Abstract
The sentinel roles of mammalian mast cells (MCs) in varied infections raised the question of their evolutionary origin. We discovered that the test cells in the sea squirt Ciona intestinalis morphologically and histochemically resembled cutaneous human MCs. Like the latter, C. intestinalis test cells stored histamine and varied heparin•serine protease complexes in their granules. Moreover, they exocytosed these preformed mediators when exposed to compound 48/80. In support of the histamine data, a C. intestinalis-derived cDNA was isolated that resembled that which encodes histidine decarboxylase in human MCs. Like heparin-expressing mammalian MCs, activated test cells produced prostaglandin D2 and contained cDNAs that encode a protein that resembles the synthase needed for its biosynthesis in human MCs. The accumulated morphological, histochemical, biochemical, and molecular biology data suggest that the test cells in C. intestinalis are the counterparts of mammalian MCs that reside in varied connective tissues. The accumulated data point to an ancient origin of MCs that predates the emergence of the chordates >500 million years ago, well before the development of adaptive immunity. The remarkable conservation of MCs throughout evolution is consistent with their importance in innate immunity.
Keywords: mast cell, Ciona intestinalis, heparin, serine protease, histamine, prostaglandin D2
1. Introduction
Mast cells (MCs) are important effector cells that participate in innate and adaptive immunity [1]. No human has been found who lacks MCs. Located at strategic sites within epithelial and mucosal surfaces, MCs perform sentinel roles in combating numerous pathogens, in part, via their exocytosed heparin•protease complexes [2–5]. Human MCs and their progenitors are highly susceptible to M-tropic strains of HIV-1 [6,7], and the loss of HIV-1-infected MCs in the gastrointestinal tract and other tissue sites [8] contributes to the development of AIDS.
When activated by complement anaphylatoxins or by varied pathogen-derived products, mammalian MCs quickly release their granule (e.g., histamine [9] and heparin•serine protease complexes [10–14]) and lipid (e.g., prostaglandin D2 [PGD2] [15] and leukotriene C4 [LTC4] [16]) mediators to initiate the acute phase of the inflammatory response against the infectious organism. Given their central roles in innate and acquired immunity, surprisingly little is known concerning the origin and evolution of different polarized subsets of MCs and their granule and lipid mediators. MCs have been identified in zebrafish [17]. Nevertheless, no protostome has been shown that possesses cells that resemble any subset of MCs in mice and humans. We therefore looked for MC-like cells in Ciona intestinalis which is a member of the chordate lineage that gave rise to vertebrates [18].
2. Materials and methods
2.1. Histochemistry and ultrastructure of the test cells in C. intestinalis, and biochemical characterization of the heparin glycosaminoglycans present in their secretory granules
C. intestinalis were collected at the Marine Biological Laboratory in Woods Hole, Massachusetts. The test cells that surround the oocytes of this sea squirt were isolated for in vitro study by mild physical trauma of the liberated eggs, following by sedimentation of their dense granulated test cells. The heparin proteoglycans and glycosaminoglycans were isolated from lysed test cells using the zwittergent 3–12 detergent/CsCl2 density-gradient method we previously developed for the isolation of proteoglycans and glycosaminoglycans from rodent and human MCs [19]. Employing the experimental procedures developed for analyzing mammalian heparin glycosaminoglycans [20], purified test cell-derived heparin was digested with heparitinases (Seikagaku Corp.) and the resulting disaccharides were subjected to high performance liquid chromatography. After chromatographic separation of the generated disaccharides, they were reacted with 2-cyanoacetoamide as a post-column reagent. They were identified and quantitated based on comparisons of their elution positions and peak heights with those of known amounts of standard disaccharides.
