Deep brain stimulation to treat Parkinson’s disease, the use of incubators for premature infants, treatments for asthma, development of drugs to control transplant rejection … these are some of the major medical advances in recent years that have depended on the use of nonhuman primates in biomedical research and testing.
Research involving nonhuman primates (NHPs) has played a vital role in many of the medical and scientific advances of the past century. NHPs are used because of their similarity to humans in physiology, neuroanatomy, reproduction, development, cognition, and social complexity – yet it is these very similarities that make the use of NHPs in biomedical research a considered decision. As primate researchers, we feel an obligation and responsibility to present the facts concerning why primates are used in various areas of biomedical research. Recent decisions in the United States, including the phasing out of chimpanzees in research by the National Institutes of Health and the pending closure of the New England Primate Research Center, illustrate to us the critical importance of conveying why continued research with primates is needed. Here we review key areas in biomedicine where primate models have been, and continue to be, essential for advancing fundamental knowledge in biomedical and biological research.
Phylogenetic context
The vast majority of biomedical research utilizes rodent models. U.S. government statistics indicate that approximately 90% of the animals used in research are mice, rats and other rodents. NHPs account for 0.28% of all laboratory animals used in research [Government Statistics from 2010]. The appropriateness of various animal models depends upon not only the species but also the ways in which the models are used. In particular, the model needs to parallel not only the clinical and biological features but also the behavioral repertoires of interest. While rodents are, and will continue to be, extremely valuable models for biomedical research, rodents do not always accurately model human behavioral and biological response [Seok et al., 2013b]. The evolutionary distance between rodents and humans [human-mouse-rat ancestor ~87 mya; [Springer et al., 2003]] presents significant differences in biological and behavioral function that may limit the immediate translational value of findings.
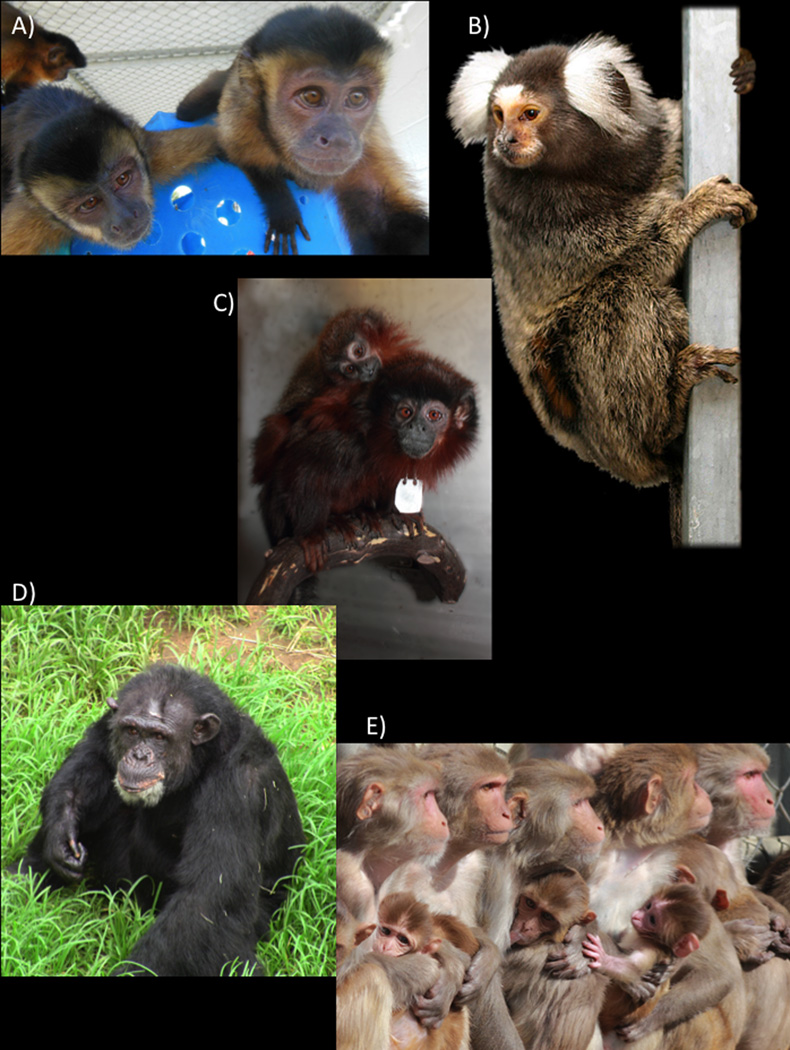
The close phylogenetic relationship of NHPs to humans makes them excellent models for particular biological phenomena. The physiological similarity between humans and NHPs means there is greater validity of the data obtained from primate models than other animal models (e.g. reproduction and pregnancy, cognition and cognitive aging). This physiological similarity also means that one can address questions using NHP models that cannot be addressed using other species (e.g. models of AIDS, lung disorders, and drug metabolism). Old World monkeys (rhesus macaques, baboons, vervet monkeys) and New World monkeys (marmosets, squirrel monkeys, titi monkeys, capuchin monkeys) are used frequently; prosimians and Great Apes (chimpanzees) are also used, though less frequently (see Figure 1). As the NIH recently decided to phase out the use of chimpanzees in most areas of research, chimpanzee models for furthering our understanding in key areas such as autism and Hepatitis C will no longer be available. In cases where NHPs are used, species selection is carefully considered, taking into account behavioral, biological, animal welfare and practical considerations [Group, 2002; Smith et al., 2001].
Figure 1.
Ethical considerations in the use of primates in biomedical research
The ability of all animals to feel pain and experience stress means that researchers have a moral obligation to conduct research in a manner that reduces negative effects and does not unnecessarily cause stress or suffering. The significant cognitive capacity and complex social behavior of NHPs raises additional issues concerning the rationale and justification for their use in biomedical research. The fundamental ethical dilemma concerning the use of primates in biomedical research is whether we can be morally justified in conducting research that benefits humans but which may cause NHPs pain, distress, and/or suffering. The core issues reflect whether NHPs count in our moral considerations, and whether they have moral standing. A being has moral standing if its interests must be given consideration in the deliberations of a moral agent. If an individual has moral standing, then the individual has a valid interest in the moral norm, and they count in a moral sense. Thus, having moral standing restricts the permissible range of conduct toward these beings. Properties that are frequently taken into account when considering questions of moral standing include being sentient, rational, and a self-conscious agent. While NHPs exhibit aspects of rationality and agency, these do not reach the level seen in humans. Sentience is what many regard as the primary trait that gives NHPs moral standing [Bentham, 1907 [1823]; Morris, 2011]. Another feature of the debate concerning the moral standing of NHPs is whether they are direct or indirect moral objects. If NHPs are direct moral objects, then we have direct duties to them. If NHPs are indirect moral objects, then we have duties regarding them but not duties to them [Aquinas, 1955–1957; Kant, 1996 [1797]; Morris, 2011]. A consensus as to the moral standing of NHPs, or whether a distinction between direct and indirect moral objects is warranted, cannot be reached even among philosophers.
