Abstract
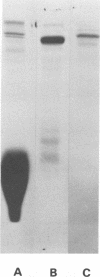
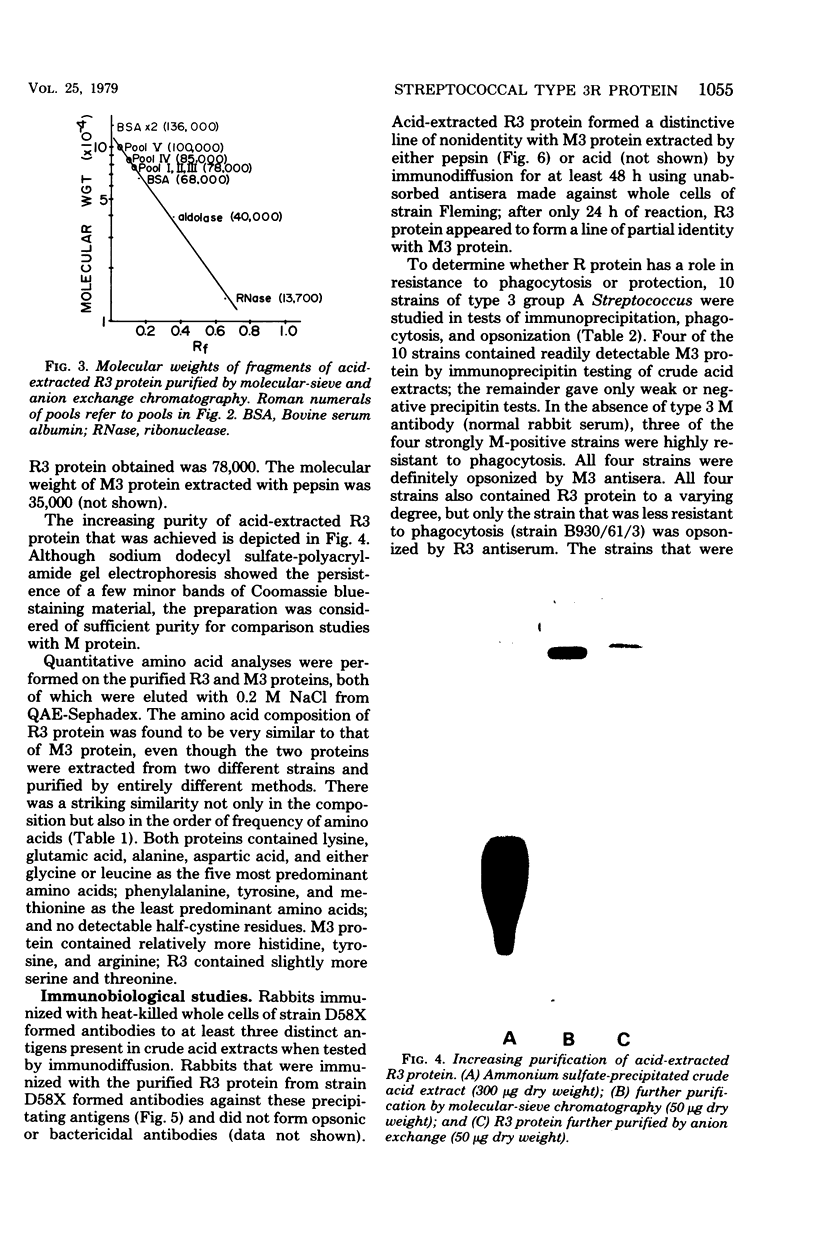
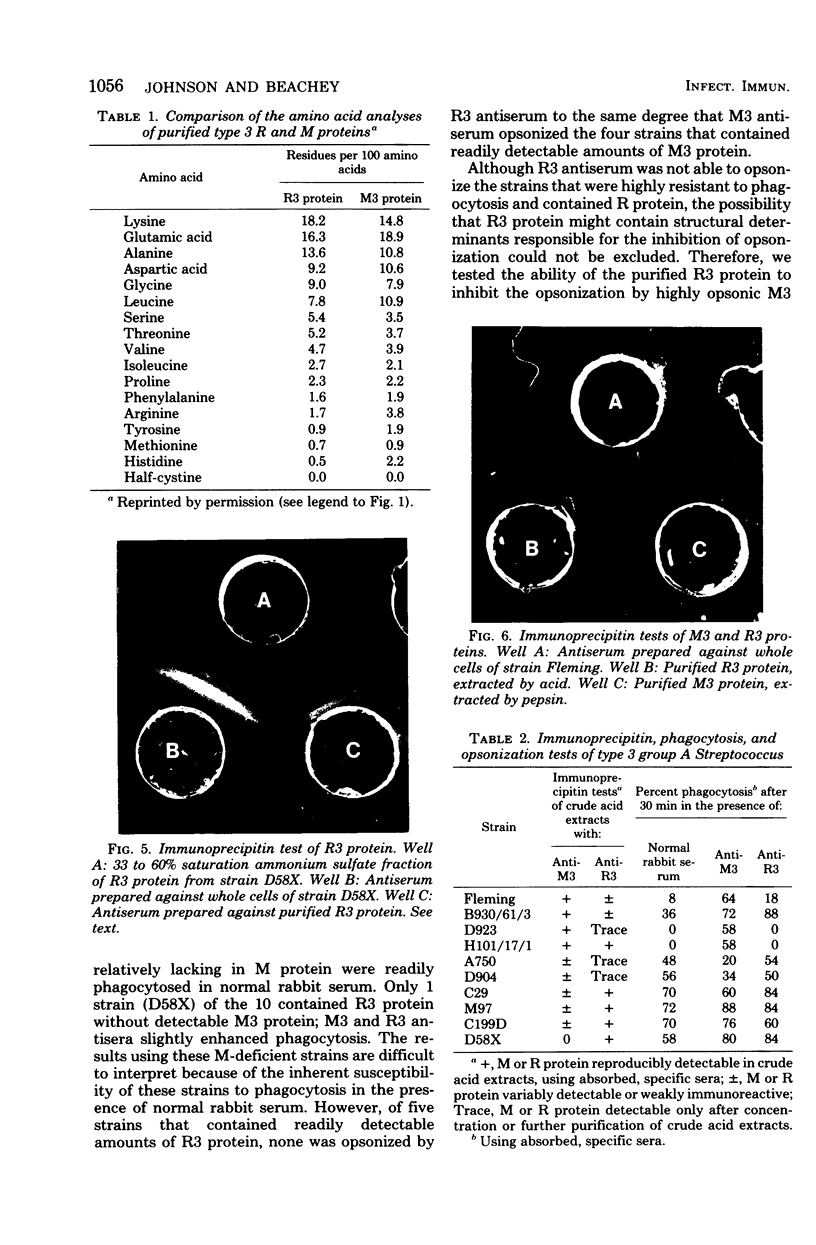
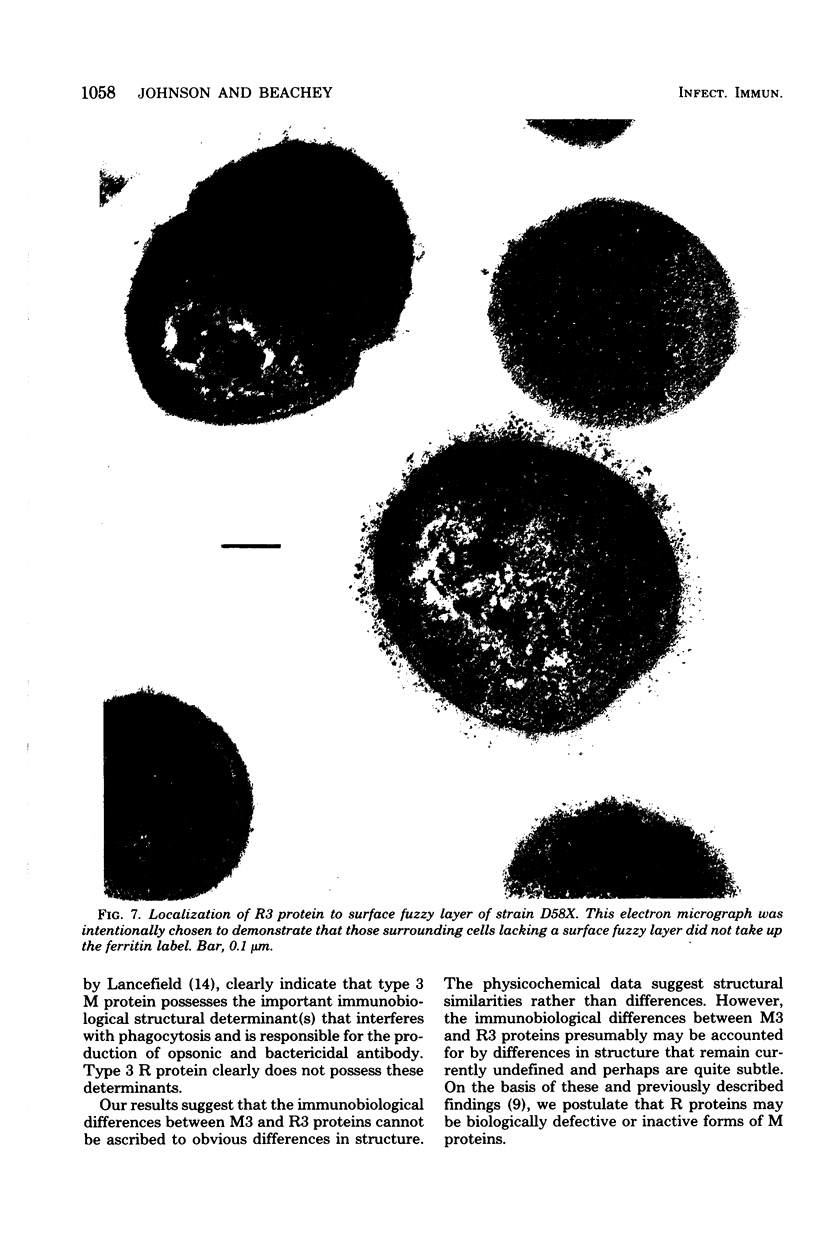
R protein was extracted from type 3 group A streptococci with hot (95°C) HCl and was purified by ammonium sulfate precipitation followed by molecular-sieve and ion exchange chromatography. Although the R3 antigen was present in a heterogeneous population of proteins ranging from 78,000 to 100,000 daltons in size, we were able to separate an R-rich fraction that contained minimal amounts of heterogeneous proteins as indicated by electrophoresis in sodium dodecyl sulfate-polyacrylamide gels. The final yield of the purified R protein was approximately 15 μg (dry weight) per g (wet weight) of washed and sedimented streptococci. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis indicated a molecular size of approximately 78,000 daltons. Amino acid analysis showed lysine, glutamic acid, alanine, and aspartic acid as the predominant amino acids. A detailed comparison of the purified R3 protein with type 3 M protein indicated a similarity in composition and order of frequency of amino acids. However, the R3 antigen was found to be distinctive from the M3 antigen in agar gel diffusion tests. In addition, R3 and M3 proteins behaved differently in opsonophagocytosis tests and opsonization inhibition tests. Thus, R3 and M3 proteins produced precipitin lines of nonidentity with an unabsorbed antiserum against whole type 3 streptococci: M3-specific antiserum, but not R3-specific antiserum, enhanced the phagocytosis of type 3 streptococci. Purified M3 but not R3 protein was capable of inhibiting the type-specific opsonization of type 3 streptococci. The physicochemical resemblance between M and R proteins in general suggests a common genetic origin. Perhaps R proteins are variant forms of M proteins from which the antiopsonic determinant has been deleted.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H., Cunningham M. Type-specific inhibition of preopsonization versus immunoprecipitation by Streptococcal M proteins. Infect Immun. 1973 Jul;8(1):19–24. doi: 10.1128/iai.8.1.19-24.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Seyer J. M., Kang A. H. Repeating covalent structure of streptococcal M protein. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3163–3167. doi: 10.1073/pnas.75.7.3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Stollerman G. H., Chiang E. Y., Chiang T. M., Seyer J. M., Kang A. H. Purification and properties of M protein extracted from group A streptococci with pepsin: covalent structure of the amino terminal region of type 24 M antigen. J Exp Med. 1977 Jun 1;145(6):1469–1483. doi: 10.1084/jem.145.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Stollerman G. H. Toxic effects of streptococcal M protein on platelets and polymorphonuclear leukocytes in human blood. J Exp Med. 1971 Aug 1;134(2):351–365. doi: 10.1084/jem.134.