Abstract
Nodal staging is of crucial importance in the management of gastric cancer (GC). The available modalities of nodal imaging in GC do not provide a high sensitivity and specificity of lymph node status. Comparative study of endoscopic ultrasonography (EUS) and multislice spiral computed tomography in GC has shown greater accuracy of EUS for N staging. EUS is not used routinely in patients with GC as it is not available at all centers, and its accuracy is operator dependent. Standard techniques of identification of nodal station (as suggested by Japanese Research Society for the Study of Gastric Cancer) by EUS have not been described so far. Identification of each nodal station by EUS requires adequate knowledge of anatomy as well as understanding the proper technique to perform EUS. This review presents a method to identify the regional nodal stations of GC by linear EUS and hence will help in appropriate N staging of GC.
Keywords: Endoscopic ultrasound, gastric cancer, lymph node, multidetector spiral computed tomography
INTRODUCTION
Gastric carcinoma is the second leading cause of cancer deaths worldwide, however, its incidence varies greatly between Eastern and Western countries.[1] The most important prognostic factors in gastric cancer (GC) include depth of invasion, lymph node (LN) involvement, and distant metastases.[2,3] Preoperative knowledge of LN status is helpful for clinical staging and for planning the optimal treatment. The Japanese Research Society for the Study of Gastric Cancer (JRSGC) has described 16 nodal stations, which surround the stomach, and depending upon the location of the primary tumor; they are grouped into N1, N2, and N3 groups [Figures 1a and b].[4] In general station N1 LNs are perigastric in location, N2 LNs lie around celiac artery (CAL) and its branches and N3 LNs are found in the ligaments surrounding stomach and in retroperitoneum.[5] Most investigators have found, high sensitivity (60-90%) and relatively low specificity of multidetector spiral computed tomography (MDCT) for nodal staging.[6,7] The accuracy of magnetic resonance imaging is considered to be inferior to MDCT for N staging.[8] Staging accuracy, and decision-making, is improved when positron emission tomography (PET) and CT are both utilized rather than either alone.[9] Currently, endoscopic ultrasonography (EUS) is accepted as the most efficient diagnostic method for T staging and has been found efficient for N staging also.[10,11,12] Comparative study of EUS and multislice spiral CT in GC has shown greater accuracy of EUS for N staging.[13] The most recent guidelines of the National Comprehensive Cancer Network introduced EUS as a preferred modality of GC staging if no evidence of M1 disease is present at CT-PET.[14] EUS guided fine-needle aspiration (FNA) cytology of LN stations defined by international association of study of lung cancer has made a significant impact in the management of lung cancer.[15,16,17,18] The use of EUS in the preoperative determination of LN status in patients with GC can have a significant impact on patient management.[19] EUS elastography significantly improves the specificity of LN staging in esophageal cancer and can be useful in GC also.[12,20] A technique for identification of individual LN stations suggested by JRSGC has not been described so far by EUS. This article presents a technique to identify the regional nodal stations of GC by EUS.
Figure 1a.
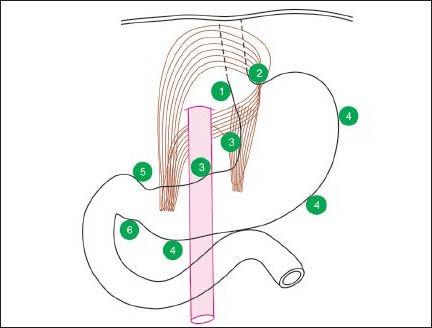
This figure shows lymph nodes near right paracardial area, station 1; lesser curvature, station 3; supra pyloric area, station 5; left paracardial area, station 2; greater curvature, station 4; and infrapyloric area station 6
Figure 1b.
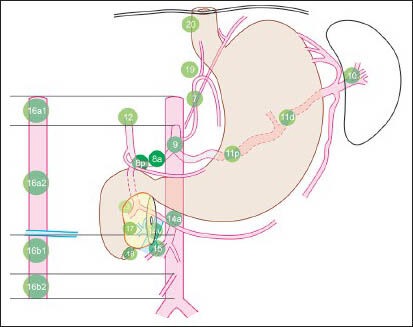
This figure shows N2 stations near; left gastric artery (station 7), common hepatic artery (station 8), celiac artery (station 9) and splenic artery (stations 10 and 11). Lymph nodes (LNs) of N3 stations are seen near hepatoduodenal ligament (station 12), the posterior aspect of the pancreas (station 13), the root of the mesentery (station 14), the transverse mesocolon (station 15) and para-aortic area (station 16). The para-aortic LN at station 16 are seen above (16a) and below (16b) the lower border of left renal vein where it crosses the aorta. Additional stations (17-20) include lower thoracic, paraesophageal and diaphragmatic nodes
PROCEDURE
Two scanning methods are used for EUS examination of the stomach and duodenum: The water-filling method (by introducing 300-500 ml of 0.9% isotonic saline solution into the stomach) and the balloon contact method. A systematic examination of retroperitoneal organs and the vessels is required. Retroperitoneal organs (kidney, spleen, pancreas and adrenal gland) are identified by their characteristic appearance. The examination of vessels of portal venous system, aorta and its branches and the inferior vena cava and its tributaries is done by conventional techniques.[21,22] During imaging of LN stations additional efforts are made to follow the course of vessels as far as possible with the help of color Doppler. This helps in tracing the course of vessels in the ligaments surrounding the stomach and duodenum where the N2 and N3 LNs are located. On EUS, the ligaments of stomach can be identified as hyperechoic areas between two adjacent organs on the lesser curvature (hepatogastric and hepatoduodenal ligament-lesser omentum)[23] and greater curvature (gastrophrenic, gastrosplenic, lienorenal and gastrocolic ligament-greater omentum). The LNs are identified by their characteristic appearance. The scanning is done in a systematic way to identify each LN station [Figures 1a and b, Video 1 and table].[4] The imaging of peri-gastric LNs (N1 stations 1-6) is done from esophagogastric junction and fundus for stations 1 and 2, from body of the stomach for stations 3 and 4 and from the antrum and the first part of the duodenum for stations 5 and 6 [Figures 2–11]. Following the course of blood vessels helps in imaging of N2 group LNs: Left gastric artery [station 7, Figure 12], common hepatic artery [station 8, Figures 13–15], CAL [station 9, Figures 16 and 17] and splenic artery [stations 10 and 11, Figures 18–20]. Imaging of N3 groups of LNs can be done from several positions. Screening of hepatoduodenal ligament is done from 1st part of the duodenum for station 12 [Figures 21–24]. The posterior aspect of the pancreas is scanned from stomach and duodenum for station 13 [Figures 25 and 26]. The root of the mesentery is seen from stomach and from descending duodenum for station 14 [Figures 27 and 28]. The transverse mesocolon and inferior aspect of PAN is seen from a third part of the duodenum after identifying the course of superior mesenteric artery for station 15 [Figures 29 and 30]. Para-aortic nodes of station 16 are visualized from stomach as well as from duodenum [Figures 31–37]. Imaging of station 17 is done from the posterior wall of the stomach for LN lying anterior to the PAN [Figure 38]. Imaging of station 18 is done from stomach and the horizontal part of the duodenum [Figure 39]. Imaging of station 19 is done from esophagogastic junction [Figure 40]. The LN of station 20 is located near the aortic hiatus [Figure 41]. Additional imaging of LN of lower thoracic, paraesophageal and diaphragmatic area is done from stomach and esophagogastric junction [Figures 42a–d]. In this case the linear EUS scope model – 3870 UTK Pentax, Tokyo, Japan and color Doppler Machine: Hitachi EUB-7500; Hitachi Medical Systems, Tokyo, Japan was used for evaluation.
