Abstract
Although microglia isolation from embryonic or postnatal mouse brain is possible using a number of different protocols, microglia isolation from adult brain is more challenging and often results in low yields. Here, we describe a protocol to isolate intact microglia from adult mouse brain for functional assays, immunocytochemistry and/or flow cytometry analysis. This protocol involves enzymatic dissociation in medium supplemented with dispase II, papain and DNase I followed by mechanical dissociation. Cell separation is achieved via percoll gradients of various densities. Microglia isolated using this protocol is suitable for flow cytometry analysis, RNA isolation for gene expression by real-time PCR or microarrays, and for functional assays including cytokine production, chemotaxis and phagocytosis.
1. Introduction
Microglia, the myeloid-derived resident macrophages of the brain, play the primary role of immune surveillance and respond to environmental stress and immunological challenges (1, 2). Initial physical or pathogenic events in the CNS can trigger microglial expansion through recruitment of peripheral macrophages to the CNS, as a result of increased permeability of the BBB, differentiation from progenitor cells or proliferation of residual microglia (3). Acute activation of microglia often results in secretion of neurotrophic factors such as glial-derived neurotrophic factor family ligands (GFLs) that limit tissue injury by protecting vulnerable populations of neurons and aiding in repair processes (4, 5). However, activated microglia can also overproduce prostaglandins, chemokines, cytokines, and reactive oxygen and nitrogen species (ROS and RNS) including nitric oxide (NO), which can have a deleterious effect on neuronal survival by enhancing oxidative stress and activating cell death pathways (6). If glial activation persists for extended periods, a cycle of chronic neuroinflammation ensues and contributes to tissue damage. This protocol describes a step-wise method (modified from one previously described (7) to isolate intact microglia from adult mouse brain. Microglia isolated using this method are suitable for in vitro studies aimed at understanding the mechanisms that regulate the balance of neuroprotective and neurotoxic microglial activities.
2. Materials
2.1 Reagents
PBS-perfused brains from adult mice (age > 8 weeks)
Hank's balanced salt solution (HBSS) without calcium and magnesium (Invitrogen, cat. No. 14175-095)
10× HBSS (Sigma Aldrich, cat. no. H4641)
DNase I (final concentration; 20 U/mL, Invitrogen,)
Dispase II (final concentration; 1.2 U/mL, Roche)
Papain (1 mg/mL, Sigma-Aldrich)
DMEM/F12
100× Pen/Strep (Sigma-Aldrich)
Percoll (Sigma-Aldrich, cat. no. P4937)
3. Media and Solutions
-
Dissection Media (50 mL)
49.5 mL of HBSS plus 0.5 mL of Pen/Strep (100×)
Sterile filter all solutions after mixing/before use.
-
Glucose
Prepare 0.45 g/mL in PBS (100X)
Add 500 μL in 50 mL reagents to make final concentration of 4,500 mg/L.
-
Serum Free Media (50 mL)
49 mL of DMEM/F12 plus 0.5 mL of Pen/Strep (100×) and 0.5 mL of Glucose (100×)
Sterile filter all solutions after mixing/before use.
-
Neutralization Media (50 mL)
44 mL of DMEM/F12 plus 0.5 mL of Pen/Strep (100×), 0.5 mL of Glucose (100×) and 5 mL of heat inactivated FBS (or FCS)
Sterile filter all solutions after mixing/before use.
-
Culture Media (50 mL)
44.5 mL of DMEM/F12 plus 0.5 mL of Pen/Strep (100×), and 5 mL of heat inactivated FBS (or FCS)
Sterile filter all solutions after mixing/before use.
-
Dispase II
Dissolve the non-sterile lyophilized enzyme in HEPES-buffered saline (50 mM HEPES/KOH pH 7.4, 150 mM NaCl) (10 mg/mL). Dilute further with the culture medium to be used for the isolated cells at the final concentration of 2.4 U/mL. Concentrations higher than 2.4 U/mL are not recommended. Sterilize through a 0.22 μm filter membrane.