2.2. Release of enzymatically active serine proteases from activated C. intestinalis test cells
C. intestinalis test cells (~1 × 106 cells/ml) by activated by exposure to 0.2 mM compound 48/80 (Sigma-Aldrich), as previously described for heparin-expressing mouse MCs [21] and Styela plicata test cells [22]. The treated cells were subjected to electron microscopy, using standard methodology [23,24]. In other experiments, supernatants were collected from the compound 48/80-activated test cells. The presence of enzymatically active serine proteases in the supernatants was detected using 5 nM of the biotinylated probe Phe-Pro-Arg-chlorometylketone (PPACK, Santa Cruz Biotech.), employing the experimental protocol recommended by the manufacturer. PPACK binds irreversibly to the active sites of serine proteases [25,26]. Thus, the PPPACK-labeled serine proteases in the test cells of C. intestinalis were separated by SDS-PAGE, transferred to Protran BA83 nitrocellulose membranes (Whatman), blocked in 5% nonfat milk for 1 h, and incubated for 3 h with horseradish peroxidase (HRP)-conjugated streptavidin which binds with high affinity to the biotin moiety of the biontinylated-PPACK probe. The treated blots were washed three times (10 min each) in phosphate-buffered saline containing 0.1% Tween 20. They were then developed in enhanced chemiluminescence (ECL) reagent (Millipore) for 2–5 min. Labeled serine proteases were detected by exposing the blots to Blue XB-1 film (Kodak).
2.3. Release of histamine from activated C. intestinalis test cells, and cloning of the putative C. intestinalis ortholog of human histidine decarboxylase (HDC)
The histamine enzyme-linked immunosorbent assay (ELISA) created by Bertin Pharma/SPI Bio and distributed by Cayman Chemical was used to measure histamine levels in lysates of enriched test cells, again using the manufacturer’s protocol. This ELISA is based on the competition between unlabeled derivatized histamine and aceylcholinesterase that has been linked to histamine (defined as the tracer) for a mouse monoclonal antihistamine antibody that is bound to a 96-well plate.
HDC [27] participates in the biosynthesis of histamine in mammalian MCs. A previously created cDNA library [28] was used to isolate its putative C. intestinalis ortholog (CiHDC). A search of the Expressed Sequence Tags (ESTs) in the library revealed a clone (designated cidg826g09) whose partial nucleotide sequence resembled that of human and mouse HDC. Using a standard molecular biology approach, the nucleotide sequence of the entire coding domain of CiHDC was determined, as well as the amino acid sequence of its translated protein. Finally, a guinea pig polyclonal anti-HDC antibody (Thermo Scientific) that recognizes human, mouse, chicken, and amphibian HDC was used to detect the presence of the putative CiHDC protein in test cells. Test cells were lysed in SDS-PAGE loading buffer. The liberated proteins were denatured at 95°C for 5 min, fractionated on a 10% NuPAGE gel (Invitrogen), and immunoblotted with the anti-HDC antibody.
2.4. Release of prostaglandin D2 (PGD2) from activated C. intestinalis test cells, and cloning of the putative C. intestinalis ortholog of human hematopoietic-type PGD2 synthase (HPGDS)
To determine if C. intestinalis test cells have the ability to generate PGD2 and/or LTC4 upon cellular activation, ~106 cells were placed in 200 μl of Hank’s buffer containing 2 mg/ml of bovine serum albumin, 5 μM calcium ionophore A23187 (Calbiochem), and 10 μM arachidonic acid. One h latter, the reactions were terminated by the addition of 2 volumes of methanol. After centrifugation at 12,000 g for 10 min, half of the supernatants were analyzed for LTC4 by reverse phase-high performance liquid chromatography [29]. The other half were dried under reduced pressure using a roto-evaporator, resuspended in ELISA buffer, and evaluated for their PGD2 content using an ELISA kit (Cayman Chemical).
Rat, mouse, and human HPGDS catalyze the conversion of prostaglandin H2 to PGD2 [30] in heparin-expressing mammalian MCs [15]. We queried the EST database to isolate its putative C. intestinalis ortholog (CiHPGDS). The nucleotide sequences of the C. intestinalis ESTs BW487468 and BW062636 revealed significant homology to the respective 5′ and 3′ ends of the human HPGDS transcript noted at GenBank accession NP_055300. The nucleotide sequences that encode the entire coding regions of allelic isoforms of CiHPGDS were next determined using a test cell-enriched mRNA preparation and primers that were based on the above C. intestinalis ESTs. To that end, total RNA (1 μg) from test cells was reverse transcribed into cDNAs using SuperScript II reverse transcriptase (Life Technologies) and random hexamers. The CiHPGDS cDNAs were amplified using high fidelity Pfu polymerase (Stratagene). The forward and reverse primers used were 5′-ATGCCAGTTTACAAGTTATACTACTTC-3′ and 5′-TTACATATTTGTCTTTGGTCTTGTGG-3′, respectively. The polymerase-chain reaction (PCR) cycling condition consisted of 35 cycles of denaturation (95°C, 15 sec), annealing (55°C, 15 sec), and extension (70°C, 1 min). The resulting PCR products (~600 bp) were separated on 1% agarose gels, purified, and inserted in the pCR2.1 TOPO cloning vector (Life Technologies). The entire inserts from multiple independent clones were subjected to DNA sequencing.