However, the growing recognition by researchers, veterinarians and other biomedical professionals that ethical issues such as these are important and relevant to primate research has led to specific changes in the way such research is conducted and regulated. Greater attention is now paid by the various participants in the research enterprise (scientists, veterinarians, and administrators) to the responsibilities that someone is accepting when they choose to engage in research using NHP. This has been an important stimulus leading to concrete, practical, and enforceable changes in the procedures and standards required, including legal restrictions and government policies that are tied to research funding. Fifty years ago, there was little formal oversight on the use of animals in research. While most research was done with appropriate concern for the health and welfare of the animal subjects, this was not always the case and a series of regulations and laws were thus enacted to ensure proper methods and housing conditions. Today, all research using NHPs (and indeed all research on vertebrates) in the United States must adhere to an extensive series of laws and/or regulations. No academic or commercial research using NHPs can be performed unless: a) the researchers have appropriate training in the use of animals, b) there is independent oversight on the procedures to be used, c) the researchers have satisfactorily justified in writing the species to be used, the number of animals to be used and the scientific significance of the data to be generated and d) there is evidence that the work does not unnecessarily duplicate prior research. One of the changes that most clearly reflects the changing attitudes, and also has immediate daily impact on the primates maintained in research colonies, is a series of regulations regarding the psychological well-being of the animals. There are now explicit and enforceable rules that go beyond requiring adequate food, housing and veterinary care to address the cognitive and psychological complexity of NHPs, providing assurance that these issues are given substantive attention. The rules apply whether or not any particular animal is actually being used in a research project1. This reflects the broad acceptance of the idea that researchers and veterinarians have a responsibility to attend to the psychological needs of the animals as well as nutritional and environmental needs.
In all research institutions, investigators must convince a set of independent experts that the work they propose to do is justified and will be performed appropriately. These committees (Institutional Animal Care and Use Committees), which are mandated by Federal law, function separately from the research team and the funding agencies and have the right and obligation to restrict or stop any primate research that the committee considers unnecessary, inappropriately designed or inadequately justified given the effects on the study subjects. These committees must include non-scientists and representatives of the community (lay members), to ensure that community standards for ethics are followed.
Most (> 95%) behavioral and biomedical research with nonhuman primates either does not involve pain, or the pain is alleviated with analgesic or anesthetic drugs2. Pain causes stress, and researchers understand that stressed animals present different biological responses which may affect the results of the study. Animal suffering and use are minimized in line with the 3Rs principle of Russell and Burch [1959]: replacement, reduction, and refinement. Researchers must specifically address the 3Rs before any research project with NHPs is approved, and guidance is available to assist researchers in implementing the 3Rs [Refinement, 2009]. Furthermore, provisions under the Animal Welfare Act3 require needs specific to NHPs be addressed. As such, efforts are made to enhance psychological well-being through social housing, addressing the specific social and development needs of infants and aged individuals, and providing environmental enrichment. The major professional societies whose members use NHPs in research - Association of Primate Veterinarians, American Society of Primatologists, and the Society for Neuroscience - endorse policies and regulations that provide for the enhancement of NHP psychological well-being. At institutions that utilize NHPs in research, considerable ongoing research and evaluation occurs to further improve the welfare of captive NHPs.
Some have argued that human rather than nonhuman primates are the more appropriate, and ethically preferable, subjects for biomedical research (e.g., Quigley, 2007). The logic behind this is that despite the similarities between humans and NHPs, small but significant biological differences exist. Therefore, conclusive results cannot be obtained from NHP models, and so the ethically preferable choice would be to experiment on a limited number of humans. This position may seem intuitive on some level. However, we should be reminded that it is essential for the protection of humans that prior research be conducted on animals. The Nuremberg Code, written as a result of the Nuremberg Trial at the end of World War II, defines a set of research ethics principles for human experimentation and states animal studies must precede research on humans.4 The use of sentient NHPs rather than other animal models certainly requires greater ethical consideration of whether a specific experiment is justified. Justification includes the appropriateness of the primate model (which we illustrate below for specific research areas; see Table 1) as well as potential costs and benefits of the research. As long as we believe that a human life is more valuable than a fish, fly, mouse, or primate, some experiments will be performed on animals before exposing humans to risk. And in some cases, the best animal model will be a NHP.
Table 1.
The major advantages of primate models for the areas of biomedical research covered in this review.
| Area of Research | Advantages of Primate Models |
|---|---|
| Atherosclerosis | Similarities in etiology and characteristics of arterial pathology, including reproductive and central nervous system characteristics that promote or protect against atherosclerosis |
| Behavior | Similarities in social and environmental complexity allows for ethologically relevant inputs to behavioral paradigms for social cognition and psychopathology |
| Cognition and Language | The relatively large brain size in NHPs compared to other mammals makes them invaluable for testing evolutionary models of human cognition |
| Cognitive aging | Greater similarities with humans in brain functional specialization associated with cognitive aging, such as the nuclear organization, projection pathways and innervation patterns of the hippocampus |
| Developmental programming | NHPs (but not rodents) share with the humans an interdependence of the fetal and maternal hypothalamo-pituitary-adrenal axis and their interactions with the placenta |
| Genetics | Share with humans fundamental genetic processes relevant to specific diseases (that other mammalian species lack), such as KLK3, the gene that produces prostate-specific antigen |
| HIV/AIDS | The only animal model for HIV/AIDS; provides the chance to control variables and conditions of infection |
| Immunology | Most similar to humans in regards to the developmental maturation of the immune system |
| Neuroscience | NHP brains closely resemble human brains in several ways, including encephalization, the number and density of cortical neurons, a large prefrontal cortex, and greater myelination; additionally, some functional areas of the NHP and human brain do not exist in the rat |
| Pharmacology | Allow for the ability to track changes in the brain and sensitivity to drugs over time, and study individual differences in these effects medications |
| Reproduction | Share with humans key characteristics of endocrine regulation of reproductive physiology not seen in other mammals that include fundamental differences in hypothalamic feedback, ovarian function, the physiology of the uterus and vagina, the establishment and control of pregnancy and menopause |
| Respiratory diseases | Reflect key features of human lung architecture and immunity |
The scientific summaries provided below for several key areas (though not exhaustive) illustrate that there is a wide range of significant and valuable biomedical information that can only be obtained by using NHPs in research. Thus, while we recognize the responsibility researchers take on themselves when they engage in research using primates, we also recognize and document that NHP research has generated results that have saved many human lives and reduced suffering in many more. The summaries focus almost exclusively on studies performed in captivity; we recognize, however, that important information in some of these topical areas has been obtained from field studies as well [Emery Thompson, 2013; Fedurek and Slocombe, 2011; Tung et al., 2010]
Atherosclerosis
Atherosclerosis of the coronary arteries and its complications are the principal pathological processes that result in coronary heart disease (CHD). Macaques (Macaca spp.) have been a well-established model of diet-induced coronary artery atherosclerosis (CAA) for several decades [Jokinen et al., 1985; Wissler and Vesselinovitch, 1977]. It is likely that the utility of this model is due to their close phylogenetic relationship to human beings which has resulted in similarities in etiology and characteristics of arterial pathology.
For example, macaques develop arterial lesions similar to those seen in human beings. Dietary manipulation results in hyperlipidemia which resembles that found in humans. Also, these species are a convenient size for diagnostic and therapeutic studies. By comparison, rats are generally resistant to atherosclerosis. The lesions they do develop are unlike those of human beings, and severe experimental conditions may be required to produce them. Atherogenic diets may result in high mortality in mice, and produce lesions that are unlike those of humans. However, the relative ease of genetic manipulation and the relatively short time frame for atherogenesis makes them useful for investigation of gene effects [Getz and Reardon, 2012]. Pigs faithfully recapitulate human atherosclerosis but their large body size makes them difficult and expensive to handle and maintain. Atherosclerosis lesions are easily induced by diet in rabbits but the resulting lesions, and cholesterol metabolism in general, are dissimilar to human beings; the latter may result in extreme hypercholesterolemia and lipid storage in organs [Jokinen et al., 1985].