Figure 2.
The gastroesophageal (GE) junction lies about 3-4 cm below the diaphragm. The GE junction demarcates the area between the paracardial stations (stations 1 and 2) and the lesser and greater curvature stations (stations 3 and 4). The lymph node (LN) of station 1 lies in the right paracardial area, and the LN of station 2 lie in left paracardial area. The LN in this figure belongs to station 1 and lies between the wall of the stomach and the left lobe of the liver and lies along esophageal branch of left gastric artery. An LN belonging to station 7 is also seen. LGA: Left gastric artery, SA: Splenic artery
Figure 11.
Imaging from the duodenal bulb can also show the portal vein and the bifurcation of common hepatic artery into the hepatic artery (HA) proper and gastroduodenal artery. The HA tends to run in a cranial direction toward the hilum of the liver and the gastroduodenal artery tends to go down behind the duodenum. In this image twolymph nodes (LNs) are seen and station 5 LN lies between duodenum and liver while station 6 LN lies between duodenum and PAN. A LN of station 12p is also seen. PAN: Pancreas, CHA: Common hepatic artery
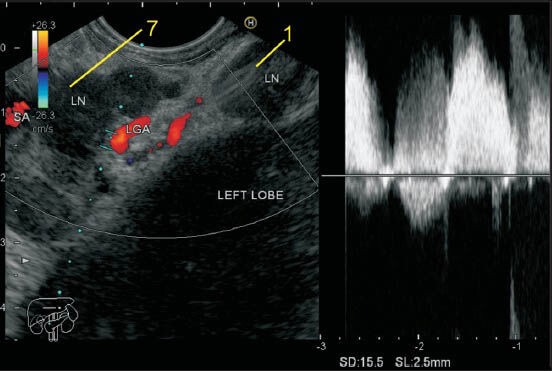
Figure 12.
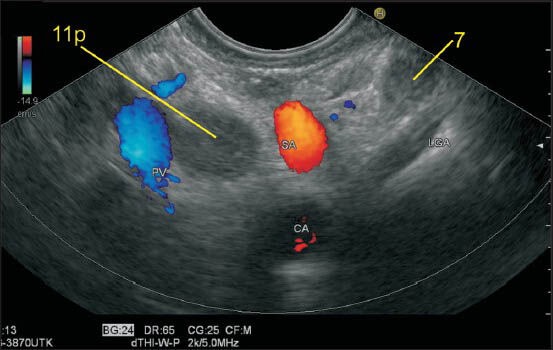
The lymph node (LN) labeled as station 7 lies a little away from the wall of stomach and close to the trunk of left gastric artery (LGA). The origin of LGA from the celiac artery is seen. The other LN belongs to station 11p. Sometimes it is possible to follow the branches of LGA to the level of the origin of esophageal branches
Figure 13.
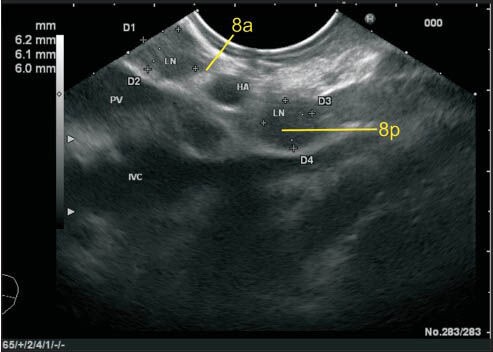
The lymph node (LN) labeled as station 8a lies close to the common hepatic artery. One LN lies anterior to hepatic artery (HA), and one LN (8p) lies anterior to inferior vena cava posterior to HA. The LN labeled as 8a can be also included in station 3b and such cases the proximity to the vessel or to the wall of the stomach will decide the station. In this case, the station 8a LN is equidistant from HA and from wall of the stomach. HA: Hepatic artery
Figure 15.
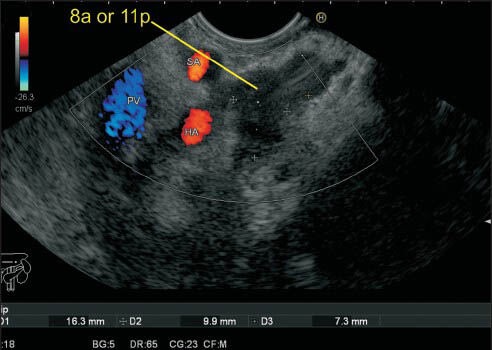
This lymph node (LN) cluster is seen anterior to hepatic artery (HA) as well as near the proximal part of the splenic artery (SA). One of the LNs is touching the SA and can be labeled as either 8a or 11p. At this point, the distance between HA and SA is only about 5 mm, and it is not possible to differentiate between 8a and 11p
Figure 16.
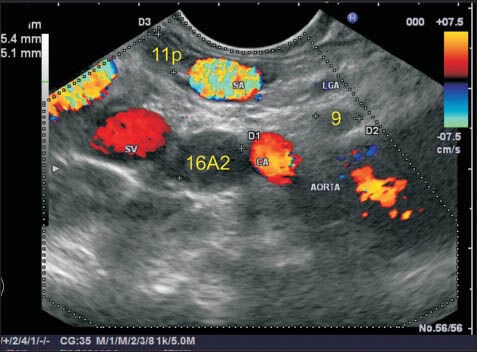
The lymph node (LN) labeled as station 9 lies just above the celiac artery (CAL) and in front of the abdominal aorta. The CAL takes a course anteriorly after taking origin from the abdominal aorta. This LN is seen just above the anterior most part of CAL. The trunk of left gastric artery near its origin is also seen, but this LN is closest to the CAL and hence is labeled as station 9. Another LN lying below the CAL lies close to the abdominal aorta and belongs to station 16A2. A LN belonging to 11p station lies near the lower border of the splenic artery
Figure 17.
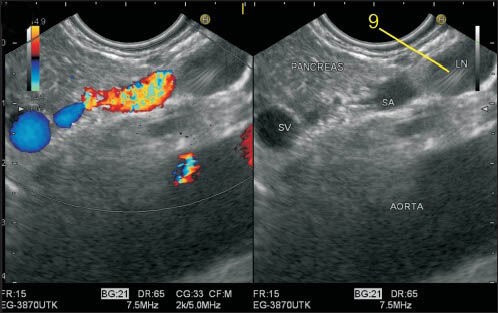
The celiac artery (CAL) takes a vertical course anteriorly after taking origin from the abdominal aorta. This lymph node (LN) is seen just above the anterior most part of CAL, which is not seen in this frame. The splenic artery (SA) is the most tortuous artery of the whole body and in this frame is seen lying anterior to aorta. The LN lying above SA is closer to CAL and anterior to aorta and thus belongs to station 9
Figure 18.