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M., Beachey E. H. Immunochemical properties of streptococcal M protein purified by isoelectric focusing. J Immunol. 1975 Oct;115(4):1002–1006. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A., Gotschlich E. C., Siviglia G., Zabriskie J. B. Streptococcal M protein extracted by nonionic detergent. I. Properties of the antiphagocytic and type-specific molecules. J Exp Med. 1976 Jul 1;144(1):32–53. doi: 10.1084/jem.144.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E. N. M proteins of group A streptococci. Bacteriol Rev. 1974 Mar;38(1):57–86. doi: 10.1128/br.38.1.57-86.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. H. Characterization of group A streptococcal R-28 antigen purified by hydroxyapatite column chromatography. Infect Immun. 1975 Oct;12(4):901–909. doi: 10.1128/iai.12.4.901-909.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. H., Vosti K. L. Purification and characterization of group A streptococcal T-1 antigen. Infect Immun. 1977 Jun;16(3):867–875. doi: 10.1128/iai.16.3.867-875.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. H., Vosti K. L. Purification of two fragments of M protein from a strain of group A, type 12 streptococcus. J Immunol. 1968 Sep;101(3):381–391. [PubMed] [Google Scholar]
- Kang A. H. Studies on the location of intermolecular cross-links in collagen. Isolation of a CNBr peptide containing -hydroxylysinonorleucine. Biochemistry. 1972 May 9;11(10):1828–1835. doi: 10.1021/bi00760a015. [DOI] [PubMed] [Google Scholar]