Perhaps the most valuable aspect of macaque models of atherosclerosis is that they provide information on important etiologic factors that promote or protect against atherosclerosis; these factors involve reproductive and central nervous system (CNS) characteristics that are unique to primates. For example, Old World Monkeys and Apes have menstrual cycles similar to those of women. Adult cynomolgus monkeys (Macaca fascicularis) are a well-characterized animal model of sex differences in susceptibility to diet-induced atherogenesis. While females are generally protected compared to males, ovariectomy results in extensive CAA. If estrogen therapy is begun right after ovariectomy, females are protected from CAA [Adams et al., 1990a; Clarkson and Mehaffey, 2009]. Likewise, the results of large observational studies such as the Nurse’s Health Study suggest that hormone therapy initiated around the time of menopause reduces the risk for a major coronary event by about 50% [Grodstein et al., 2000; Stampfer et al., 1985]. However, in monkeys, if estrogen therapy is not initiated until two years after ovariectomy (approximately equivalent to six human years), there is no beneficial effect on atherosclerotic plaque size [Clarkson and Mehaffey, 2009; Register et al., 1998; Williams et al., 1995]. This observation essentially predicted the outcomes of both the Heart and Estrogen/Progestin Replacement Study (HERS) and the Women’s Health Initiative (WHI), in which delayed initiation of estrogen therapy for about 15 years after menopause was associated with no overall cardiovascular benefit [Hulley et al., 1998; Rossouw et al., 2002].
Likewise, intact cynomolgus monkeys with poor ovarian function develop extensive CAA like that of ovariectomized females [Adams et al., 1985]. This is not surprising, because females with low progesterone concentrations in the luteal phase also have low estradiol concentrations in the follicular phase, i.e. they are estrogen-deficient. The effects of premenopausal ovarian dysfunction on CHD risk in women are difficult to evaluate, because long-term characterization of hormone levels over the menstrual cycle is problematic. However, women with a history of irregular menses are at increased risk for CHD [Solomon et al., 2002]. Thus, ovarian function, and in particular estradiol, confers protection from CHD in women and CAA in female cynomolgus macaques.
Psychosocial stress is a well-recognized CHD risk factor that doubles the risk of myocardial infarction in human beings with traditional risk factors [Steptoe and Kivimäki, 2012]. Macaques depend on their social relationships, and psychosocial factors also affect their health. The stress of low social status results in a two-fold increase in CAA in females due, at least in part, to the social suppression of ovarian function [Kaplan, 2008; Kaplan et al., 2009].
In female cynomolgus monkeys consuming a Western-like diet, 60% of subordinates and 10% of dominants develop depressive-like behavior [Willard and Shively, 2012]. This social-stress associated depressive behavior is accompanied by perturbations in the CNS including small hippocampi and decreased serotonin 1a receptor binding affinity, autonomic perturbations manifested as high 24 hour heart rates, perturbed hypothalamic-pituitary-adrenal function, poor ovarian function, dyslipidemia, and low activity levels. These characteristics are reminiscent of major depressive disorder in human beings and most have not been reported in other animal models of depression [Shively and Willard, 2012]. Furthermore, cortical areas known to be critically involved in depression in human beings are not elaborated or differentiated in nonprimate species [Hamilton et al., 2012]. In human beings, depression and CHD are highly comorbid, and when depression is present the prognosis for CHD is greatly worsened [Leung et al., 2012]. Adult female cynomolgus monkeys that display depressive behavior develop four times more CAA than their nondepressed counterparts, faithfully recapitulating the depression – CHD comorbidity in human beings [Shively et al., 2009]. Thus, this species may be used as an animal model in which to study the comorbidity of depression and CHD.
In summary, macaque models of CAA are unique in that they recapitulate the pathobiology of CAA, and the primate-specific etiology of reproductive and CNS effects on CAA/CHD risk in human beings.
Behavior
In health-related research, behavior is most often used as an output measure for models of psychopathology, or as treatment responses to psychoactive drugs. In this context, there are a number of behaviors that are both upwardly and downwardly translatable – i.e. quantifiable in rodents, NHPs, and humans – as well as some behavioral paradigms that are uniquely available in NHPs and humans.
Social deficits are a feature of many mental health disorders, including autism, schizophrenia and social anxiety [APA, 2013; Baird et al., 2003]. The availability of NHP models with widely differing social systems and behavior makes them suitable for a number of different questions. The most common biomedical model, the rhesus monkey (Macaca mulatta), lives in large multi-male/multi-female groups with strong dominance hierarchies [Capitanio et al., 2006]. As Old World monkeys, they are more closely related evolutionarily to humans than any group except the apes and display high levels of social intelligence [Thierry et al., 2004], as well as personality dimensions remarkably similar to those of humans [Capitanio, 1999]. They have proven particularly useful in studies of faces, both of facial expression [Parr et al., 2013], and facial recognition, due to their ability to recognize individuals from photos or video [Habbershon et al., 2013; Silwa et al., 2011]. In particular, new and sophisticated eye-tracking techniques [Machado and Nelson, 2011], very similar to those used in humans, allow a measure of social attention that is not available in rodents. Abnormal eye gaze is commonly found in studies of humans with autism spectrum disorders [Guastella et al., 2008] and schizophrenia [Morris et al., 2009]. Visual tracking of eye gaze in NHPs has been designated as one of two priority behavioral paradigms for social cognition in animal models of schizophrenia by the CNTRICS initiative [Millan and bales, 2013].
Other primate taxa, while not as closely related to humans, provide opportunities for alternative translational models of social behavior. Socially monogamous New World monkey species which form long-term, heterosexual social bonds such as titi monkeys (Callicebus spp.) [Fernandez-Duque et al., 1997; Mason and Mendoza, 1998; Mendoza and Mason, 1997] and owl monkeys (Aotus spp.) [Fernandez-Duque and Huck, 2013; Fuentes, 1999], allow researchers to examine the basic neurobiology and permit pharmacological manipulation of social behaviors such as male parenting, food-sharing, and adult male social bonds. Marmosets and tamarins (family Callitrichidae), while not displaying traditional social monogamy [Fuentes, 1999] display other social behaviors shared with humans, such as alloparenting behavior [Bales et al., 2000; Tardif et al., 1992]. Compared to larger non-human primate species, these New World monkey species are smaller in size, easier to handle, and do not carry zoonotic diseases such as Herpes B virus [Tardif et al., 2006].
Many non-social aspects of behavior can be modeled in rodents, NHPs, and humans, and thus provide continuity of interpretation across species and studies. Startle responses, and the ability to inhibit these responses (prepulse inhibition, or PPI) based on an acoustic prepulse, are commonly used as measures of sensorimotor gating [Millan et al., 2012], and reveals multimodal deficits in schizophrenia [Thoma et al., 2007]. Startle amplitude and PPI are modeled in rodents using startle boxes with either acoustic or tactile (air-puff) stimuli, and in NHPs and humans using the effects of acoustic or air-puff stimuli on whole body startle or eye blink [Davis et al., 2008]. Despite the comparability of behavioral startle paradigms, the neural substrates differ between rodents and primates, being independent of the amygdala in primates but not in rodents [Antoniadis et al., 2007]. Other, simple non-social behaviors that can be compared across species might include locomotion and stereotypical behaviors [Novak et al., 2013].
In comparison to rodent models such as rats (Rattus rattus), mice (Mus musculus), and prairie voles (Microtus ochrogaster), all primate models display a more extended, human-like period of development [Walters, 1987]. In many species, offspring often remain in the natal group well past the age of sexual maturity. This can be particularly important in studying behavioral aspects of juvenile and adolescent periods.
In addition to mental health research, primate social behavior has been the basis for a new group of biologically inspired computational models [Bales and Kitzmann, 2011; Zhang et al., 2009]. Scent-marking behavior, in particular, has been used as a model for mobile sensor communication in which delayed information can be relayed [Xiao et al., 2011]. Cooperation between animals, including primates, has also been used in a number of engineering applications [Liang and Xiao, 2012]. Social hierarchies are of special interest to engineering [Markham, 2011], and primate social hierarchies have been previously modeled using agent-based modeling, a technique frequently utilized in engineering and computing applications [Bryson, 2007].
