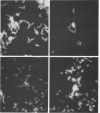
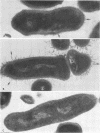
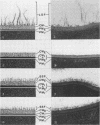
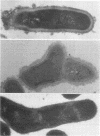
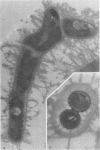
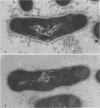
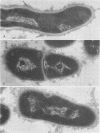
Abstract
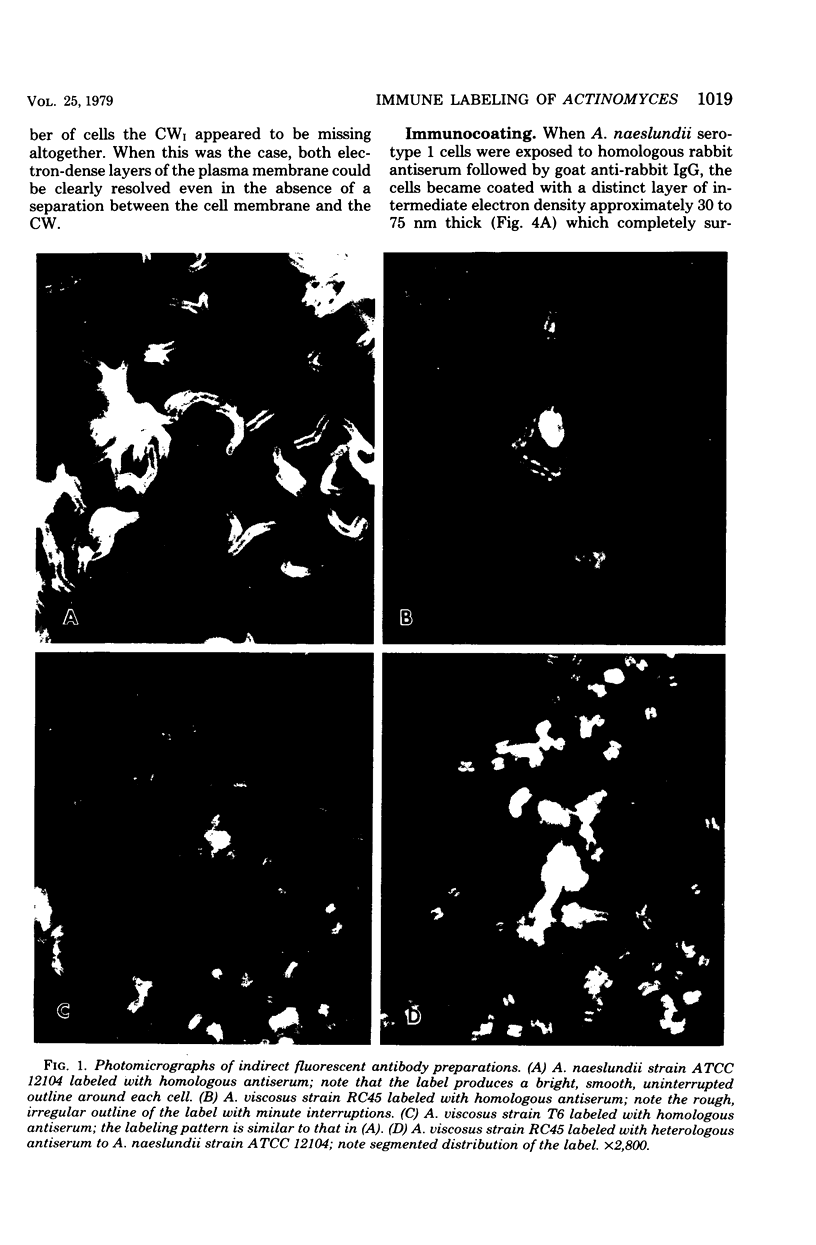
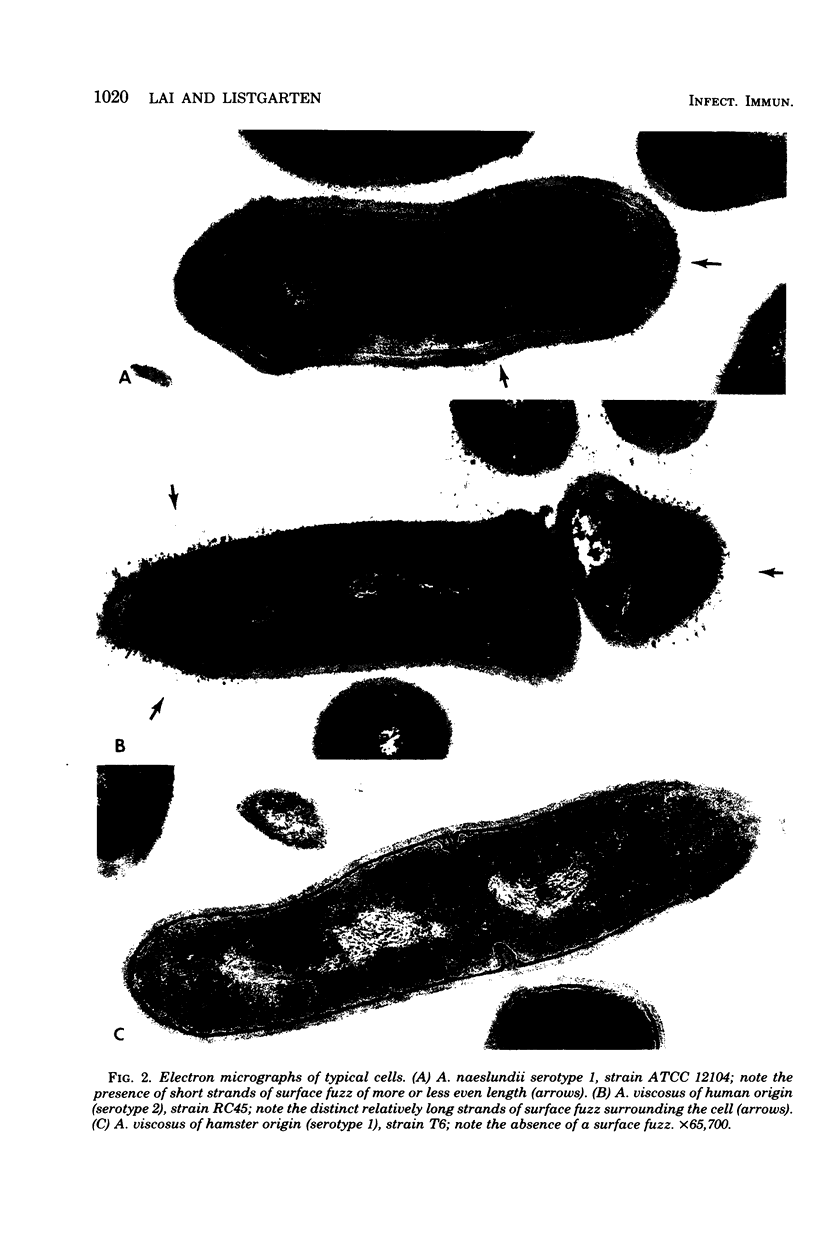
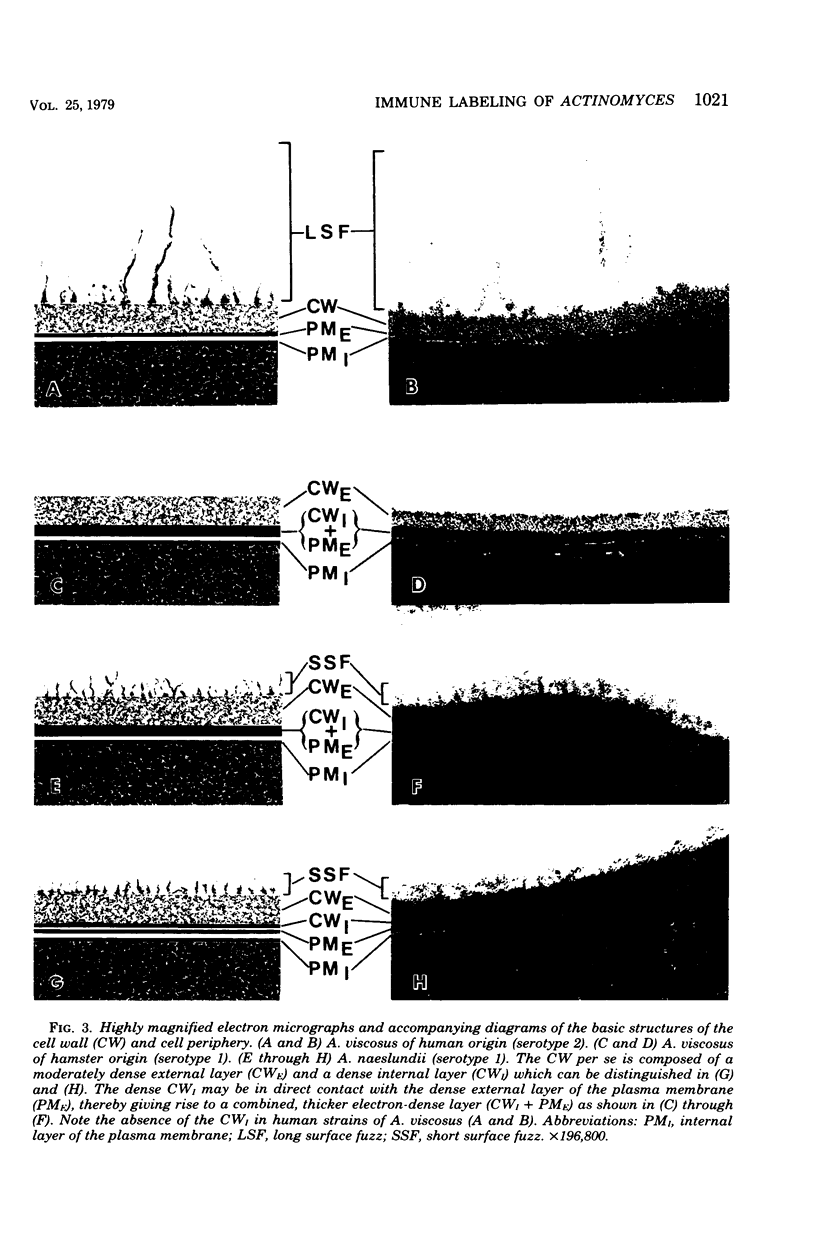
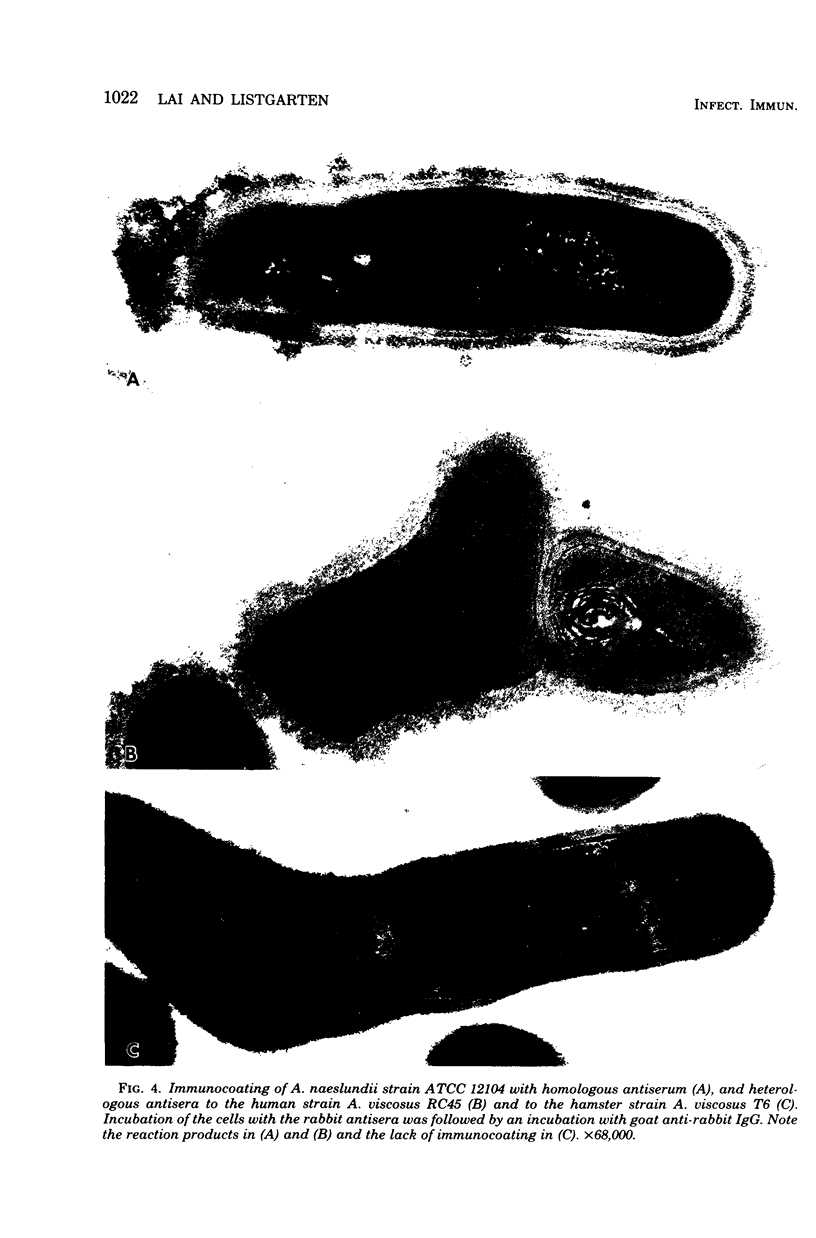
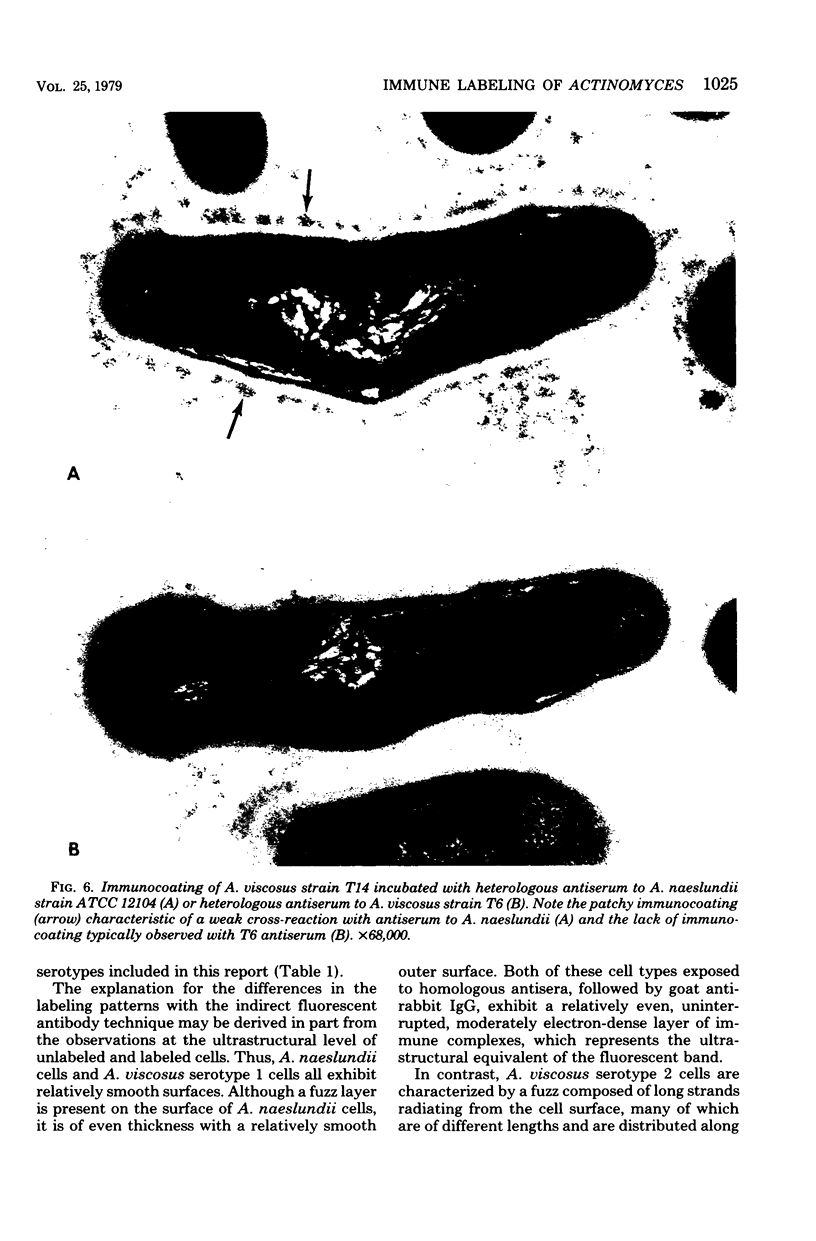
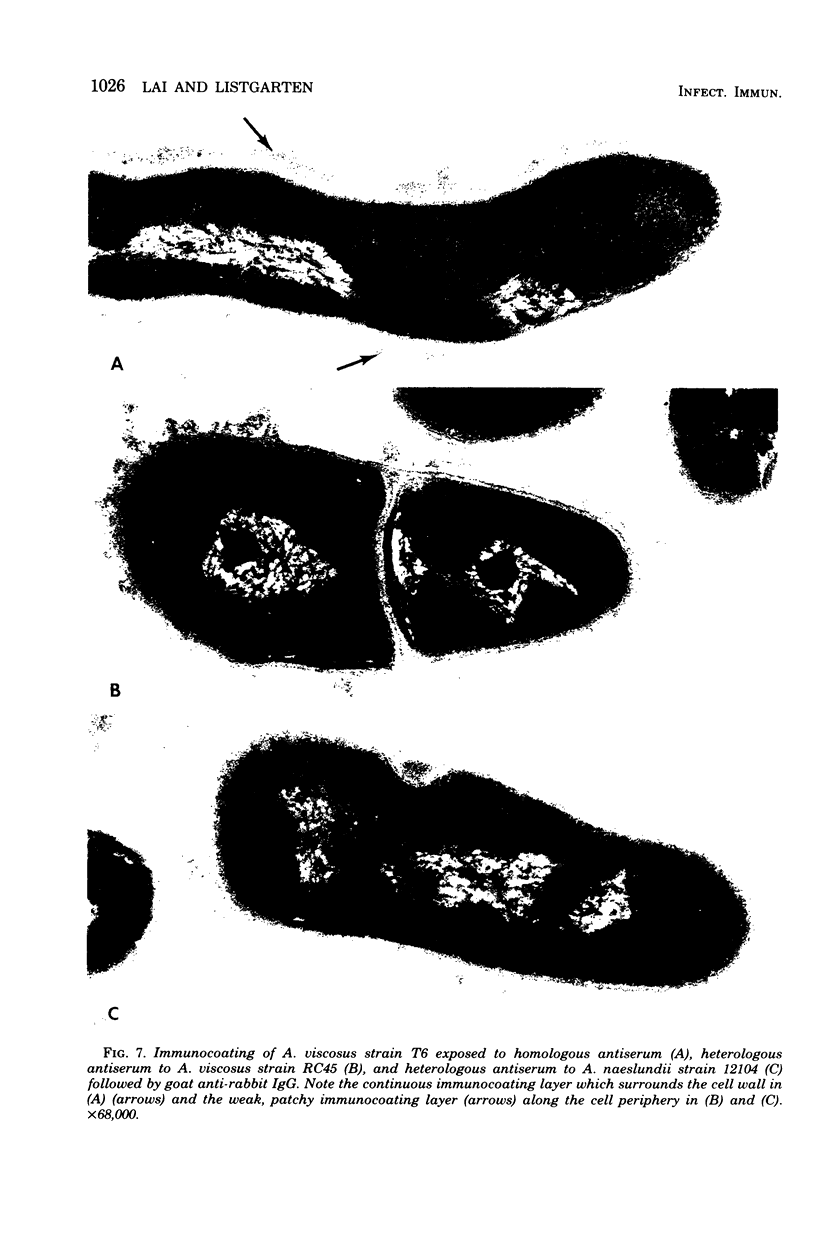
A total of 12 well-characterized strains of Actinomyces viscosus and A. naeslundii grown on Trypticase soy agar plates supplemented with sheep erythrocytes were examined by light microscopy and transmission electron microscopy after treatment with appropriately labeled antisera to homologous and heterologous strains. Cells incubated with homologous rabbit antisera followed by fluorescein-isothiocyanate (FITC)-conjugated goat anti-rabbit immunoglobulin G (IgG) exhibited a completely