-
Dissociation Medium
-
Prepare Dispase DNase Papain (DDP) solution
0.028 g Papain (final concentration 1 mg/mL)
14 mL of DMEM/F12
14 mL premade dispase II (final concentration 1.2 U/mL)
Aliquot in -20 °C.
Add DNAse I (final concentration 20 U/mL) right before use.
-
Percoll Preparation
Prepare stock isotonic percoll (SIP); Mix nine parts of percoll with one part 10× HBSS.
70 % percoll; 7.0 mL of SIP plus 2.0 ml of 1×HBSS.
37 % percoll: 3.7 mL of SIP plus 5.3 ml of 1×HBSS.
30 % percoll: 3.0 mL of SIP plus 6.0 ml of 1×HBSS.
3. Methods
Place the freshly perfused adult brain in 2 mL serum free media into the 35mm dish and mince the freshly perfused adult brain as finely as possible using the 15 T scalpel blade. Make sure the amount of serum free media is equivalent to the amount of dissociation medium.
Transfer minced brain to 15 mL tube containing 3 mL of dissociation medium.
Gently rock (or invert the tube every 5 min) cell suspension in tissue culture incubator for 20 min.
Neutralize the enzymes in dissociation media by adding 5 mL of neutralization medi.
Centrifuge 5 minutes at 250×g in room temperature.
Aspirate the media slowly (be extremely cautious not to disturb the pellet as it can easily be aspirated by the aspirating pipet).
Resuspend the pellet in 5 mL of serum free media. Repeat steps 5 and 6.
Add 3 mL DMEM/F12 and pipette up and down with polished large size-hole Pasteur pipette against bottom of tube until large clumps of tissue are broken up. Let it sit for 1 min until large clumps settle. Transfer upper part (containing dissociated cells) into new 15 mL conical tube and keep it on ice.
Add 3 mL of DMEM/F12 and pipette up and down with polished Pasteur pipette (medium-size hole) against bottom of tube until large clumps of tissue are broken up. Let it sit for 1-2 min until large clumps settle. Transfer upper part into collecting tube with previous cell suspension and keep it on ice.
Add 2 mL DMEM/F12 and pipette up and down with polished Pasteur pipette (small size-hole) to break clumps. Let it sit for 1 min until large clumps settle.
Combine upper part (containing dissociated cells) into previous cell suspension.
Wet 40 μm cell strainer with 2ml of DMEM/F12 and then filter cell suspension through cell strainer.
Spin at 250×g for 4 min.
Resuspend cells in 5 mL DMEM/F12, spin 250×g for 4 min and remove supernatant.
Resuspend cell pellet in 4 mL per brain of 37% SIP.
Transfer 4 mL of the 37% SIP (from Step 15) to 15 mL conical tubes and slowly underlay 4 mL of 70% SIP (see Note 1). Then on top of the 37% layer slowly pipette 4 mL of 30% SIP, followed by 2 mL of HBSS(Fig. 1).
-
Centrifuge gradient 40 min at 300×g (18 °C) with no brake.
***important!! Make sure centrifuge will stop with no brake so that the interphase is not disturbed.
Using a transfer pipette, gently remove layer of debris and collect 2.0–2.5 mL of the 70–37% interphase into a clean 15-mL conical tube.
Add 6 mL of HBSS for each 2 mL of interphase volume collected to ensure the percoll containing the interphase is diluted about three times.
Centrifuge 7 min at 500×g at 4 °C.
Resuspend pellet in 500 μL of HBSS and transfer to small 0.6-mL or 1.5-mL tubes and wash 3 times in a volume of 500 μL, using a micro-centrifuge at 800×g at 4 °C.
Count cells using a hemocytometer and seed cells on the culture plate for immunocytochemistry or functional assays (see Note 2, 3 and 4).
Figure 1.