Cognition and Language
The use of nonhuman primates has been central to elucidating principles underlying learning and memory as well as more advanced cognitive function. Further to this point, Harlow developed the Wisconsin General Testing Apparatus as a direct consequence of his early effort to studying discrimination learning and memory in primates [Harlow, 1949]. Indeed, primate behavior and cognition has been a central focus in biology and particularly in psychology, almost from their conceptions as scientific disciplines. The early works on learning theory by Thorndike, Watson, and Skinner were dominated by studies in rats and other more distantly related species with little recognition or acknowledgment for potential species differences in cognition. Yet, even early on, many questioned strictly operant explanations for cognitive phenomena in primates, notably the infamous studies describing “insight” learning by Kohler [1925]. What distinguishes cognition from traditional views of animal learning is the role that reinforcement history has on the behavioral performance. In this realm, studies from nonhuman primates have been particularly significant. For instance, Gallup reported that when chimpanzees were confronted with mirrors, they treated the image as a reflection of themselves rather than another conspecific chimpanzee [Gallup, 1970]. Interestingly, subsequent studies have shown that other apes show self-recognition but this ability appears absent in other more distantly related primates [Povinelli, 1987; Povinelli et al., 1997]. The evidence of self-recognition in apes compared to other primates has been the foundation for neurobiological studies that aim to identify uniquely ape and human characteristics of the brain, which may explain these abilities. One such neurobiological landmark is Von Economo neurons (VENs), which are found in abundance in the anterior cingulate and insular cortex of humans and apes but are rather sparsely found in the regions in most Old and New World monkeys [Allman et al., 2011]. Whether VENs play a role in self-recognition or self-awareness is unclear but the critical point is that the only valid model for demonstrating their potential role in these aspects of cognition is primates.
The value of primates for testing evolutionary models of human cognition is not restricted to self-recognition. It is now well recognized that primates are much better animal models of human cognition in a variety of domains including inhibitory control and delay of gratification [Rosati et al., 2007], meta-cognition (Beran, Smith, & Purdue, 2013), cognitive representation of motor actions [Christel, 1994{Frey, 2012 #8393], planning [Menzel and menzel, 2007], and lateralization of structure and function [Hopkins, 2013]. All of these abilities are likely attributable to the relatively large brain size in primates compared to other mammals.
Perhaps no other domain of cognitive research has been more influential and recognized in the public domain as the studies of the linguistic capacities of apes. Initial attempts to teach apes to speak failed miserably, but starting in the 1960’s, efforts to teach apes language using alternative communication systems involving sign language, plastic chips, or visual graphic symbols were all highly successful in demonstrating a variety of basic language skills including symbolization, basic semantic representation [Savage-Rumbaugh et al., 1993], categorical representation, spoken English comprehension [Savage-Rumbaugh and Lewin, 1994], and rudimentary grammar [Greenfield and Savage-Rumbaugh, 1990]. From a theoretical standpoint, the ape-language research, as well as studies on vocal and gestural communication in monkeys and apes [Call and Tomasello, 2007], has no doubt helped in defining what characteristics of language are unique to humans and those that are shared. For instance, apes and humans both seem capable of learning and using symbolic communication systems, it has become increasingly evident that only humans seem to combine these symbols into multiword utterances in order to create new meanings. Pragmatically, the ape language work has been instrumental in the development of technology and methods used to assist disabled children in learning to communicate [Rumbaugh, 1977].
Cognitive aging
With advancing age, cognitive functions begin to decline in both humans and nonhumans. The specific cognitive domains that become altered with age and the brain mechanisms that underlie these declines have been the subject of investigation for many decades. NHPs are critical animal models that have provided valuable and unique contributions to our understanding of cognitive aging and to our search for possible treatments for cognitive decline with age.
NHPs are closest to humans phylogenetically [Finch and Austad, 2012; Kumar and Hedges, 1998] and the structure and function of human and NHP brains are very similar (see also Neuroscience section below). The rhesus monkey hippocampus more closely resembles the human hippocampus in terms of nuclear organization, projection pathways, and innervation patterns than does the rodent hippocampus [Amaral and Lavenex, 2007], and NHP and human brains are especially similar in cortical development and organization [Hutchison and Everling, 2012; Petrides et al., 2012]. The neocortex comprises 80% of the human brain and 72% of the macaque brain, but only 20% of the rat brain [Hutchison and Everling, 2012; Passingham, 2009]. Importantly, there are functional areas of the primate brain that do not exist in the rat, including visual cortical functional divisions [Uylings et al., 2003] and prefrontal cortex subdivisions [Preuss, 1995; Uylings et al., 2003]. These points are critical for studies of cognitive aging that are focused on cognitive processes dependent upon cortical regions, e.g., prefrontal cortex.
Besides similarities in brain functional specialization, NHPs share other vital similarities with humans that distinguish this animal model from rodent species and makes it a significantly unique model for translational investigation of cognitive aging. Humans and NHPs are primarily visually-oriented, unlike rodents. Using NHPs to study cognitive aging allows one the ability to examine visual non-spatial and spatial cognitive processes, thus providing examination of critical cognitive functions relevant to the human conditions being modeled. Considerable translational advantages of using NHPs to study cognitive aging include the shared complexity and breadth of their cognitive abilities with that of humans, especially higher order cognitive functions, e.g., the ability to perform numerical operations (e.g., [Okuyama et al., 2013]). Using NHPs facilitates the investigation of cognitive aging because it enables the use of established neuropsychological tests that were developed originally to evaluate human cognition to be used to evaluate NHP cognitive abilities [Voytko, 2003] and likewise, the use of experimental paradigms developed originally to evaluate NHP cognition to be used to evaluate human cognition [Voytko, 2003]. For example, the Wisconsin Card Sorting Task (WCST) is the gold standard for assessing cognitive flexibility in humans. Using a version of WCST (without the numerosity category), executive function deficits were noted in middle-age and aged rhesus monkeys [Moore et al., 2003; Moore et al., 2005; Moore et al., 2006] and in menopausal middle-aged rhesus monkeys [Voytko et al., 2009]. The ability to use operationally similar behavioral tasks in both humans and monkeys allows for greater and more reliable extrapolation between these species. Also of important note, identical pieces of equipment and technology can be used interchangeably in cognitive studies of humans and NHPs, e.g. computer driven behavioral testing apparati [Nagahara et al., 2010] and brain radiological equipment and procedures for both imaging [Voytko et al., 2001; Wey et al., 2013] and irradiation [Sundgren and Cao, 2009; Voytko et al., 2012] studies. Besides the already noted unique qualities and aspects of using NHPs to study cognitive aging, female NHPs are the ideal animal models in which to investigate the effects of reproductive aging on both cognitive and brain function. Female NHPs share many reproductive and endocrine features with women; unlike many common laboratory rodents which have a four-day estrus cycle and cessation of ovarian function that does not closely resemble primate menopause [Steger and Peluso, 1987]. Of particular note, female macaque monkeys have 1) 28 day menstrual cycles and patterns of ovarian hormones similar to women [Goodman et al., 1977; Jewitt and Dukelow, 1972], 2) a similar menopause to that of women [Downs and Urbanski, 2006; Gilardi et al., 1997; Johnson and Kapsalis, 1995; Shidler et al., 2001], 3) physiological responses to surgical menopause and estrogen therapy that are similar to women [Adams et al., 1990b; Jayo et al., 1998; Jerome et al., 1994], and 4) improvements in cognitive function with estrogen similar to women [Lacreuse, 2006; Rapp et al., 2003; Voytko et al., 2008; Voytko et al., 2009]. Moreover, one is able to use novel hormone therapy schedules in NHPs that closely mirror the hormonal fluctuation patterns that occur over the course of a normal primate menstrual cycle [Voytko et al., 2008; Voytko et al., 2009]. Thus, female NHPs enable examination of cognitive processes, as well as their modulation by menopause and hormone therapy, that are essentially identical to those found in women.