The splenic artery (SA) runs a tortuous course and sometimes dips behind and above the pancreas. In this image, two lymph node (LN) are seen, and the LN labeled as 11d is seen close to the distal part of SA near the upper border of the body of PAN. The upper LN is at station 4
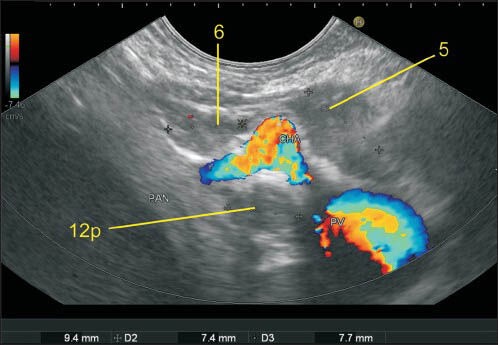
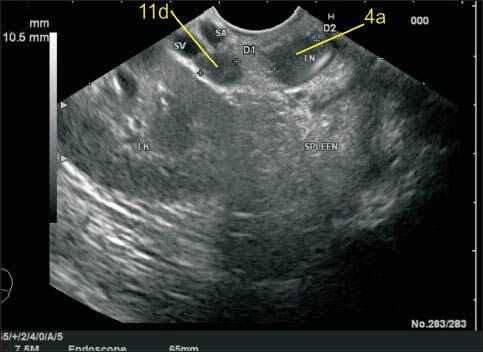
Figure 20.
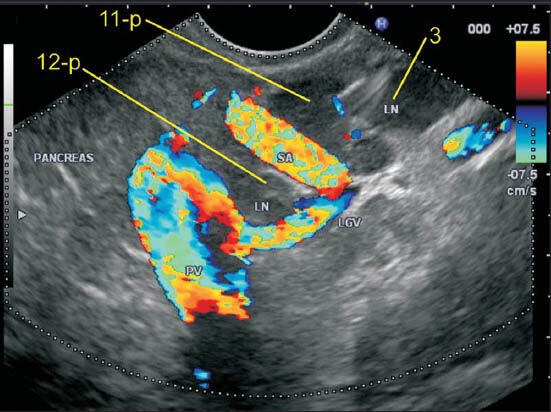
In this case one-lymph node (LN) labeled as 11p lies above the splenic artery as it takes a tortuous course and dips down. The LN labeled as 12p lies in the hepatoduodenal ligament just above the upper border of head of the pancreas as the portal vein exits near the upper border on its way to the hilum of the liver
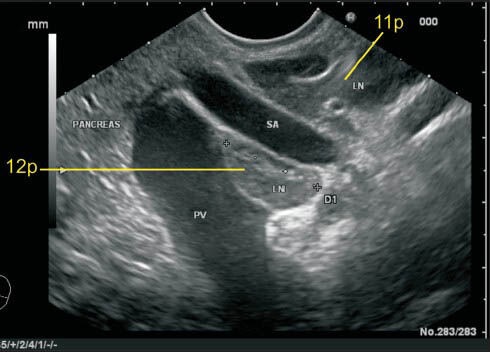
Figure 21.
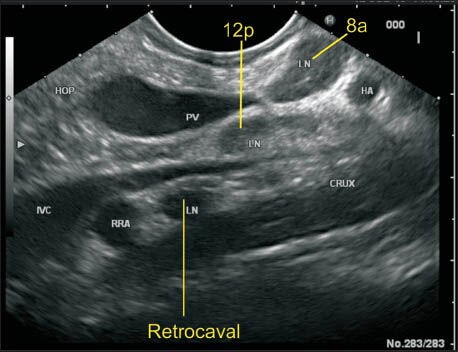
The portal vein (PV) near its formation lies anterior to the inferior vena cava (IVC) and maintains this position till it reaches the hilum. In this case the lymph node (LN) labeled as 12p lies posterior to PV and anterior to IVC. The location of this LN can be also included as precaval because it lies on the anterior surface of the proximal half of IVC. This LN is equidistant from both vessels. The retrocaval LN lies behind IVC anterior to the crux of the diaphragm. The LN of station 8a is also seen lying anterior to hepatic artery
Figure 24.
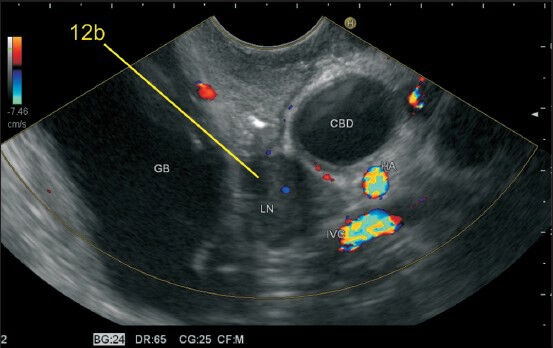
The lymph node labeled as 12b lies along the common bile duct in the curve below union of the cystic duct with gallbladder. This lies in the hepatoduodenal part of lesser omentum and is best visualized from the bulb. CBD: Common bile duct, GB: Gall bladder
Figure 25a.
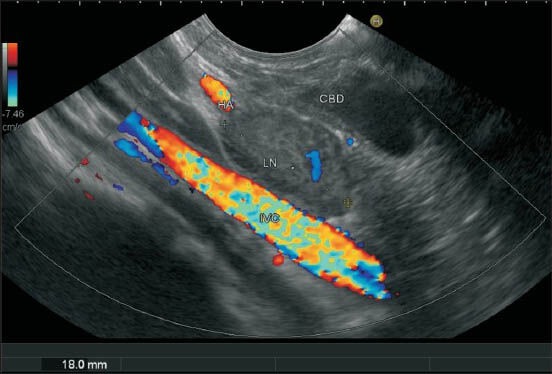
The lymph node (LN) labeled as 13 lies behind the pancreas just close to the lower end papilla and anterior to inferior vena cava. The can be included in station 12b (close to common bile duct) or precaval LN also
Figure 26.
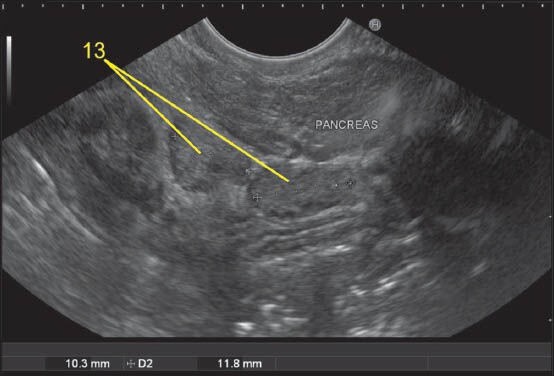
Imaging from stomach can sometimes push the scope below the level of head and body of pancreas. A lymph node (LN) lying cranial to duodenal papilla during imaging from 2nd part of the duodenum can be considered as station 13 whereas an LN lying below the papilla will lie close to the lower border of PAN and will belong to station 18
Figure 27.
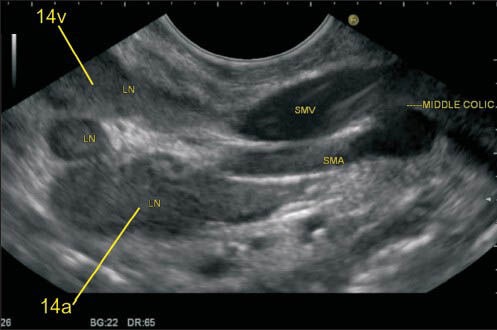
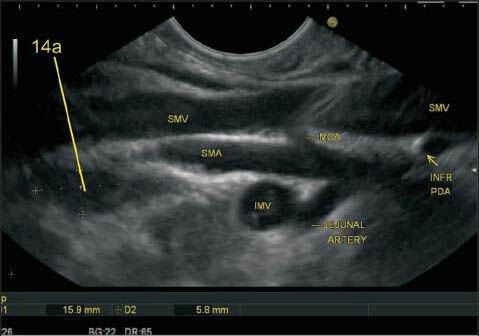
The superior mesenteric artery (SMA) is the second ventral branch of the aorta, given off slightly below the celiac trunk, which descends in a groove on the posterior surface of the neck of the pancreas. Below the inferior margin of the neck of the PAN, it goes anterior to the uncinate process and the horizontal portion of the duodenum and enters the root of the mesentery. It is easy to identify lymph node (LN) close to SMA and superior mesenteric vein (SMV) from the horizontal portion of the duodenum. In this image, the LN is seen near the root of mesentery close to the origin of SMA and also close to the place where the SMV is about to join splenic vein for formation of the portal vein. The SMA nodes (14a) can lie behind the body of PAN, on the uncinate process of PAN or near the root of mesentery
Figure 28.