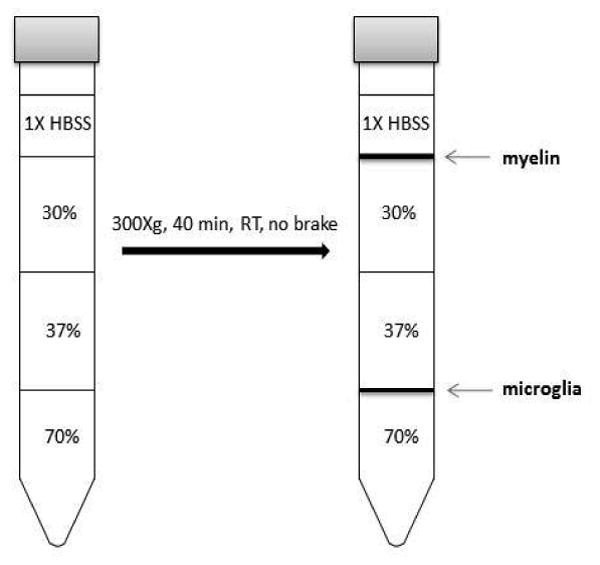
Schematic of the percoll gradient set-up for isolation of adult mouse microglia.
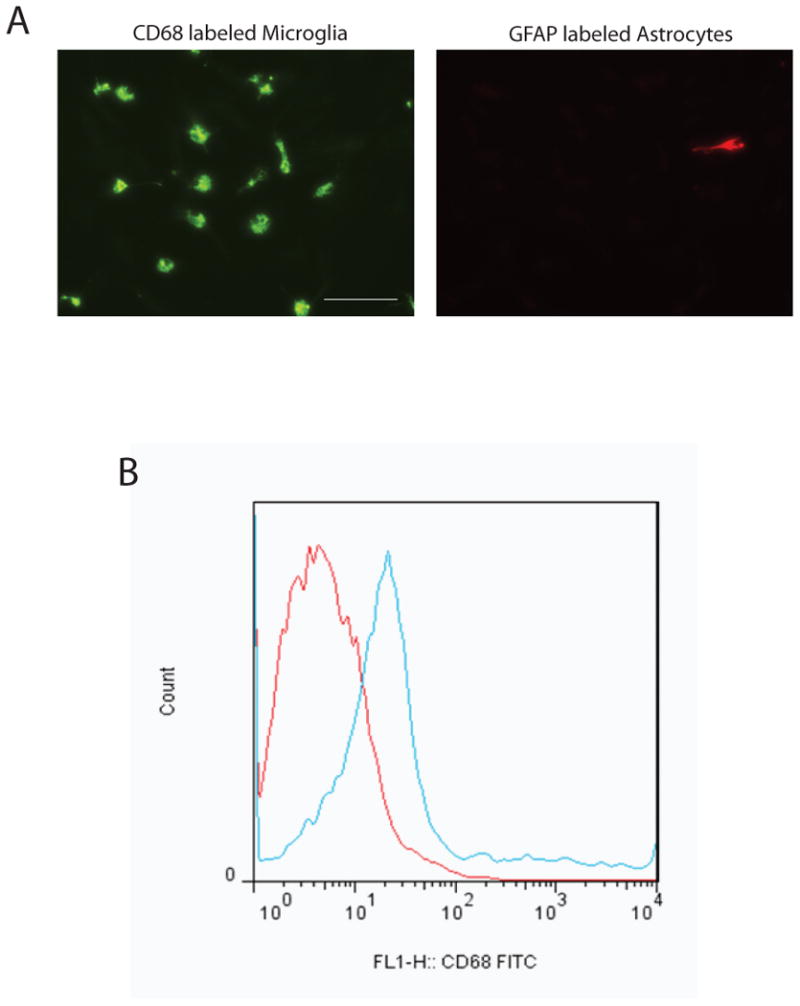
Figure 2. Purity of isolated adult mouse microglia population.