smooth fluorescent outline in the case of A. naeslundii and and interrupted, irregular fluorescent outline in the case of human strains of A. viscosus. The different labeling patterns appeared to be related to the presence at the ultrastructural level of long, unevenly distributed strands of "fuzz" on the surface of human A. viscosus cells, whereas A. naeslundii cells had a narrower layer of fuzz, or more even thickness. The immunocoating reaction revealed homologous antibody binding to the irregular strands of fuzz on the surface of human A. viscosus cells, whereas homologous antisera to A. naeslundii coated A. naeslundii cells with a moderately electron-dense coating of antibody of even thickness. Human strains of A. viscosus incubated with heterologous antiserum to A. naeslundii followed by FITC-labeled goat anti-rabbit IgG exhibited a segmented fluorescent outline, which differed from that produced with homologous antisera. A. naeslundii incubated with heterologous rabbit antisera to human A. viscosus strains and FITC-labeled anti-rabbit IgG exhibited a completely smooth fluorescent outline similar to that produced with homologous antiserum. A. viscosus strains of hamster origin differed from A. viscosus strains of human origin by the absence of a surface fuzz and the comparatively smooth, even fluorescence produced by incubating these cells with homologous rabbit antiserum followed by FITC-labeled goat anti-rabbit IgG. Antiserum to a hamster strain did not cross-react with A. naeslundii or human strains of A. viscosus. Under the growth conditions of this experiment, ultrastructural features and labeling patterns with the indirect fluorescent technique may be useful in differentiating these serotypes from one another.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellack S., Jordan H. V. Serological identification of rodent strains of Actinomyces viscosus and their relationship to Actinomyces of human origin. Arch Oral Biol. 1972 Jan;17(1):175–182. doi: 10.1016/0003-9969(72)90145-8. [DOI] [PubMed] [Google Scholar]
