pract 2013. 2013:390821. doi: 10.1155/2013/390821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azad JS, Verma D, Kapadia AS, et al. Can U.S. GI fellowship programs meet American Society for Gastrointestinal Endoscopy recommendations for training in EUS? A survey of U.S. GI fellowship program directors. Gastrointest Endosc. 2006;64:235–41. doi: 10.1016/j.gie.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 3.Fritscher-Ravens A, Topalidis T, Bobrowski C, et al. Endoscopic ultrasound-guided fine-needle aspiration in focal pancreatic lesions: A prospective intraindividual comparison of two needle assemblies. Endoscopy. 2001;33:484–90. doi: 10.1055/s-2001-14970. [DOI] [PubMed] [Google Scholar]
- 4.Gress F, Gottlieb K, Sherman S, et al. Endoscopic ultrasonography-guided fine-needle aspiration biopsy of suspected pancreatic cancer. Ann Intern Med. 2001;134:459–64. doi: 10.7326/0003-4819-134-6-200103200-00010. [DOI] [PubMed] [Google Scholar]
- 5.Harewood GC, Wiersema MJ. Endosonography-guided fine needle aspiration biopsy in the evaluation of pancreatic masses. Am J Gastroenterol. 2002;97:1386–91. doi: 10.1111/j.1572-0241.2002.05777.x. [DOI] [PubMed] [Google Scholar]
- 6.Erickson RA, Garza AA. Impact of endoscopic ultrasound on the management and outcome of pancreatic carcinoma. Am J Gastroenterol. 2000;95:2248–54. doi: 10.1111/j.1572-0241.2000.02310.x. [DOI] [PubMed] [Google Scholar]
- 7.Suits J, Frazee R, Erickson RA. Endoscopic ultrasound and fine needle aspiration for the evaluation of pancreatic masses. Arch Surg. 1999;134:639–42. doi: 10.1001/archsurg.134.6.639. [DOI] [PubMed] [Google Scholar]
- 8.Cahn M, Chang K, Nguyen P, et al. Impact of endoscopic ultrasound with fine-needle aspiration on the surgical management of pancreatic cancer. Am J Surg. 1996;172:470–2. doi: 10.1016/S0002-9610(96)00222-X. [DOI] [PubMed] [Google Scholar]
- 9.Tummala P, Junaidi O, Agarwal B. Imaging of pancreatic cancer: An overview. J Gastrointest Oncol. 2011;2:168–74. doi: 10.3978/j.issn.2078-6891.2011.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pancreas Cancer. Clinical manifestations, diagnosis, and surgical staging of exocrine pancreatic cancer. [Last accessed on 2014 Feb 5]. http://www.aboutcancer.com/pacreas_utd_807.htm .
- 11.Eloubeidi MA, Jhala D, Chhieng DC, et al. Yield of endoscopic ultrasound-guided fine-needle aspiration biopsy in patients with suspected pancreatic carcinoma. Cancer. 2003;99:285–92. doi: 10.1002/cncr.11643. [DOI] [PubMed] [Google Scholar]
- 12.Săftoiu A, Vilmann P. Role of endoscopic ultrasound in the diagnosis and staging of pancreatic cancer. J Clin Ultrasound. 2009;37:1–17. doi: 10.1002/jcu.20534. [DOI] [PubMed] [Google Scholar]
- 13.Siddiqui AA, Brown LJ, Hong SK, et al. Relationship of pancreatic mass size and diagnostic yield of endoscopic ultrasound-guided fine needle aspiration. Dig Dis Sci. 2011;56:3370–5. doi: 10.1007/s10620-011-1782-z. [DOI] [PubMed] [Google Scholar]
- 14.Krishna NB, Mehra M, Reddy AV, et al. EUS/EUS-FNA for suspected pancreatic cancer: Influence of chronic pancreatitis and clinical presentation with or without obstructive jaundice on performance characteristics. Gastrointest Endosc. 2009;70:70–9. doi: 10.1016/j.gie.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 15.Varadarajulu S, Tamhane A, Eloubeidi MA. Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc. 2005;62:728–36. doi: 10.1016/j.gie.2005.06.051. [DOI] [PubMed] [Google Scholar]
- 16.Turner BG, Cizginer S, Agarwal D, et al. Diagnosis of pancreatic neoplasia with EUS and FNA: A report of accuracy. Gastrointest Endosc. 2010;71:91–8. doi: 10.1016/j.gie.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 17.Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000;51:184–90. doi: 10.1016/s0016-5107(00)70416-0. [DOI] [PubMed] [Google Scholar]
- 18.Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, et al. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol. 2011;106:1705–10. doi: 10.1038/ajg.2011.119. [DOI] [PubMed] [Google Scholar]
- 19.Möller K, Papanikolaou IS, Toermer T, et al. EUS-guided FNA of solid pancreatic masses: High yield of 2 passes with combined histologic-cytologic analysis. Gastrointest Endosc. 2009;70:60–9. doi: 10.1016/j.gie.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Nakai Y, Isayama H, Chang KJ, et al. Slow pull versus suction in endoscopic ultrasound-guided fine-needle aspiration of pancreatic solid masses. Dig Dis Sci. 2014;59:1578–85. doi: 10.1007/s10620-013-3019-9. [DOI] [PubMed] [Google Scholar]