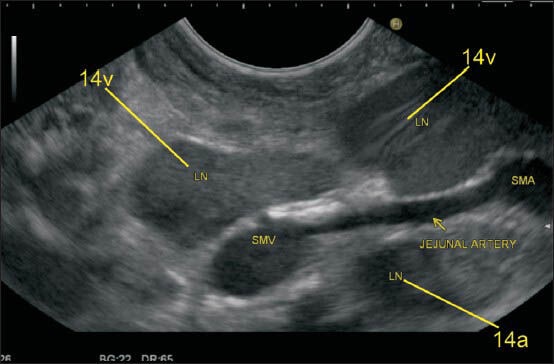
The station 14v nodes lie close to superior mesenteric vein
Figure 29a.
Within the mesentery, the main arterial stem of superior mesenteric artery (SMA) describes an arc that spans the distance between the horizontal duodenum and the ileocecal junction, where the SMA terminates by anastomosing with one of its own branches, the ileocolic artery. Of the arteries to the large intestine, the middle colic artery (MCA) is the first to arise from the right side of the SMA. This branch is usually given off at the inferior margin of the neck of the pancreas before the SMA enters the mesentery. The MCA passes into the transverse mesocolon. In this image, the jejunal branches are seen going away from the probe, and the inferior pancreaticoduodenal artery is seen as the first branch arising from the right side of the SMA coming toward the probe. The second branch coming from the right side of SMA is MCA, which is seen to enter the transverse mesocolon. MCA: Middle colic artery, INFR PDA: Inferior pancreaticoduodenal artery, IMV: Inferior mesenteric vein
Figures 30a and b.
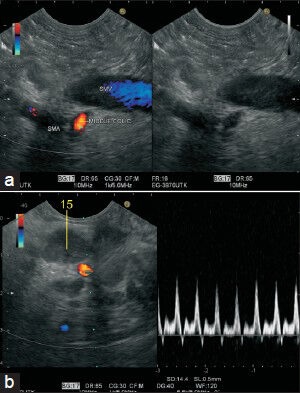
The middle colic artery (MCA) is seen taking origin from the right side of superior esenteric artery from the horizontal part of the duodenum. The course of the MCA is followed-up to the lymph node present in transverse mesocolon (station 15)
Figure 31.
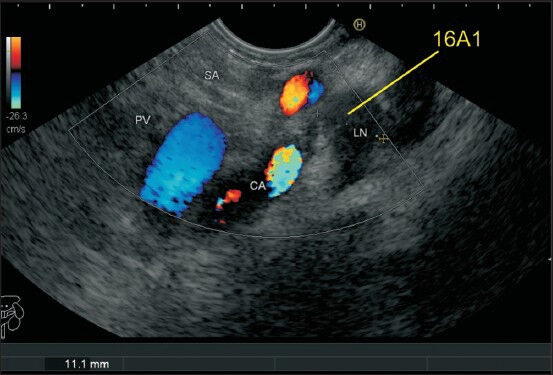
The distance between the esophageal hiatus and the aortic hiatus is about 1.5 cm, and the crux of diaphragm comes in between the two. A lymph node (LN) lying between the esophageal and aortic hiatus can be differentiated by the location: A LN between crux and aorta will be called as aortic hiatus node (16A1) whereas an LN between esophageal hiatus and crux will be called as esophageal hiatal node belonging to station 20
Figure 37.
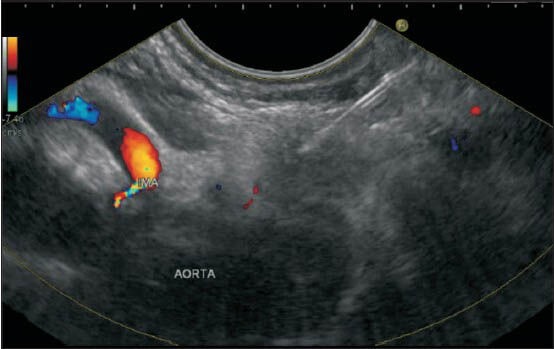
The fine-needle aspiration cytology was done from the 16B1station
Figure 38.
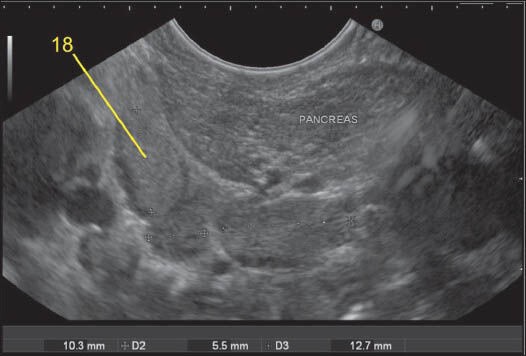
Imaging from stomach can sometimes push the scope below the level of head and body of pancreas. A lymph node (LN) lying cranial to duodenal papilla can be considered as station 13 whereas a LN lying below the papilla will lie near the lower border of PAN and will belong to station 18. In this case, the papilla is not seen but two LN lies behind the PAN in retroperitoneum and belongs to station 13 and another LN, which belong to station 18 lies along the inferior border of the body of PAN
Figure 39.
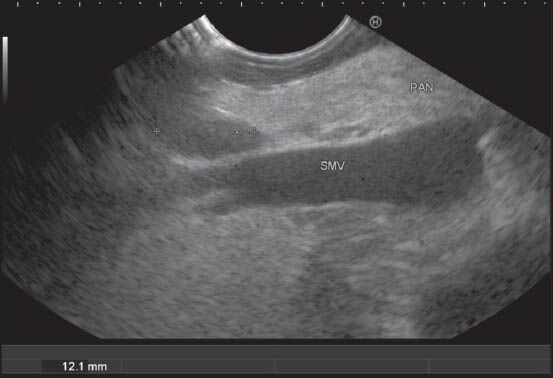
The station 18 lymph nodes (LNs) are retroperitoneal and lie along the inferior border of the body of pancreas. In this case, the LN is seen from the horizontal part of duodenum anterior to the superior mesenteric vein (SMV) just below the place where it crosses the uncinate process. The part of PAN lying in front of SMV belongs to the uncinate process
Figure 40.
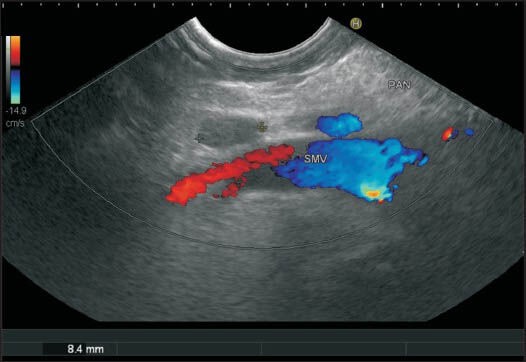
A color Doppler shows the superior mesenteric vein and the lymph node belonging to station 18 from the horizontal part of the duodenum
Figure 41.