- LANCEFIELD R. C. Differentiation of group A streptococci with a common R antigen into three serological types, with special reference to the bactericidal test. J Exp Med. 1957 Oct 1;106(4):525–544. doi: 10.1084/jem.106.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANCEFIELD R. C. Occurrence of R antigen specific for Group A type 3 streptococci. J Exp Med. 1958 Sep 1;108(3):329–341. doi: 10.1084/jem.108.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANCEFIELD R. C., PERLMANN G. E. Preparation and properties of a protein (R antigen) occurring in streptococci of group A, type 28 and in certain streptococci of other serological groups. J Exp Med. 1952 Jul;96(1):83–97. doi: 10.1084/jem.96.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa S., Yaegashi T., Nakayama Y. Existence of 28R-antigen in a certain strain of group F streptococcus. Microbiol Immunol. 1978;22(5):263–267. doi: 10.1111/j.1348-0421.1978.tb00371.x. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Antigen-antibody reactions in gels. IV. Types of reactions in coordinated systems of diffusion. Acta Pathol Microbiol Scand. 1953;32(2):230–240. [PubMed] [Google Scholar]
- Vosti K. L., Johnson R. H., Dillon M. F. Further characterization of purified fractions of M protein from a strain of group A, type 12 Streptococcus. J Immunol. 1971 Jul;107(1):104–114. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wilkinson H. W. Comparison of streptococcal R antigens. Appl Microbiol. 1972 Oct;24(4):669–670. doi: 10.1128/am.24.4.669-670.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]