- Cisar J. O., Vatter A. E., McIntire F. C. Identification of the virulence-associated antigen on the surface fibrils of Actinomyces viscosus T14. Infect Immun. 1978 Jan;19(1):312–319. doi: 10.1128/iai.19.1.312-319.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford J. M., Taubman M. A., Smith D. J. The effects of local immunization with periodontopathic microorganisms on periodontal bone loss in gnotobiotic rats. J Periodontal Res. 1978 Sep;13(5):445–459. doi: 10.1111/j.1600-0765.1978.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. J., Montgomery P. C. Characterization of an antibody directed against a surface component of normal and pleomorphic cells of Streptococcus sanguis. Infect Immun. 1975 Sep;12(3):668–678. doi: 10.1128/iai.12.3.668-678.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garant P. R. Light and electron microscopic observations of osteoclastic alveolar bone resorption in rats monoinfected with Actinomyces naeslundii. J Periodontol. 1976 Dec;47(12):717–723. doi: 10.1902/jop.1976.47.12.717. [DOI] [PubMed] [Google Scholar]
- Gerencser M. A., Slack J. M. Identification of human strains of Actinomyces viscosus. Appl Microbiol. 1969 Jul;18(1):80–87. doi: 10.1128/am.18.1.80-87.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerencser M. A., Slack J. M. Serological identification of Actinomyces using fluorescent antibody techniques. J Dent Res. 1976 Jan;55:A184–A191. doi: 10.1177/002203457605500110011. [DOI] [PubMed] [Google Scholar]
- Holmberg K., Forsum U. Identification of Actinomyces, Arachnia, Bacterionema, Rothia, and Propionibacterium species by defined immunofluorescence. Appl Microbiol. 1973 May;25(5):834–843. doi: 10.1128/am.25.5.834-843.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan H. V., Keyes P. H., Bellack S. Periodontal lesions in hamsters and gnotobiotic rats infected with actinomyces of human origin. J Periodontal Res. 1972;7(1):21–28. doi: 10.1111/j.1600-0765.1972.tb00627.x. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C., Listgarten M., Rosan B. Serology of Streptococcus sanguis: localization of antigens with unlabeled antisera. Infect Immun. 