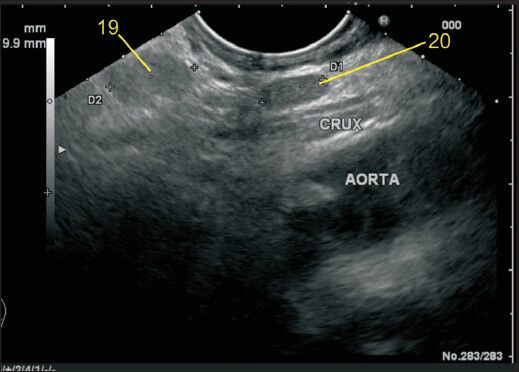
In this case the station 19 and 20 lymph node (LN) are seen. The station 19 LN is retroperitoneal and lies on the inferior surface of the diaphragm. In this case, the LN is much away from the esophageal hiatus and also away from the aorta. The diaphragm is not seen in this image, but the location anterior to crux, below the esophageal hiatus suggest this LN as station 19. The station 20 LN lies between the esophageal hiatus and crux of diaphragm
Figure 42.
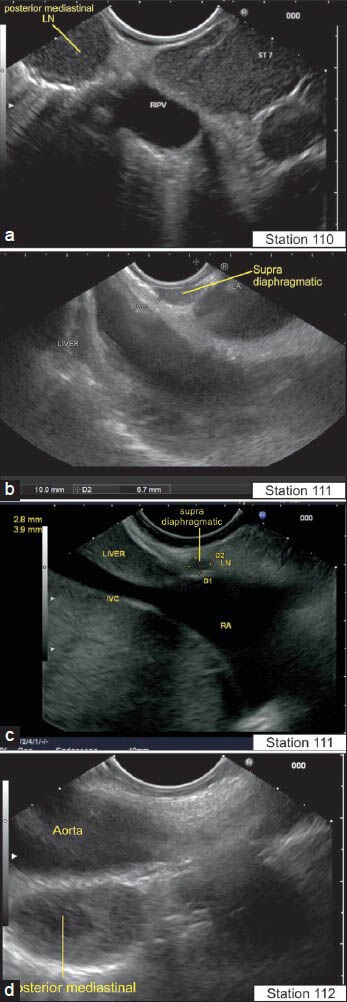
The lymph node (LN) in supra diaphragmatic location lie near the esophagus: Paraesophageal below the pulmonary vein (110) (a), below the left atrium (LA) (111) (b), close to inferior vena cava (supra diaphragmatic) (c) and close to aorta in a posterior mediastinal location (112) (d). The International Association for the Study of Lung Cancer classification of lung cancer staging identifies these LNs in a different manner as station 8 and station 9 LN. RIPV: Right inferior pulmonary vein, ST7: Station 7 of international association of study of lung cancer classification for LN. LA: Left atrium, RA: Right atrium
Figure 3.
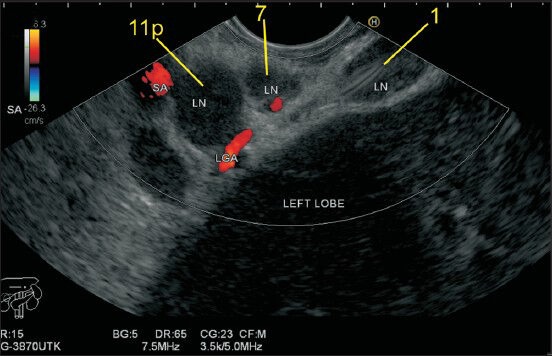
The Lymph node (LN) of station 1 lies on the right side of esophagogastric junction. This LN lies between the wall of the stomach and the left lobe of the liver in the hepatogastric ligament, which lies between the liver and stomach. Two LNs belonging to station 7 and 11p are also seen. The identification of hepatogastric ligament is done as the hyperechoeic area between liver and stomach during anticlockwise rotation after visualizing the aorta
Figure 4.
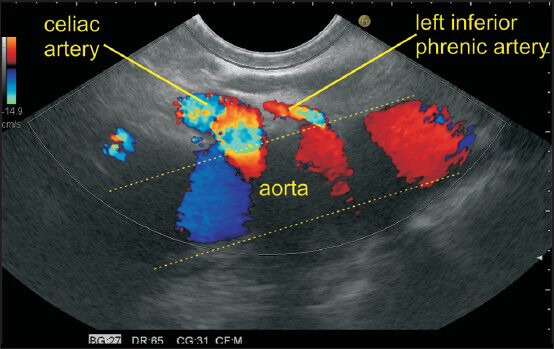
The imaging of the left paracardial and right paracardial areas can be done along a plane which lies posteriorly along the anterior border of aorta and anteriorly goes through the segments 2 and 3 of the left lobe of the liver. Initially, the segments 2 and 3 of liver are visualized in an open position by echoendoscope. A clockwise rotation of the echoendoscope from this position traces the right paracardial area till it reaches the hilum of the liver and traces it on further rotation till the aorta is visualized after a 180° rotation. An anticlockwise rotation from segments 2 and 3 of liver traces the left paracardial area where the origin of left inferior phrenic artery is seen as the first branch from the anterolateral surface of the abdominal aorta above the origin of the celiac artery. The gastrophrenic ligament lies in left paracardial area where lymph node of station 2 lies in at the left margin of the gastroesophageal junction along the esophagocardiac branches of left inferior phrenic artery
Figure 5a.
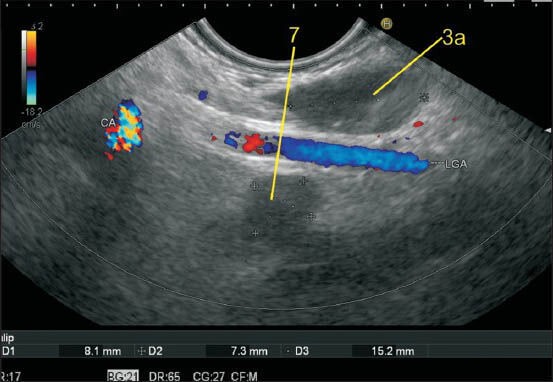
The lesser curvature of the stomach is approximately 10 cm in distance and can be divided into two parts. The branches of left gastric artery lie in the upper part of the lesser curvature in the hepatogastric ligament near the upper part of the lesser curvature (about 5 cm below the esophagogastric junction). The station 3 lymph nodes (LNs) lie along the lesser curvature of the stomach. The LNs up to 5 cm below the junction are included in the upper part of the lesser curvature. CAL: Celiac artery
Figure 5b.
In this image, the left gastric vein is seen joining the upper part of the portal vein (PV) and this lymph node (LN) lies very close to the upper part of the lesser curvature. The left gastric artery and vein run together in the hepatogastric ligament and a LN, which lies close to either, can be included in 3a. The LN labeled as 11p lies more close to the splenic artery than to the lesser curvature and hence is not included in station 3a. An LN lying near the PV (12p) is also seen. The LNs of the 3b (not seen in this image) station lie along the right gastric artery in the lower part of the lesser curvature. The origin and course of right gastric artery is difficult to identify on endoscopic ultrasonography. LGV: Left gastric vein, PV: Portal vein
Figure 6.