3. Results and discussion
3.1. Histochemistry and ultrastructure of the test cells C. intestinalis test cells, and biochemical characterization of their granular heparin•protease complexes
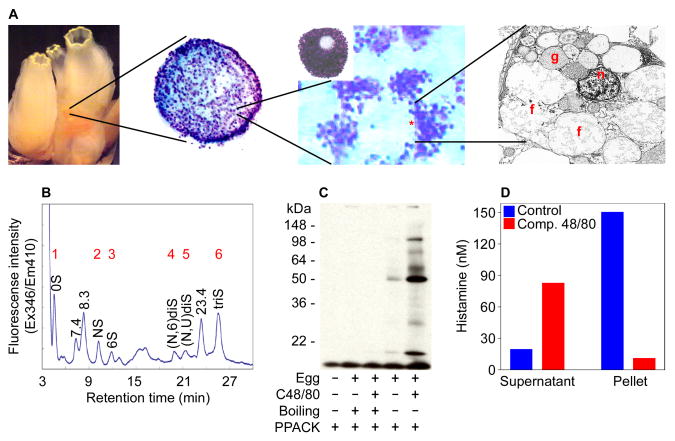
All mammalian MCs possess non-segmented nuclei [23] and become metachromatic when stained with toluidine blue [31]. We discovered that the test cells in C. intestinalis histochemically and morphologically resembled the MCs that reside in the skin and other connective tissues of mammals in that they contained non-segmented nuclei and electron-dense granules that became metachromatic when stained with toluidine blue (Fig. 1A).
Fig. 1.
The test cells that surround the oocyte in C. intestinalis resemble the MCs that reside in the skin and other connective tissues of humans and mice. (A) Depicted are increasing magnifications of C. intestinalis test cells. The cells in the middle panels were stained with toluidine blue. The circled area corresponds to a test cell. At the electron microscopic level, these cells contained a non-segmented nucleus (n) and electron-dense granules (g). When activated, many of these intracellular granules fused (f) before their contents were exocytosed analogous to activated mammalian MCs. (B) High performance liquid chromatography was carried out on the disaccharides obtained when purified test cell-derived heparin was incubated with heparitinases I–III. Peaks 1 to 6 correspond to the 0S, NS, 6S, (N,6)diS, (N,U)diS, and triS uronic acid-glucosamine disaccharides, respectively, based on their elution positions relative to that of known standards. The peak at 23.4 min also is present in a heparitinase digest of porcine heparin. Although its structure has not yet been deduced, almost certainly it is a disulfated disaccharide. The unknown peaks at 7.4 and 8.3 min most likely are monosulfated disaccharides. (C–D) Compound 48/80-treated test cell-enriched oocytes also exocytose enzymatically active serine proteases (C) and histamine (D).
The T-cell-independent polarized subset of MCs that constitutively reside in the peritoneal cavity, skin, and other connective tissues of mice preferentially store heparin in their granules [32,33]. S. plicata contains a heparin glycosaminoglycan that structurally resembles that in the latter population of mammalian MCs [22]. In this urochordate, heparin is released upon cellular activation. We discovered that the test cells in C. intestinalis also contain classical heparin (Fig. 1B).