1973 Sep;8(3):475–481. doi: 10.1128/iai.8.3.475-481.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner T., Challacombe S. J., Wilton J. M., Ivanyi L. Immunopotentiation by dental microbial plaque and its relationship to oral disease in man. Arch Oral Biol. 1976;21(12):749–753. doi: 10.1016/0003-9969(76)90065-0. [DOI] [PubMed] [Google Scholar]
- Patters M. R., Genco R. J., Reed M. J., Mashimo P. A. Blastogenic response of human lymphocytes to oral bacterial antigens: comparison of individuals with periodontal disease to normal and edentulous subjects. Infect Immun. 1976 Nov;14(5):1213–1220. doi: 10.1128/iai.14.5.1213-1220.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J. T., Fischlschweiger W., Birdsell D. C. Modification of surface composition of Actinomyces viscosus T14V and T14AV. Infect Immun. 1978 Dec;22(3):934–944. doi: 10.1128/iai.22.3.934-944.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky S. S., Hubersak C., Propas D. Induction of periodontal destruction in gnotobiotic rats by a human oral strain of Actinomyces naeslundii. Arch Oral Biol. 1970 Oct;15(10):993–995. doi: 10.1016/0003-9969(70)90095-6. [DOI] [PubMed] [Google Scholar]
- Syed S. A., Loesche W. J., Pape H. L., Jr, grenier E. Predominant cultivable flora isolated from human root surface caries plaque. Infect Immun. 1975 Apr;11(4):727–731. doi: 10.1128/iai.11.4.727-731.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkateswaran P. S., Stanton N., Buettger C., Austrian R. Filamentous capsulated streptococci from the human respiratory tract: chemical and immunochemical characterization of a glycoprotein capsular antigen of provisional binary capsular type 87. Infect Immun. 1978 Apr;20(1):180–193. doi: 10.1128/iai.20.1.180-193.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. L., Pantalone R. M., Sherris J. C. Subgingival microflora and periodontitis. J Periodontal Res. 1976 Feb;11(1):1–18. doi: 10.1111/j.1600-0765.1976.tb00045.x. [DOI] [PubMed] [Google Scholar]