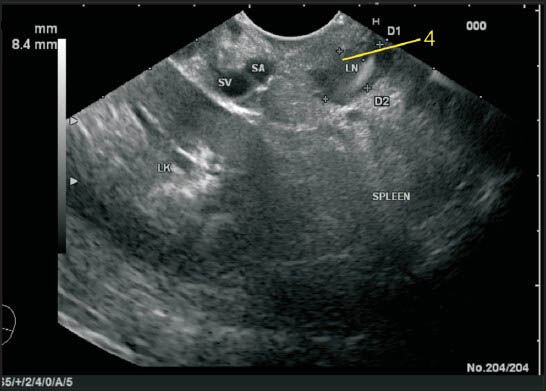
The lienorenal ligament is seen as a structure between the splenic hilum and the left kidney and includes the tail of pancreas. In this ligament the splenic artery and splenic vein traverse toward the hilum of the spleen but the lymph node (LN) is away from the vessels. In this case, a LN lying at the hilum of the spleen close to the tail of PAN is seen and belongs to station 4. LK: Left kidney, D1, D2: Diameter of node
Figure 7.
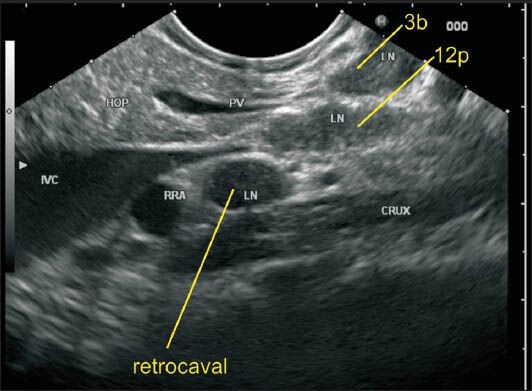
The station 3 lymph nodes (LNs) lie along the lower part of the lesser curvature of the stomach where the pancreas is also seen. This LN belongs to station 3b as it lies close to the lower part of the lesser curvature. In this image the portal vein (PV) is seen behind the PAN and an LN of station 12p lies close to PV. An LN lying behind inferior vena cava belonging to retrocaval group is also seen. RRA: Right renal artery, HOP: Head of pancreas
Figure 8.
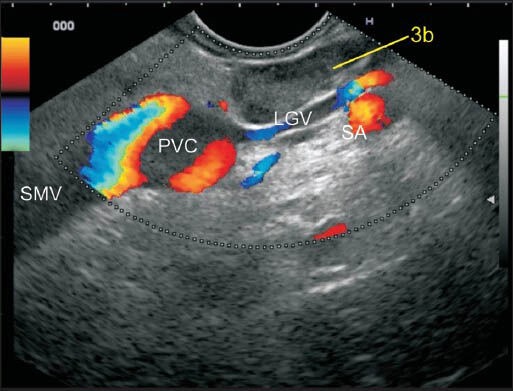
In this image, the LN lies at the upper border of the pancreas between the lesser curvature and the left gastric vein (LGV). The LGV is seen joining the upper border of portal vein (PV) near the portal venous confluence. The splenic artery (SA) is seen. This lymph node (LN) is in proximity of two vessels; the PV and SA. However, this LN lies very close to the lower part of the lesser curvature of the stomach and belongs to station 3b. PVC: Portal venous confluence
Figure 9.
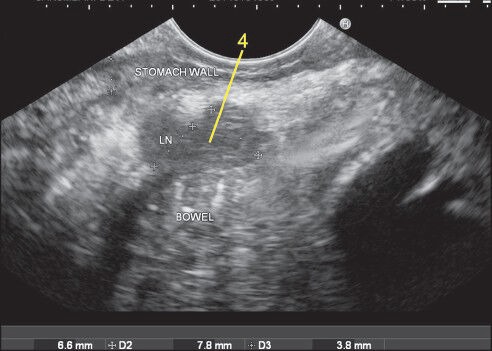
Two lymph nodes are seen adjacent to the greater curvature. They belong to station 4. It is difficult to identify the blood vessels along the greater curvature because of their smaller caliber
Figure 10.
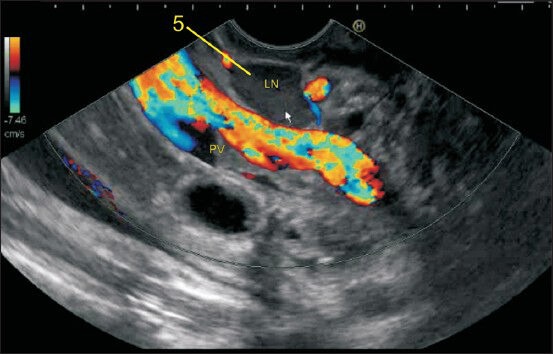
Imaging from the duodenal bulb can show the portal vein (PV) and the bifurcation of common hepatic artery into the hepatic artery proper and gastroduodenal artery. In this image, the lymph node is seen between the supra pancreatic part of PV and the wall of the duodenum and belongs to station 5
Figure 14.
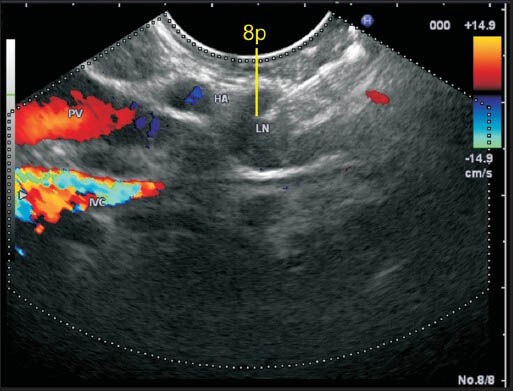
The lymph node (LN) labeled as station 8p lies close to the common hepatic artery. This LN lies anterior to inferior vena cava and posterior to hepatic artery
Figure 19.
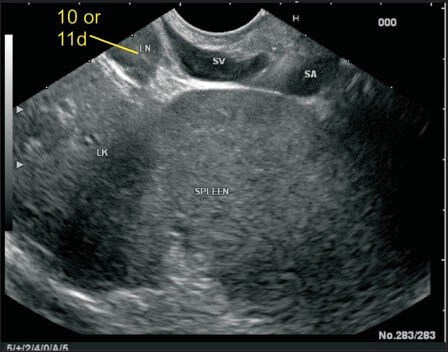
This image shows another lymph node (LN) in the lienorenal ligament close to the hilum of the spleen and can be included either in station 11d or 10. The station 10 LN lies more close to the pancreatic tail whereas station 11d lies near the upper border of the body of pancreas. On endoscopic ultrasonography a demarcation of the point where the body of PAN becomes tail can be seen when the renal artery and renal vein are no longer visualized because of the entry into to the hilum of the left kidney. So the inclusion of this LN can be done in station 10
Figure 22.
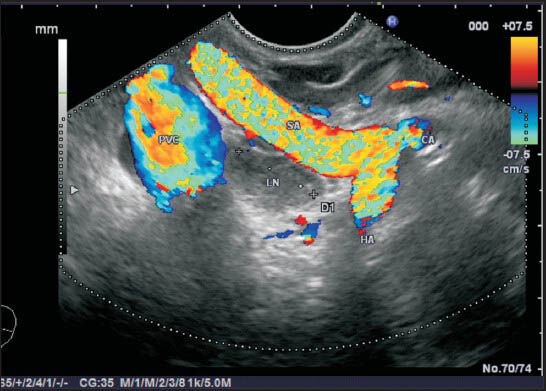
The bifurcation of the celiac artery into the hepatic artery (HA) and splenic artery (SA) produces a vertical seagull like appearance with vascular signals on endoscopic ultrasonography from stomach where the limb coming toward the probe is SA and the limb going away from the probe is HA. In between the two vessels lies the highest point of pancreas, which is called tuber omentale
Figure 23.