Although rodents are commonly used for studies of cognitive aging, there are critical neural, reproductive, and endocrine disparities between rodents and primates that likely contribute to the differences in behavioral observations that have been found between NHP and rodent models of aging. Collectively, these factors highlight the continued importance of using NHPs to investigate aspects of human cognitive aging and age-related disease.
Developmental Programming
The Developmental Programming hypothesis states that – responses to challenges during critical developmental time windows alter development with persistent effects on phenotype. Extensive human epidemiologic and precisely controlled animal studies show that reduced maternal nutrition, both global calories or protein intake, and other challenges such as maternal obesity and maternal stress during fetal and neonatal development alter the trajectory of organ differentiation and development, predisposing offspring to a wide variety of chronic diseases including cardiovascular disease, obesity, diabetes and behavioral disorders [Ainge et al., 2011; Armitage et al., 2004; Armitage et al., 2008; Armitage et al., 2005; Beall et al., 2005; Desai et al., 2005; Fernandez-Twinn and Ozanne, 2010; Li et al., 2011; Morimoto et al., 2011; Papadopoulou et al., 2003; Tosh et al., 2010; Vega et al., 2013; Vickers and Sloboda, 2012; Zambrano et al., 2010].
Controlled experimental studies on developmental programming have almost entirely been conducted in the common polytocous, altricial rodent laboratory species that have a very different developmental trajectory and maternal nutritional load in pregnancy and lactation compared to relatively precocial, mostly monotocous, species including humans. One central feature of perinatal development in which primates and the common polytocous laboratory animals differ is the interdependence of the fetal and maternal hypothalamo–pituitary–adrenal axis and their interactions with the placenta. One of the most significant differences between precocial and altricial species is the extent to which maternal glucocorticoids can cross the placenta and influence fetal development. Glucocorticoids act as a general orchestrator of late gestational fetal differentiation and maturation playing a central role in the preparations the fetus makes for independent life [Fowden et al., 2006].
The major precocial animal investigated in the field of developmental programming has been the sheep which had great advantages in the ease of accessibility of the fetus, extensive documentation on fetal development and the ability to conduct interventions to determine mechanisms and indicate potential markers in human development [Fowden et al., 2006; Nijland et al., 2008; Tuersunjiang et al., 2013]. However, the sheep has different placentation from primates including humans. Therefore nonhuman primate studies of programming, in ways that allow translation to human development, are needed. To date the major approaches to developmental programming that have been conducted in nonhuman primates have included; global nutrient reduction during pregnancy and lactation in the baboon [Antonow-Schlorke et al., 2011; Cox et al., 2013; Cox et al., 2006b; Cox et al., 2006c; Keenan et al., 2013; Nijland et al., 2010; Tchoukalova et al., 2013]; feeding high fat, high energy diets to Japanese macaque monkeys [Sullivan et al., 2010; Sullivan et al., 2011] or baboons [Maloyan et al., 2013]; the study of spontaneously growth restricted monkeys [Emerald et al., 2011] and studies on effects of fetal exposure to concentrations of glucocorticoids higher than appropriate for the current stage of gestation as a result of maternal administration of exogenous, synthetic glucocorticoids [Rodriguez et al., 2011]. This last model is of importance because excessive glucocorticoid exposure can produce organs that are both smaller and contain the wrong balance of different cell types.
The central role of glucocorticoids is further indicated by the observation that different challenges to the developing mammal can result in similar outcome phenotypes. Thus a variety of exposures in the perinatal period such as bilateral uterine ligations to mimic intrauterine growth restriction (IUGR) due to disruption of placental blood flow, chronic fetal hypoxia, excess glucocorticoid exposure, environmental insults (tobacco and endocrine-disrupting chemicals), maternal diet restrictions (caloric, iron and protein restriction) and over nutrition (high fat diets and obesity), can result in very similar phenotypes that include obesity, hypertension, insulin resistance, type 2 diabetes and cardiovascular disease in the offspring, suggesting common mechanistic pathways such as exposure to glucocorticoids at higher levels than normal for the current stage of gestation. Several other candidate mechanisms have been proposed that are either dependent on epigenetic mechanisms [Wadhwa et al., 2009; Wang et al., 2012] or oxidative stress [Sen and Simmons, 2010]
The ability to produce experimental models of IUGR is important since IUGR results in much perinatal morbidity and mortality. IUGR occurs not only with poor maternal nutrition but also in maternal obesity especially in primigravidae [Nelson et al., 2010], in teenage pregnancies where the growing mother competes with her fetus for nutrients [Wallace et al., 2006] and in pregnancies associated with placental disease, pre-eclampsia or maternal vascular disease [Roberts and Post, 2008]. Cohorts of male and female baboon offspring of mothers fed either ad lib or 70% of the ad lib global diet in pregnancy and lactation have been developed, resulting in IUGR and reduced growth in early life [Xie et al., 2013]. Three-year-old male IUGR baboon offspring (human equivalent 12 years) show signs of incipient hypertension and metabolic syndrome (MS) [Choi et al., 2011]. Data from nonhuman primate models such as this model and the obese Japanese macaque model studied by investigators at the Oregon Regional Primate Center [Grayson et al., 2006; Grayson et al., 2010; Sullivan and Grove, 2010; Suter et al., 2011; Suter et al., 2012] are needed to remove barriers to progress in development of human clinical diagnostic markers, preventative and therapeutic strategies.
An invaluable and powerful practical advantage of studies in both the monkey and baboon is the availability of extensive information on human gene and protein structure that can be extrapolated to these species. Human reagents, such as gene probes and antibodies, generally cross-react in these nonhuman primate species and are available for molecular studies addressing mechanism. For example the normal baboon gene expression phenotype has been extensively characterized at mid- and late-gestation as well as responses to reduced fetal nutrition and IUGR in the placenta [Cox et al., 2013; Li et al., 2009] fetal liver [Li et al., 2009], kidney [Cox et al., 2006a; Nijland et al., 2007] adipose tissue [Tchoukalova et al., 2009] and brain [Antonow-Schlorke et al., 2011] as well as the protein phenotype in liver, frontal cortex of the brain, hypothalamus and kidney. Using these approaches it has been demonstrated that several metabolic pathways are altered in IUGR particularly those involved in mTOR nutrient sensing and the IGF system [McDonald et al., 2007; Nijland et al., 2007; Xie et al., 2013]. In a similar way, studies by another group of investigators noted differential expression of 1,973 genes by microarray between neonates of average or low birth weight. Gene ontology studies showed changes in several metabolic pathways including carbohydrate metabolism [Emerald et al., 2011]. Alterations that are likely to have persistent epigenetic effects have been described in the Japanese macaque [Suter et al., 2012; Suter et al., 2013].
In summary information of great value in understanding the challenges, exposures, mechanisms and outcomes that lead to developmental programming in humans requires a synthesis of data from common, altricial experimental species as well as precocial nonhuman primates. Much can be learned from the similarities and differences that will be of great value in identifying markers that will enable the choice of preventative interventions and the design of therapies.
Genetics
The study of nonhuman primates has been and will continue to be a critical aspect of the broader field of genetics and genomics. There are many reasons why investigators study the genetics and genomics of humans and other organisms. Among the major motivations are the desires to understand how genetic variation influences individual differences in risk for or treatment of disease, and the genetic basis of human and primate evolution. Analyses of NHPs contribute much valuable and unique information to these two areas.