3.2. Release of enzymatically active serine proteases from activated C. intestinalis test cells
Heparin is essential for the packaging of tetramer-forming tryptases and other serine proteases inside the secretory granules of cutaneous mouse and human MCs [33,34], and the exocytosed heparin•tryptase complexes are essential for combating Klebsiella pneumonia [3] and Trichinella spiralis [5]. Mammalian MCs are heterogeneous and compound 48/80 is a potent secretagogue of the subset of MCs that reside in the peritoneal cavity, skin, and connective tissues of mice due to the drug’s ability to bind to varied members of the MAS family of G-protein coupled receptors [35,36]. C. intestinalis contain a large repertoire of genes (~170) that encode various classes of the G-protein coupled receptors [37]. When our enriched preparations of C. intestinalis test cells were exposed to compound 48/80, the cell’s granules quickly fused intracellularly. Their contents were then released en mass into the extracellular environment.
As occurs in activated mammalian MCs, we detected substantial amounts of enzymatically active serine proteases in the resulting supernatants of the compound 48/80-treated cells using PPACK (Fig. 1C). hTryptase-β [12] and mMCP-6 [13] are tetramer-forming tryptases stored in abundance in the secretory granules of human and mouse MCs, respectively, ionically bound to heparin [33]. Through genome sequence comparison, we identified numerous C. intestinalis genes and transcripts that encode proteins that are 32–38% identical to mMCP-6 and hTryptase-β (e.g., XP_002130273, XP_002120017, XP_002126930, and XP_002129039), suggesting that one or more of these tryptic-like serine proteases resides in the heparin-rich secretory granules of the sea squirt’s test cells.
3.3. Release of histamine from activated C. intestinalis test cells, and cloning of the putative C. intestinalis ortholog of HDC
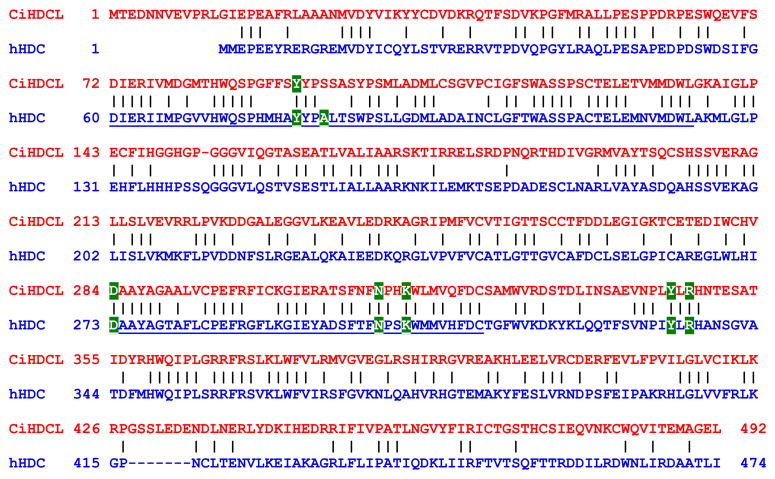
While mammalian MCs also store substantial amounts of histamine in their secretory granules, Dehal and coworkers failed to identify a histidine decarboxylase-like (CiHDCL) gene in their initial draft of the C. intestinalis genome [38]. While these unanticipated data led to the tentative conclusion that sea squirts lack histamine and therefore no MCs, we discovered that histamine was exocytosed from our compound 48/80-treated test cells (Fig. 1D). In support of these data, Cavalcante and coworkers [39] discovered that the corresponding test cells in S. plicata also contained histamine. To resolve these discrepancies, we cloned a novel cDNA that encodes a previously uncharacterized C. intestinalis protein (Fig. 2) that remarkably was ~50% identical to human HDC [27]. The nucleotide sequence of the CiHDC-like cDNA was deposited in GenBank database (accession number EF125183}. We also identified an immunoreactive protein in lysates of the test cell-enriched oocytes of C. intestinalis that cross-reacted with a polyclonal antibody that recognizes mammalian and amphibian HDC (data not shown).
Fig. 2.