A, Primary microglia cells C57BL/6 mice were plated into a 4-well chamber plate at the density of 8,000 cells/well in growth medium. Twenty-four hours after seeding, cells were fixed with 4% paraformaldehyde for 15 min. Cells were then stained with CD68 (as microglia marker, green) and GFAP (as astrocyte marker, red) antibodies followed by incubating with Alexa fluorophore conjugated secondary antibody (Invitrogen). B, Primary microglia cells were labeled with fluorescently conjugated CD68-FITC antibodies (green), fixed and analyzed by flow cytometry. Unstained microglia (red) were used as negative controls. Histogram plot indicates the presence of a single-labeled population. Scale bar represents 100 μm.
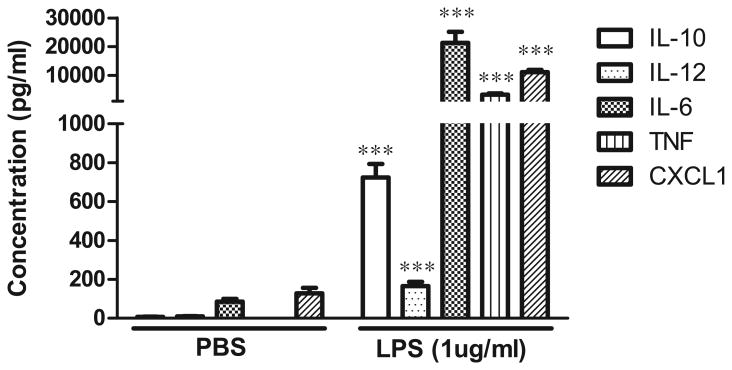
Figure 3. Isolated adult mouse microglia secrete cytokines upon stimulation with LPS.
Primary microglia cells were plated at a density of 3000 cells per well in 96 well plate. Cells were treated with 1 μg/mL of LPS for 18 hrs. Conditioned media was collected and analyzed for inflammatory factor production by multiplexed immunoassay (Meso Scale Discovery). Student t-test was used for statistical analysis. ***denotes significant differences between PBS and LPS treatment at p < 0.001.
Acknowledgments
This work was supported by grant 1R01 NS072467 (MGT) from NIH/NINDS.
Footnotes
Percoll should be kept at room temperature.
Typical yield is from 300,000 to 500,000 microglial cells per mouse brain (see Fig. 2).
All the procedures need to be performed under the hood and reagents needs to be filtered to prevent contamination.
Cells can be maintained in the culture media for functional assays (see Fig. 3).
References
- 1.Puntambekar SS, Doose JM, Carson MJ. In: Central Nervous System Diseases and Inflammation. Lane TE, C M, Bergmann C, Wyss-Coray T, editors. New York: Springer; 2008. pp. 1–12. [Google Scholar]
- 2.Tansey MG, Wyss-Coray T. In: Central Nervous System Diseases and Inflammation. Lane TE, C M, Bergmann C, Wyss-Coray T, editors. New York: Springer; 2008. pp. 59–106. [Google Scholar]
- 3.Bjorkqvist M, Wild EJ, Thiele J, Silvestroni A, Andre R, Lahiri N, Raibon E, Lee RV, Benn CL, Soulet D, et al. A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington's disease. J Exp Med. 2008;205(8):1869–77. doi: 10.1084/jem.20080178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease--a double-edged sword. Neuron. 2002;35(3):419–32. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- 5.Marchetti B, Abbracchio MP. To be or not to be (inflamed)--is that the question in anti-inflammatory drug therapy of neurodegenerative disorders? Trends Pharmacol Sci. 2005;26(10):517–25. doi: 10.1016/j.tips.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 6.McGeer PL, McGeer EG. Inflammation and neurodegeneration in Parkinson's disease. Parkinsonism Relat Disord. 2004;10(Suppl 1):S3–7. doi: 10.1016/j.parkreldis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Cardona AE, Huang D, Sasse ME, Ransohoff RM. Isolation of murine microglial cells for RNA analysis or flow cytometry. Nat Protoc. 2006;1(4):1947–51. doi: 10.1038/nprot.2006.327. [DOI] [PubMed] [Google Scholar]