A variety of animal species have proven valuable as model organisms for research related to human health and disease. However, recent progress provides numerous examples of specific circumstances in which a fundamental genetic process relevant to a disease can only be modeled in a NHP, i.e. where no other species can provide a valid substitute. For example, prostate cancer is a major public health problem causing substantial mortality in the US and other countries. For years, one of the most commonly performed tests to detect cancer has been the prostate-specific antigen (PSA) test, performed to detect prostate cancer early and thus improve treatment outcomes. But the utility of the PSA test has recently been debated, and a more complete understanding of the function and expression of the gene that produces PSA (gene symbol KLK3) is important for further research progress. However, the KLK3 gene occurs only in Old World monkeys, apes and humans, and only these species produce the PSA protein [Karr et al., 1995; Mubiru et al., 2008]. Thus, only primates can be used to investigate the biology of PSA and its correlations with pathology.
A second example of the necessity of primate genetic studies for biomedical research involves psychiatric illness. Anxiety disorders and depression affect millions of people each year, leading to substantial suffering and disability that affects patients, their families, and the wider society. Susceptibility to anxiety disorders and depression is influenced by various factors, but it is clear that some people inherit a genetic predisposition to these psychiatric problems by virtue of inheriting genetic variation that can reduce their ability to effectively cope with various stressful experiences (Binder and Nemeroff 2010). A recent study using the rhesus macaque model investigated variation in the corticotrophin releasing hormone receptor 1 gene (CRHR1), which has previously been implicated as exerting significant influence on differences among people in their response to stress [Binder and Nemeroff, 2010; Liu et al., 2006]. This study of macaques identified specific mutations in the CRHR1 gene that are associated with differences in behavioral responses to mild stress, and also with differences in functional activation of specific neuronal structures (the hippocampus, intra-parietal sulcus and others) in the macaque brain that are in part responsible for the outward expression of anxiety-related behaviors [Rogers et al., 2012]. The hippocampus is well established as a central component of the neural circuitry that underlies emotion and reactivity to stress in humans and other mammals. While the CRHR1 gene is found in many mammalian species, the specific portion of the gene affected by the newly discovered mutations (exon 6) is a relatively new evolutionary innovation, found in Old World monkeys, apes and humans. Non-primate mammals do not exhibit the same gene structure or protein sequence, and therefore experimental analysis of the influence of these CRHR1 mutations on neurobiology and risk of psychopathology can only be performed in nonhuman primate models.
Numerous other examples of disease processes that are specific to primates and are significantly influenced by genetic differences among individuals could be described. For example, polycystic ovary syndrome is a common disorder that causes anovulation and infertility in women, is associated with increased risk for obesity and diabetes and is significantly influenced by genetic differences among women [Kosova and Urbanek, 2013]. This disorder is also well documented in rhesus macaques but cannot be adequately modeled in non-primate species [Abbott et al., 2013]. Primates are also uniquely suited to modeling the influence of genetics on immunobiology and risk for infectious disease. Susceptibility to infection by HIV and subsequent progression to AIDS is significantly affected by genetic differences among people [Guergnon and Theodorou, 2011]. Rhesus macaques are the premier animal model for studying HIV/AIDS, and macaques also exhibit individual differences in response to infection by SIV [Loffredo et al., 2007]. Only these NHP can be used to investigate the genetic basis of individual variation among hosts in response to challenge with SIV and related viruses. In other cases, especially circumstances related to neurobiology and immunology, particular disease processes depend on genetic mechanisms that are shared between humans and NHPs, but not with other species [Barr et al., 2003; Lesch et al., 1997; Seok et al., 2013a].
One aspect of comparative biology that fascinates both scientists and the general public is the question of human origins. What are the genetic differences that account for uniquely human characteristics including our expanded brain, increased cognitive complexity, extended lifespan, spoken language, bipedal locomotion and others? It is obvious that efforts to identify the particular genetic changes underlying unique human traits must compare the content and function of the human genome with that of closely related species. Comparative analyses of the human, chimpanzee, gorilla, and orangutan genomes are beginning to identify specific DNA sequence changes that seem to account in part for specific aspects of human evolution [Charrier et al., 2012; O'Bleness et al., 2012; Prabhakar et al., 2008], but much more research is needed. In addition, comparisons across a wider set of primates, including Old World monkeys, New World monkeys, and strepsirrhines are essential for development of a comprehensive understanding of how our hominoid relatives (chimpanzees, gorillas, orangutans and gibbons) arose out of more primitive non-hominoid ancestors. Only through detailed analysis and comparison of multiple primate genomes will we reconstruct the history and processes of genetic change that produced our species and our close relatives. This research also generates information about the genetic basis of more widely shared fundamental aspects of primate biology, thus increasing our knowledge of basic biology and evolution [Jolly et al., 2011; Prado-Martinez et al., 2013; Roos et al., 2011; Zinner et al., 2013].
HIV/AIDS
Human immunodeficiency virus (HIV), the etiologic agent of AIDS, evolved as a result of cross-species transmissions of simian immunodeficiency viruses (SIV) from African NHP species [Gao et al., 1999; Sharp and Hahn, 2011]. Although HIV infection is endemic in human populations, its host range is highly restricted. Only a handful of great ape species are susceptible to HIV infection [Alter et al., 1984] and AIDS-like diseases have only been observed in sporadic cases of experimentally infected chimpanzees [O'Neil et al., 2000]. As such, there is currently no experimental animal model that can capture the full spectrum of HIV infection in humans and its clinical sequelae. Despite this obstacle, substantial progress in HIV/AIDS research has been made in the past two decades with “surrogate” models such as SIV infection of macaques [Evans and Silvestri, 2013; Lifson and Haigwood, 2012; Van Rompay, 2012; Veazey, 2013].
SIV was first isolated in 1985 from rhesus macaques that presented with AIDS-like conditions, including CD4+ T cell depletion, opportunistic infection and neoplastic diseases [Daniel et al., 1985]. This virus was later found to be closely related to a primate lentivirus (SIVsm) endemic in populations of sooty mangabeys in Africa [Hirsch and Johnson, 1992]. Although SIVsm infection in their natural hosts is generally non-pathogenic, experimental inoculation of Asian macaques can result in AIDS-like diseases [Apetrei et al., 2005]. Because of its ability to induce AIDS-like diseases in relatively accessible NHP species, infection of macaques with SIVsm and its derivatives (e.g. SIVmac, SIVsmm, SIVmne, etc.) has been the animal model of choice for HIV/AIDS research.
SIVsm is believed to have evolved from a common ancestor with HIV type 2 (HIV-2), with which it shares similar virion structures, genomic organization, cellular tropism, and replication strategies [Hirsch et al., 1989]. However, SIVsm is only distantly related to HIV-1 (~40% genetic homology with HIV-1, vs. ~80% with HIV-2). Significant differences exist in their coreceptor usage, accessory genes, and sensitivity to host restriction factors and antiviral drugs [Hatziioannou et al., 2009]. Because of these differences and because HIV-1 does not readily establish infection in macaques, chimeric viruses have been developed, in which specific HIV-1 genes (e.g., envelope, Env; or reverse transcriptase, RT) [Ambrose et al., 2007; Hatziioannou et al., 2009; Shibata et al., 1997; Uberla et al., 1995] were inserted into the genome of a pathogenic SIV clone, SIVmac239. Inoculation of macaques with these SHIV chimera resulted in persistent infection, and, usually after serial in vivo passages, rapid CD4+ T-cell depletion and AIDS-like diseases. SHIV shares many of the advantages of SIV/macaque models, but also allows direct testing of specific HIV-1 vaccines (e.g., HIV-1 Env-based vaccines), or antiviral drugs (e.g., some HIV-1 RT inhibitors).