Isolation and characterization of a C. intestinalis transcript that encodes a HDC-like protein. A C. intestinalis cDNA was isolated (see GenBank accession number EF125183) that encoded a novel 492-mer protein that was ~50% identical to residues 1-474 of human histidine decarboxylase (hHDC). Additional information on this cDNA (accession number cidg826g09) can be found at http://ghost.zool.kyoto-u.ac.jp/. Shown is a comparison of the C. intestinalis HDC-like protein (CiHDCL) and hHDC. Using an expression/site-directed mutagenesis approach, two regions (corresponding to underlined residues 60-123 and 273-313 in hHDC) and seven amino acids (i.e., Y80, A83, D273, N302, K305, Y334, and R336) have been identified in hHDC that are important for the enzymatic activity of this decarboxylase. CiHDCL possesses those regions as well as the amino acids that are essential for histamine biosynthesis. hHDC is abundant in the gastric mucosa of humans as well as their tissue MCs. The fact that the CiHDCL cDNA was isolated from the sea squirt’s digestive gland is further evidence that it is the likely ortholog of hHDC. Only one CiHDCL EST was present in our library of ~600,000 C. intestinalis ESTs. Thus, CiHDCL is a highly restricted transcript in C. intestinalis as is HDC in humans.
3.4. Release of PGD2 from activated C. intestinalis test cells, and cloning of the putative C. intestinalis ortholog of human HPGDS
Activated rodent heparin+ MCs preferentially metabolize arachidonic acid to PGD2 [15], whereas activated rodent heparin− MCs produce primarily LTC4 [16]. A search of the C. intestinalis genome and EST database revealed HPGDS-like genes, but no LTC4 synthase-like gene. Using primers that reside in the 5′ and 3′ ends of one of these HPGDS-like genes, we isolated full-length CiHPGDS-like transcripts in a test cell-enriched mRNA preparation. Cloning and sequencing of the ~600-bp PCR products revealed three closely related cDNAs (GenBank accession numbers DQ789056, DQ789057, and DQ789058) that encode isoforms of the 197-mer CiHPGDS whose translated products differ only at amino acids 28, 57, 116, and 166. These cDNAs likely represent allelic isoforms of the enzyme since the genome of C. intestinalis is highly polymorphic [40]. At the protein levels, CiHPGDS is 38% and 39% identical to mouse and human HPGDS, respectively. Consistent with our DNA and RNA data, substantial amounts of PGD2 (but not LTC4) were generated in a time-dependent manner when C. intestinalis test cells were given arachidonic acid and calcium ionophore ex vivo (data not shown).
Our morphological, histochemical, biochemical, and molecular biology data suggest that the PGD2 pathway of arachidonic acid metabolism developed in evolution before the LTC4 pathway, that heparin evolved to store histamine and varied enzymatically active serine proteases in the MC’s secretory granules, and that heparin-expressing MCs appeared >500 million years ago before the development of adaptive immunity.
Highlights.
Test cells of Ciona intestinalis resemble human cutaneous mast cells
Activated test cells release histamine and heparin•serine protease complexes
We isolated a Ciona cDNA that encodes a histidine decarboxylase-like protein
Activated test cells produce and release prostaglandin D2
We cloned Ciona cDNAs that encode PGD synthase-like proteins.
Acknowledgments
We thank Yuhui Xu (Brigham and Women’s Hosp.) for electron microscopy and Jay Dimond (Woods Hole Marine Biol. Lab.) for C. intestinalis samples. These studies were supported by grants NIH grants AI54950 and by grants from the Nitto Foundation of Japan and The Mastocytosis Foundation of America.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 2.Huang C, De Sanctis GT, O’Brien PJ, Mizgerd JP, Friend DS, Drazen JM, Brass LF, Stevens RL. Evaluation of the substrate specificity of human mast cell tryptase β1 and demonstration of its importance in bacterial infections of the lung. J Biol Chem. 2001;276:26276–26284. doi: 10.1074/jbc.M102356200. [DOI] [PubMed] [Google Scholar]