In this case a lymph node (LN) is seen just above the tuber omentale and is close to four vessels, which are celiac artery, hepatic artery, splenic artery and portal vein. This LN lies in the lower part of the gastro hepatic omentum and can be included in station 9, 11p, 8a and 12p
Figure 25b.
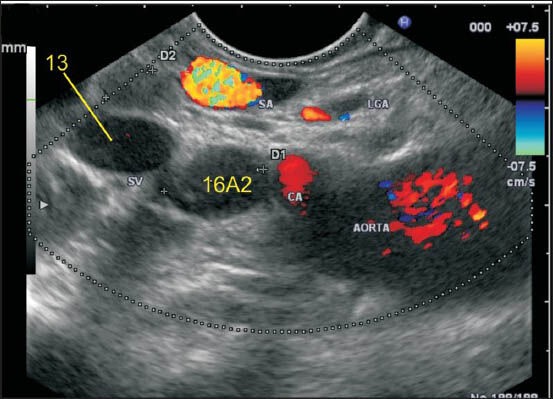
In this case two-lymph nodes (LNs) are seen. Station 13 LN lies behind pancreas but lies away from the aorta. Station 16A2 lies below the celiac artery above the renal vein anterior to aorta
Figure 29b.
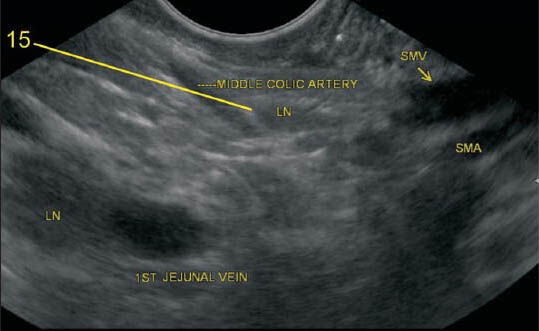
The lymph node (LN) lying along middle colic artery belongs to station 15. In the second image, a LN is also seen along the first-jejunal vein
Figure 32.
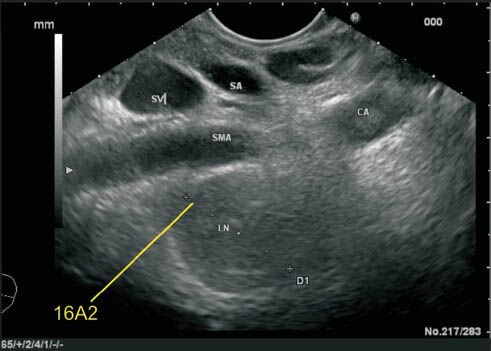
This lymph node (LN) belonging to station 16A2 lies below the celiac artery and posterior to the origin of superior mesenteric artery (SMA). Although this LN is very close to the posterior surface of SMA, the LN lies anterior to aorta and this LN is not included in the root of mesentery LN (station 14a)
Figure 33.
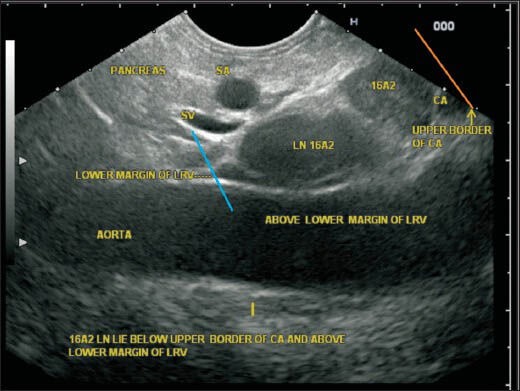
In this image, the upper and lower boundary of station A2 are seen as the upper border of the celiac artery (CAL) (orange line) and lower border of left renal vein (LRV) (blue line). The lymph node (LN) belonging to station 16A2 lies below the CAL and above the lower margin of LRV anterior to the aorta. Although the LNs lie posterior to the pancreas, the location near the aorta places them in para-aortic group
Figure 34.
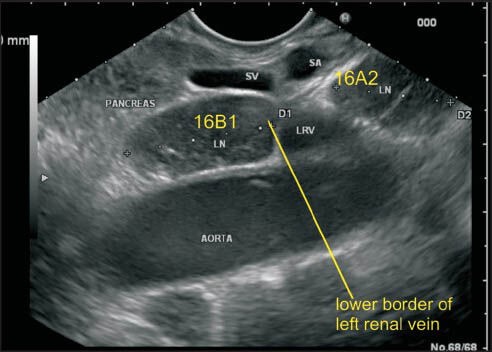
The lower border of left renal vein (LRV) demarcates the upper border of station 16B1. This image shows a lymph node anterior to aorta and below the lower border of LRV belonging to station 16B1. The lower border of station 16B1 is identified by origin of inferior mesenteric artery, which is not seen in these images. LRV: Left renal vein
Figure 35.
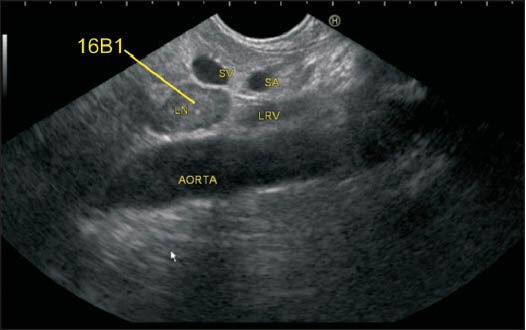
This image shows 16B1 lymph node in a different patient
Figure 36.
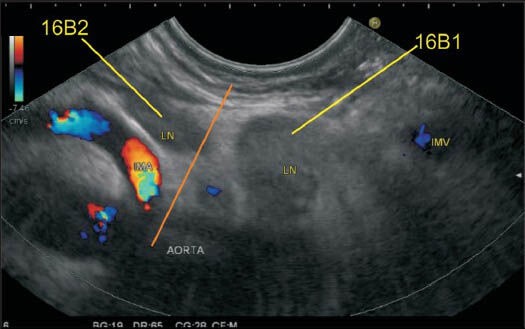
In this case two-lymph nodes (LNs) are seen: One LN lies above the origin of inferior mesenteric artery from the aorta, and the second LN lies below the level of the upper margin of origin of IMA. The upper LN belongs to station 16B1, and the lower LN belongs to station 16B2. It is uncommon to see the origin of IMA from aorta by endoscopic ultrasonography but in this image the LNs can be seen from the stomach below the level of the pancreas. IMA: Inferior mesenteric artery
DISCUSSION
The nomenclature of nodal stations in abdomen is based primarily on the relationship of the lymphatic drainage (LNs) that follows the accompanying vessel (artery and vein) or a direct relationship with the regional organ. The accompanying vessels traverse through the ligaments surrounding stomach and the metastatic nodes commonly reside in ligaments rather than the retroperitoneum.[24] Soon after the first descriptions of the five-layer structure of the gastric wall,[25] EUS became a standard technique for the staging of GC. Until a few years ago, the impact of EUS in GC was limited by the lack of therapeutic options, surgery being the only recourse either with curative or with palliative intent. The clinical arena of GC has changed substantially in recent years as treatments have become more numerous. Besides the traditional surgical approach, endoscopic mucosal resection and submucosal dissection are adopted for the early stages of the disease, and neoadjuvant therapies are used for the advanced stages. As a consequence, the potential role of EUS in GC has become much more attractive to identify the patients suitable for minimally invasive treatment, those who should undergo primary surgery, and those who need neoadjuvant therapy.[10] Under- or over-staging of LN disease has been noted when EUS is used alone[26] and a multimodality imaging with CT, PET scan along with EUS and EUS-FNA of nodal stations may provide a more detailed staging. The additional value of EUS-FNA over EUS alone for N and M staging of GC are emphasized in a study where distant LN and liver metastases were detected by EUS-FNA in 42% of the patients, and CT of the abdomen or thorax had previously failed to show any abnormality.[27] Although preliminary reports have yielded conflicting results in this respect, GC restaging after neoadjuvant treatment is likely to emerge as another clinical task for endosonographers.[2] The practical impact and use of EUS in treatment decisions in GC patients is lower than would have been expected from the EUS. This may be due to lack of application of EUS for N staging. EUS-FNA should be considered an integral part of the EUS staging procedure for GC in the near future. This review elaborately describes the technique for proper identification of nodal stations by EUS.