The primary advantage offered by the NHP model is the opportunity it affords to control the experimental conditions of infection and to collect tissues, especially those at early stages of infection, that are otherwise difficult or impossible to obtain from humans. Thus, NHP studies have been instrumental in shaping our understanding of the pathogenic mechanism of primate lentivirus infection in general and the early events after transmission in particular. The NHP model has also been an important tool for proof-of-concept studies of novel therapeutic and prophylactic approaches against HIV infection and disease [Clements et al., 2011; Del Prete and Lifson, 2013; Garcia-Lerma and Heneine, 2012; Lifson and Haigwood, 2012; Van Rompay, 2012; Veazey, 2013]. Summarized below are a few examples of how NHP models have contributed to the field of AIDS research.
Because of the ability to control experimental conditions, such as the timing, the route and the composition of the virus inoculum, the NHP model has played an important role in informing us of the early events in lentivirus transmission [Haase, 2011]. For example, intrarectal or intravaginal inoculation of macaques with low dose SIV have shown that infection through mucosa is often initiated with only a small number of “transmitted/founder” viruses, similar to the “bottleneck” observed in sexual transmission of HIV in humans [Keele et al., 2009; Shaw and Hunter, 2012]. In 1996, Marx [Marx et al., 1996] and colleagues reported progesterone implants enhanced mucosal transmission of SIV in macaques, most likely due to the thinning of vaginal mucosa resulting from the hormone treatment. Similarly, subsequent clinical trials indicated that injectable contraceptives may be a risk factor for HIV-1 transmission through direct effects on genital mucosal HIV-1 replication [Heffron et al., 2012]. NHP models therefore offer a highly relevant experimental platform to study factors that influence HIV transmission and to evaluate approaches to prevent acquisition.
NHP models have also provided important insights on the pathogenic mechanism of HIV infection. Studies of early events after SIV infection of macaques helped identify central memory CD4+ T cells (Tcm) and gut-associated lymphoid tissues (GALT) as the primary targets of infection [Heise et al., 1994; Mattapallil et al., 2005; Veazey et al., 1998]. The rapid and early depletion of Tcm in GALT, coupled with the dysregulation of homeostatic signals and the destruction of the gut mucosa, results in microbial translocation, inflammatory responses, activation of target cells and enhanced viral replication. These cyclical events set in motion an irreversible loss of gut Tcm and ultimately the collapse of the immune system. Similar observations made in clinical studies and NHP models [Brenchley et al., 2007; Brenchley et al., 2004; Klatt et al., 2010; Mehandru et al., 2004] inform our current understanding of the pathogenic mechanism of HIV infection and point to potential novel therapeutic approaches [Klatt et al., 2013].
NHP models played an important role in the development of prophylactic treatment concepts and topical microbicides against HIV acquisition. Using an SIV model, Tsai [Tsai et al., 1995] and colleagues protected macaques against SIV infection and disease by treatment with an antiviral drug pre- or post-exposure. They further showed that the timing of the initiation and duration of treatment was critical [Tsai et al., 1998]. These early proof-of-concept studies in NHP models predicted the success of prophylactic use of antiviral drugs [Grant et al., 2010; Van Damme et al., 2008] and provided much of the basis for the development of post-exposure prophylaxis as a treatment regimen in the clinic [Grant, 2010].
Studies in NHP models also predicted the efficacy of topical microbicide to reduce vaginal transmission of HIV [Abdool Karim et al., 2010; Dobard et al., 2012; Veazey, 2013]. Despite controversies over the discrepancy between findings from NHP models and early clinical trials, recent studies have shown that, if the studies were designed and interpreted properly, results from NHP models are highly predictive of the clinical outcomes. For instance, nonoxynol-9, a non-specific antiviral compound, was shown to be efficacious in vitro and in animal models [Hillier et al., 2005]. However, studies in the clinic showed increased HIV acquisition with the use of nonoxynol-9, most likely due to the inflammatory responses it causes in the vaginal/cervical mucosa [Hillier et al., 2005; Van Damme et al., 2008]. This result cast significant doubt on the value of NHP models in general. However, when repeated nonoxynol-9 dosing in the clinical trial was modeled in macaques, similar findings of inflammatory responses in the vaginal/cervical mucosa were observed [Van Rompay, 2012; Veazey, 2013]. Thus, proper interpretation of results from animal models requires considerations not only of the intrinsic differences between experimental systems, but also the comparability of the trial designs.
Natural history studies of HIV exposed individuals do not support the notion that protective immunity against HIV infection and diseases can be acquired through natural exposure, as has been demonstrated in many vaccine-preventable diseases. Until the report of the RV144 trial in 2009 [Rerks-Ngarm et al., 2009], the only direct evidence supporting the feasibility of vaccine induced protection against primate lentivirus infection and disease was provided by NHP models. Since the late 1980’s, a number of vaccine concept and immunization approaches have been shown to induce different levels of protective immunity against primate lentiviruses in a variety of NHP models. Live attenuated vaccine, long considered as the “gold standard” vaccine approach against viral diseases, was shown to be effective against SIVmac infection [Daniel et al., 1992]. However, this live attenuated vaccine was subsequently shown to induce AIDS in infant macaques, demonstrating the usefulness of the NHP model to address safety concern of this vaccine approach [Baba et al., 1995; Baba et al., 1999]. Protective efficacy of the “prime-boost” strategy, using poxvirus for priming and subunit protein for boosting, was also first demonstrated in a NHP model in 1992 [Hu et al., 1992]. Seventeen years later, a similar strategy, using canarypox viruses and subunit gp120 proteins in a “prime-boost” regimen, provided the first indication that vaccine protection against HIV acquisition is possible [Rerks-Ngarm et al., 2009]. Although the efficacy of this vaccine regimen still needs to be confirmed, understood and improved upon, a number of “prime-boost” immunization approaches, using various replicative or non-replicative vectors (including DNA) and different boosting immunogens, are being developed and evaluated in the clinic. More recently, Picker and colleagues [Hansen et al., 2011; Hansen et al., 2013], using a NHP model, showed that significant protection and durable antiviral immunity can be achieved by a cytomegalovirus (CMV) vector based vaccine.
Despite its limitation as a surrogate model, NHP represents the most relevant animal model for HIV/AIDS research to date. Studies in NHP models have contributed much to our understanding of the early events of HIV infection and its pathogenic mechanisms. NHP have also been proven useful in the development of therapeutic and prophylactic treatment concepts and microbicides. Better understanding and judicious use of NHP models will continue to inform HIV vaccine development and the search for a cure for AIDS.
Immunology
The immune system has a central regulatory role in the maintenance of homeostasis within the body and is involved in almost all aspects of human health and disease. Outside the usual suspect disorders - infection, asthma/allergy, autoimmunity, and transplant rejection – the immune system has a role in neurodegenerative diseases (Alzheimer, Parkinsonism) and even in psychiatric diseases (schizophrenia) and mood disorders (depression).
According to the classical dogma, the immune system learns during fetal development to respond only against non-self (e.g., foreign agents or a transplanted organ) but not against self, i.e. it tolerates its own body. However, more recent insights show that the distinction made by the immune system is between real threats (danger) against which an immediate response is required and relatively harmless disturbances of homeostasis, which can be ignored [Matzinger, 2002]. The immune system perceives danger via innate receptors expressed on the surface of professional antigen presenting cells (APC) -- dendritic cells and macrophages, for example -- which detect conserved molecular structures on pathogens, such as bacterial lipopolysaccharide or viral RNA [Mills, 2011]. The detection of danger induces activation of the APC whereby they acquire the capacity to mobilize effector T and B lymphocytes and tailor their response [Iwasaki and Medzhitov, 2010]. This innate part of the immune system is present throughout the animal kingdom. Information on encountered pathogens is stored within the adaptive part of the immune system, which is only present in vertebrate species. The adaptive system comprises T and B cell lymphocytes, which store immunological memory via expansion of the responding clonal specificities and molecular imprinting, ensuring a quicker and more effective reaction upon re-encounter of the pathogen.