- 3.Thakurdas SM, Melicoff E, Sansores-Garcia L, Moreira DC, Petrova Y, Stevens RL, Adachi R. The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J Biol Chem. 2007;282:20809–20815. doi: 10.1074/jbc.M611842200. [DOI] [PubMed] [Google Scholar]
- 4.McNeil HP, Adachi R, Stevens RL. Mast cell-restricted tryptases: structure and function in inflammation and pathogen defense. J Biol Chem. 2007;282:20785–20789. doi: 10.1074/jbc.R700017200. [DOI] [PubMed] [Google Scholar]
- 5.Shin K, Watts GF, Oettgen HC, Friend DS, Pemberton AD, Gurish MF, Lee DM. Mouse mast cell tryptase mMCP-6 is a critical link between adaptive and innate immunity in the chronic phase of Trichinella spiralis infection. J Immunol. 2008;180:4885–4891. doi: 10.4049/jimmunol.180.7.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Li L, Wadley R, Reddel SW, Qi JC, Archis C, Collins A, Clark E, Cooley M, Kouts S, Naif HM, Alali M, Cunningham A, Wong GW, Stevens RL, Krilis SA. Mast cells/basophils in the peripheral blood of allergic individuals who are HIV-1 susceptible due to their surface expression of CD4 and the chemokine receptors CCR3, CCR5, and CXCR4. Blood. 2001;97:3484–3490. doi: 10.1182/blood.v97.11.3484. [DOI] [PubMed] [Google Scholar]
- 7.Bannert N, Farzan M, Friend DS, Ochi H, Price KS, Sodroski J, Boyce JA. Human mast cell progenitors can be infected by macrophagetropic human immunodeficiency virus type 1 and retain virus with maturation in vitro. J Virol. 2001;75:10808–10814. doi: 10.1128/JVI.75.22.10808-10814.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irani AM, Craig SS, DeBlois G, Elson CO, Schechter NM, Schwartz LB. Deficiency of the tryptase+, chymase− mast cell type in gastrointestinal mucosa of patients with defective T lymphocyte function. J Immunol. 1987;138:4381–4386. [PubMed] [Google Scholar]
- 9.Riley JF, West GB. The presence of histamine in tissue mast cells. J Physiol. 1953;120:528–537. doi: 10.1113/jphysiol.1953.sp004915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yurt RW, Leid RW, Spragg J, Austen KF. Immunologic release of heparin from purified rat peritoneal mast cells. J Immunol. 1977;118:1201–1207. [PubMed] [Google Scholar]
- 11.Lagunoff D, Benditt EP. Proteolytic enzymes of mast cells. Ann NY Acad Sci. 1963;103:185–198. doi: 10.1111/j.1749-6632.1963.tb53698.x. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz LB, Lewis RA, Austen KF. Tryptase from human pulmonary mast cells: purification and characterization. J Biol Chem. 1981;256:11939–11943. [PubMed] [Google Scholar]
- 13.Reynolds DS, Gurley DS, Austen KF, Serafin WE. Cloning of the cDNA and gene of mouse mast cell protease 6: transcription by progenitor mast cells and mast cells of the connective tissue subclass. J Biol Chem. 1991;266:3847–3853. [PubMed] [Google Scholar]
- 14.Stevens RL, Adachi R. Protease-proteoglycan complexes of mouse and human mast cells and importance of their β-tryptase-heparin complexes in inflammation and innate immunity. Immunol Rev. 2007;217:155–167. doi: 10.1111/j.1600-065X.2007.00525.x. [DOI] [PubMed] [Google Scholar]
- 15.Lewis RA, Soter NA, Diamond PT, Austen KF, Oates JA, Roberts LJ. Prostaglandin D2 generation after activation of rat and human mast cells with anti-IgE. J Immunol. 1982;129:1627–1631. [PubMed] [Google Scholar]