Videos are available at www.onlinejets.org
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–62. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park SR, Kim MJ, Ryu KW, et al. Prognostic value of preoperative clinical staging assessed by computed tomography in resectable gastric cancer patients: A viewpoint in the era of preoperative treatment. Ann Surg. 2010;251:428–35. doi: 10.1097/SLA.0b013e3181ca69a7. [DOI] [PubMed] [Google Scholar]
- 3.Jeong JY, Kim MG, Ha TK, et al. Prognostic factors on overall survival in lymph node negative gastric cancer patients who underwent curative resection. J Gastric Cancer. 2012;12:210–6. doi: 10.5230/jgc.2012.12.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–12. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 5.Kajitani T. The general rules for the gastric cancer study in surgery and pathology. Part I. Clinical classification. Jpn J Surg. 1981;11:127–39. doi: 10.1007/BF02468883. [DOI] [PubMed] [Google Scholar]
- 6.Ahn HS, Lee HJ, Yoo MW, et al. Diagnostic accuracy of T and N stages with endoscopy, stomach protocol CT, and endoscopic ultrasonography in early gastric cancer. J Surg Oncol. 2009;99:20–7. doi: 10.1002/jso.21170. [DOI] [PubMed] [Google Scholar]
- 7.Kwee RM, Kwee TC. Imaging in assessing lymph node status in gastric cancer. Gastric Cancer. 2009;12:6–22. doi: 10.1007/s10120-008-0492-5. [DOI] [PubMed] [Google Scholar]
- 8.Hallinan JT, Venkatesh SK. Gastric carcinoma: Imaging diagnosis, staging and assessment of treatment response. Cancer Imaging. 2013;13:212–27. doi: 10.1102/1470-7330.2013.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Cheong JH, Yun MJ, et al. Improvement in preoperative staging of gastric adenocarcinoma with positron emission tomography. Cancer. 2005;103:2383–90. doi: 10.1002/cncr.21074. [DOI] [PubMed] [Google Scholar]
- 10.Caletti G, Fusaroli P. The rediscovery of endoscopic ultrasound (EUS) in gastric cancer staging. Endoscopy. 2012;44:553–5. doi: 10.1055/s-0032-1309770. [DOI] [PubMed] [Google Scholar]
- 11.Mocellin S, Marchet A, Nitti D. EUS for the staging of gastric cancer: A meta-analysis. Gastrointest Endosc. 2011;73:1122–34. doi: 10.1016/j.gie.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 12.Filip M, Iordache S, Sâftoiu A. Gastric cancer staging by endoscopic ultrasound – Contrast enhancement and real-time elastography. Video J Encyclopedia GI Endosc. 2013;1:164–6. [Google Scholar]
- 13.Feng XY, Wang W, Luo GY, et al. Comparison of endoscopic ultrasonography and multislice spiral computed tomography for the preoperative staging of gastric cancer – Results of a single institution study of 610 Chinese patients. PLoS One. 2013;8:e78846. doi: 10.1371/journal.pone.0078846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NCCN. Clinical Practice Guidelines in Oncology (NCCN guidelines).Gastric Cancer (including cancer in the proximal 5 cm of the stomach) Version 2.2011. National Comprehensive Cancer Network. 2011 [Google Scholar]
- 15.Harris CL, Toloza EM, Klapman JB, et al. Minimally invasive mediastinal staging of non-small-cell lung cancer: Emphasis on ultrasonography-guided fine-needle aspiration. Cancer Control. 2014;21:15–20. doi: 10.1177/107327481402100103. [DOI] [PubMed] [Google Scholar]
- 16.Sharma M, Arya CL, Somasundaram A, et al. Techniques of linear endobronchial ultrasound imaging. J Bronchology Interv Pulmonol. 2010;17:177–87. doi: 10.1097/LBR.0b013e3181dca122. [DOI] [PubMed] [Google Scholar]
- 17.Sharma M, Chittapuram R, Rai P. Endosonography of the normal mediastinum: The Experts Approach. Video J Encyclopedia GI Endosc. 2013;1:56–9. [Google Scholar]
- 18.Sharma M, Rameshbabu CS, Mohan P. Standard techniques of imaging of IASLC borders by endoscopic ultrasound. J Bronchology Interv Pulmonol. 2011;18:99–110. doi: 10.1097/LBR.0b013e318207e6d5. [DOI] [PubMed] [Google Scholar]
- 19.de Melo SW, Jr, Panjala C, Crespo S, et al. Interobserver agreement on the endosonographic features of lymph nodes in aerodigestive malignancies. Dig Dis Sci. 2011;56:3204–8. doi: 10.1007/s10620-011-1725-8. [DOI] [PubMed] [Google Scholar]
- 20.Knabe M, Günter E, Ell C, et al. Can EUS elastography improve lymph node staging in esophageal cancer? Surg Endosc. 2013;27:1196–202. doi: 10.1007/s00464-012-2575-y. [DOI] [PubMed] [Google Scholar]
- 21.Sharma M, Babu CS, Garg S, et al. Portal venous system and its tributaries: A radial endosonographic assessment. Endosc Ultrasound. 2012;1:96–107. doi: 10.7178/eus.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rameshbabu CS, Wani ZA, Rai P, et al. Standard imaging techniques for assessment of portal venous system and its tributaries by linear endoscopic ultrasound: A pictorial essay. Endosc Ultrasound. 2013;2:16–34. doi: 10.7178/eus.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desai G, Filly RA. Sonographic anatomy of the gastrohepatic ligament. J Ultrasound Med. 2010;29:87–93. doi: 10.7863/jum.2010.29.1.87. [DOI] [PubMed] [Google Scholar]
- 24.Vikram R, Balachandran A, Bhosale PR, et al. Pancreas: Peritoneal reflections, ligamentous connections, and pathways of disease spread. Radiographics. 2009;29:e34. doi: 10.1148/rg.e34. [DOI] [PubMed] [Google Scholar]
- 25.Kimmey MB, Martin RW, Haggitt RC, et al. Histologic correlates of gastrointestinal ultrasound images. Gastroenterology. 1989;96:433–41. doi: 10.1016/0016-5085(89)91568-0. [DOI] [PubMed] [Google Scholar]
- 26.Kutup A, Vashist YK, Groth S, et al. Endoscopic ultrasound staging in gastric cancer: Does it help management decisions in the era of neoadjuvant treatment? Endoscopy. 2012;44:572–6. doi: 10.1055/s-0032-1308950. [DOI] [PubMed] [Google Scholar]
- 27.Hassan H, Vilmann P, Sharma V. Impact of EUS-guided FNA on management of gastric carcinoma. Gastrointest Endosc. 2010;71:500–4. doi: 10.1016/j.gie.2009.10.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