- 16.Heavey DJ, Ernst PB, Stevens RL, Befus AD, Bienenstock J, Austen KF. Generation of leukotriene C4, leukotriene B4, and prostaglandin D2 by immunologically activated rat intestinal mucosa mast cells. J Immunol. 1988;140:1953–1957. [PubMed] [Google Scholar]
- 17.Dobson JT, Seibert J, Teh EM, Da’as S, Fraser RB, Paw BH, Lin TJ, Berman JN. Carboxypeptidase A5 identifies a novel mast cell lineage in the zebrafish providing new insight into mast cell fate determination. Blood. 2008;112:2969–2972. doi: 10.1182/blood-2008-03-145011. [DOI] [PubMed] [Google Scholar]
- 18.Canestro C, Bassham S, Postlethwait JH. Seeing chordate evolution through the Ciona genome sequence. Genome Biol. 2003;4:208.1–208.5. doi: 10.1186/gb-2003-4-3-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens RL, Fox CC, Lichtenstein LM, Austen KF. Identification of chondroitin sulfate E proteoglycans and heparin proteoglycans in the secretory granules of human lung mast cells. Proc Natl Acad Sci USA. 1988;85:2284–2287. doi: 10.1073/pnas.85.7.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Habuchi H, Nagai N, Sugaya N, Atsumi F, Stevens RL, Kimata K. Mice deficient in heparan sulfate 6-O-sulfotransferase-1 exhibit defective heparan sulfate biosynthesis, abnormal placentation, and late embryonic lethality. J Biol Chem. 2007;282:15578–15588. doi: 10.1074/jbc.M607434200. [DOI] [PubMed] [Google Scholar]
- 21.Paton WD. Compound 48/80: a potent histamine liberator. Br J Pharmacol Chemother. 1951;6:499–508. doi: 10.1111/j.1476-5381.1951.tb00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavalcante MC, Allodi S, Valente AP, Straus AH, Takahashi HK, Mourao PA, Pavao MS. Occurrence of heparin in the invertebrate Styela plicata (Tunicata) is restricted to cell layers facing the outside environment; an ancient role in defense? J Biol Chem. 2000;275:36189–6. doi: 10.1074/jbc.M005830200. [DOI] [PubMed] [Google Scholar]
- 23.Enerbäck L, Lundin PM. Ultrastructure of mucosal mast cells in normal and compound 48/80-treated rats. Cell Tissue Res. 1974;150:95–105. doi: 10.1007/BF00220383. [DOI] [PubMed] [Google Scholar]
- 24.Friend DS, Ghildyal N, Austen KF, Gurish MF, Matsumoto R, Stevens RL. Mast cells that reside at different locations in the jejunum of mice infected with Trichinella spiralis exhibit sequential changes in their granule ultrastructure and chymase phenotype. J Cell Biol. 1996;135:279–290. doi: 10.1083/jcb.135.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harmon JT, Jamieson GA. Activation of platelets by alpha-thrombin is a receptor-mediated event. D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone-thrombin, but not N alpha-tosyl-L-lysine chloromethyl ketone-thrombin, binds to the high affinity thrombin receptor. J Biol Chem. 1986;261:15928–15933. [PubMed] [Google Scholar]
- 26.McHowat J, Corr PB. Thrombin-induced release of lysophosphatidylcholine from endothelial cells. J Biol Chem. 1993;268:15605–15610. [PubMed] [Google Scholar]
- 27.Yamauchi K, Sato R, Tanno Y, Ohkawara Y, Maeyama K, Watanabe T, Satoh K, Yoshizawa M, Shibahara S, Takishima T. Nucleotide sequence of the cDNA encoding L-histidine decarboxylase derived from human basophilic leukemia cell line, KU-812-F. Nucleic Acids Res. 1990;18:5891. doi: 10.1093/nar/18.19.5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satou Y, Yamada L, Mochizuki Y, Takatori N, Kawashima T, Sasaki A, Hamaguchi M, Awazu S, Yagi K, Sasakura Y, Nakayama A, Ishikawa H, Inaba K, Satoh N. A cDNA resource from the basal chordate Ciona intestinalis. Genesis. 2002;33:153–154. doi: 10.1002/gene.10119. [DOI] [PubMed] [Google Scholar]
- 29.Lam BK, Penrose JF, Freeman GJ, Austen KF. Expression cloning of a cDNA for human leukotriene C4 synthase, an integral membrane protein conjugating reduced glutathione to leukotriene A4. Proc Natl Acad Sci USA. 1994;91:7663–7667. doi: 10.1073/pnas.91.16.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanaoka Y, Ago H, Inagaki E, Nanayama T, Miyano M, Kikuno R, Fujii Y, Eguchi N, Toh H, Urade Y, Hayaishi O. Cloning and crystal structure of hematopoietic prostaglandin D synthase. Cell. 1997;90:1085–1095. doi: 10.1016/s0092-8674(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 31.Ehrlich P. Doctoral thesis. University of Leipzig; Germany: 1878. Beitrage zur Theorie und Praxis der Histologischen Farbung. [Google Scholar]
- 32.Yurt RW, Leid RW, Jr, Austen KF. Native heparin from rat peritoneal mast cells. J Biol Chem. 1977;252:518–521. [PubMed] [Google Scholar]
- 33.Humphries DE, Wong GW, Friend DS, Gurish MF, Qiu WT, Huang C, Sharpe AH, Stevens RL. Heparin is essential for the storage of specific granule proteases in mast cells. Nature. 1999;400:769–772. doi: 10.1038/23481. [DOI] [PubMed] [Google Scholar]
- 34.Forsberg E, Pejler G, Ringvall M, Lunderius C, Tomasini-Johansson B, Kusche-Gullberg M, Eriksson I, Ledin J, Hellman L, Kjellén L. Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature. 1999;400:773–776. doi: 10.1038/23488. [DOI] [PubMed] [Google Scholar]
- 35.Tatemoto K, Nozaki Y, Tsuda R, Konno S, Tomura K, Furuno M, Ogasawara H, Edamura K, Takagi H, Iwamura H, Noguchi M, Naito T. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem Biophys Res Commun. 2006;349:1322–1328. doi: 10.1016/j.bbrc.2006.08.177. [DOI] [PubMed] [Google Scholar]
- 36.Kashem SW, Subramanian H, Collington SJ, Magotti P, Lambris JD, Ali H. G protein coupled receptor specificity for C3a and compound 48/80-induced degranulation in human mast cells: roles of Mas-related genes MrgX1 and MrgX2. Eur J Pharmacol. 2011;668:299–304. doi: 10.1016/j.ejphar.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamesh N, Aradhyam GK, Manoj N. The repertoire of G protein-coupled receptors in the sea squirt Ciona intestinalis. BMC Evol Biol. 2008;8:129. doi: 10.1186/1471-2148-8-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dehal P, Satou Y, Campbell RK, Chapman J, Degnan B, De Tomaso A, Davidson B, Di Gregorio A, Gelpke M, Goodstein DM, Harafuji N, Hastings KE, Ho I, Hotta K, Huang W, Kawashima T, Lemaire P, Martinez D, Meinertzhagen IA, Necula S, Nonaka M, Putnam N, Rash S, Saiga H, Satake M, Terry A, Yamada L, Wang HG, Awazu S, Azumi K, Boore J, Branno M, Chin-Bow S, DeSantis R, Doyle S, Francino P, Keys DN, Haga S, Hayashi H, Hino K, Imai KS, Inaba K, Kano S, Kobayashi K, Kobayashi M, Lee BI, Makabe KW, Manohar C, Matassi G, Medina M, Mochizuki Y, Mount S, Morishita T, Miura S, Nakayama A, Nishizaka S, Nomoto H, Ohta F, Oishi K, Rigoutsos I, Sano M, Sasaki A, Sasakura Y, Shoguchi E, Shin-i T, Spagnuolo A, Stainier D, Suzuki MM, Tassy O, Takatori N, Tokuoka M, Yagi K, Yoshizaki F, Wada S, Zhang C, Hyatt PD, Larimer F, Detter C, Doggett N, Glavina T, Hawkins T, Richardson P, Lucas S, Kohara Y, Levine M, Satoh N, Rokhsar DS. The draft genome of Ciona intestinalis: insights into chordate and vertebrate origins. Science. 2002;298:2157–2167. doi: 10.1126/science.1080049. [DOI] [PubMed] [Google Scholar]
- 39.Cavalcante MC, de Andrade LR, Du BS-P, Straus AH, Takahashi HK, Allodi S, Pavao MS. Colocalization of heparin and histamine in the intracellular granules of test cells from the invertebrate Styela plicata (Chordata-Tunicata) J Struct Biol. 2002;137:313–321. doi: 10.1016/s1047-8477(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 40.Satou Y, Shin-i T, Kohara Y, Satoh N, Chiba S. A genomic overview of short genetic variations in a basal chordate, Ciona intestinalis. BMC Genomics. 2012;13:208. doi: 10.1186/1471-2164-13-208. [DOI] [PMC free article] [PubMed] [Google Scholar]