Many of our current concepts on the architecture and functioning of the human immune system comes from well-characterized inbred and specific pathogen-free mouse strains. Although the blueprint of the mouse innate and adaptive immune system is representative for the human system, translation of immunological principles from laboratory mice to humans has been notoriously difficult. This is in part explained by basic immunological differences between mice and humans [Mestas and Hughes, 2004], but it is also due to the immunological immaturity of the very clean (SPF) laboratory mouse [Sachs, 2003]. The direct consequence of immaturity is that the mouse immune system is much more amenable to experimental manipulation than the robust, pathogen-educated immune system of humans [Sachs, 2003].
Although as many as 11 ground-breaking immunological discoveries have been awarded with a Nobel prize, making immunology one of the most successful disciplines in medicine and physiology, rather few discoveries in basic immunology could be incorporated in clinical practice. Indeed, we have now effective vaccines against some infectious diseases, we have monoclonal antibodies for diagnosis and treatment of autoimmune diseases and cancer, and we can successfully replace certain dysfunctional body organs (skin, heart, kidney, liver, lung) and tissues (bone marrow). However, these evident successes are contrasted by a long list of new treatments for immune-mediated inflammatory disorders that fail to reproduce beneficial effects observed in mouse models when they were tested in the clinic. Not only are the investment losses due to the high attrition rates enormous [Kola and Landis, 2004], but it also shows how little we understand of the human immune system [Davis, 2008].
Obstacles to the translation of pathogenic and therapeutic principles from mouse to man are in part related to the artificial nature of the disease models, which often do not replicate the essence of the human disease, but certainly also to the considerable immunological gap between a clean laboratory mouse and humans. Another bias is the short life span of a laboratory mouse that makes it a less suitable model for diseases associated with aging. Despite these obvious limitations, the inbred/SPF laboratory mouse is the standard experimental model for the vast majority of immunologists in academia and industry. Frequently heard arguments in support are the abundance of reagents, availability of well-characterized genetically modified animals, the relatively low costs, the reliability of the models implying high reproducibility of experiments, and the fact that the standard disease models are accepted in the field implying easier acceptance by reviewers and editors of the leading journals [Steinman and Mellman, 2004].
Nonetheless, the notion that a nonhuman primate may be the more relevant model for human biology and disease - due to their closer genetic, immunological and anatomical proximity to humans and the fact that their housing in outdoor enclosures allows exposure to immune shaping environmental cues – is (slowly) gaining acceptance. In the field of transplantation, the nonhuman primate is an inevitable model for proving the efficacy of a new treatment before it can be tested in the clinic [Sachs, 2003]. It is difficult to understand why the same argumentation would not be applicable to the autoimmune disease field, where the nonhuman primate is much less accepted as a relevant preclinical model. However, efforts to develop the experimental autoimmune encephalomyelitis (EAE) model in common marmosets, as a generic autoimmune disease model for exploratory research into ethiopathogenic mechanisms and applied research into novel therapies for multiple sclerosis, seem to be bearing fruit ['t Hart et al., 2011].
Neuroscience
NHPs provide important models for neuroscience research for a variety of reasons Chief among these is the similarity with humans in both central and peripheral nervous system structure and organization. Compared to other mammals, such as rodents, nonhuman primates’ brains resemble human brains most closely on a variety of criteria including encephalization (a measure of brain size relative to a taxonomic standard), number and density of cortical neurons, a large prefrontal cortex, and greater myelination [Roth and Dicke, 2005; Semendeferi et al., 2002; Ventura-Antunes et al., 2013]. For example, the encephalization quotient for humans is 7.4 – 7.8. For Old World monkeys, the values range from 1.7 – 2.7, and for capuchin monkeys, the values range from 2.4 – 4.8. In contrast, encephalization quotients for rats and mice are in the 0.4 – 0.5 range [Roth and Dicke, 2005]. Important cytoarchitectural differences between primate and rodent brains have also been reported in areas associated with adult neurogenesis [Brus et al., 2013], and particular structural and functional areas, such as the frontal and temporal poles, appear to be unique to primates [Insausti, 2013; Tsujimoto et al., 2011]. Differences between rodents and primates exist in spinal cord anatomy as well [Courtine et al., 2007].
Humans and Old World monkeys (which are most commonly used as model species in neuroscience research) also share important aspects of their lifestyles (e.g., diurnality, terrestriality, omnivory), sensory/perceptual abilities (e.g., color vision, greater reliance on vision than olfaction), anatomical specializations (e.g., use of hands and thumbs, rather than vibrissae, for tactile perception), and genetics. The similarities between human and NHPs in these features are reflected in brain organization. For example, comparative studies of a variety of mammalian taxa have shown that all species possess primary and secondary sensory areas [Krubitzer, 2007]. The internal organization of these areas, however, can reflect broader anatomical differences, with a relatively higher proportion of primary somatosensory cortex devoted to the hand in primates, compared to a high proportion devoted to the vibrissae in rats (e.g., [Seelke et al., 2012]. Because of anatomical similarities and specializations, nonhuman primates are important subjects in the emerging field of neuroprosthetics [O'Doherty et al., 2011], which may eventually result in an exoskeleton that could restore mobility to paralyzed humans. Considerable research is ongoing with nonhuman primates in the areas of sensory neuroscience, focusing on basic questions of how color is processed in the cortex [Hass and Horwitz, 2013], and what neurological mechanisms are associated with age-related hearing loss [Engle et al., 2013]. Advances in genetics have likewise shown associations between genetic polymorphisms that are much more conserved in anthropoid primates than among mammals in general (such as those coding for the corticotropin-releasing hormone receptor 1), and metabolic activity that is relevant to understanding brain mechanisms associated with anxious temperament [Rogers et al., 2013].
Similarity in aspects of life-history also makes NHPs valuable models. Year-round sociality, relatively long gestations, singleton births, the lengthy period of postnatal development, long lifespan, and for some species, the development and persistence of adult pair bonds, permit questions to be asked about the neuropeptide basis of monogamy [Jarcho et al., 2011], the role of early experience in the development of brain systems subserving affiliation [Winslow et al., 2003], and the importance of the social environment in affecting sympathetic nervous system innervation of lymphoid tissue [Sloan et al., 2007].
NHP models are also making significant contributions in the understanding and treatment of diseases and injuries that affect large numbers of humans, including Alzheimer’s Disease, Parkinson’s Disease, and NeuroAids [Capitanio and Emborg, 2008]. Some of these studies have led to clinical trials (e.g., [Tuszynski, 2007]. New models continue to be developed (e.g., Huntington’s Disease: [Yang et al., 2008], and thoughtful discussion about the development of valid nonhuman primate models for pathological neurological conditions and treatments is ongoing (e.g., [Cook and Tymianski, 2012; Kimmelman et al., 2009]. Advances in imaging technologies, including diffusion spectrum imaging and resting-state functional magnetic resonance imaging (e.g., [Koo et al., 2013; Kroenke, 2010] and references therein), enable study of human and nonhuman primates using the same methodologies. Importantly, however, the greater access to the brains of nonhuman primates permits validation of the imaging data through comparison with data obtained from more invasive measures, and provides a level of resolution (e.g., down to the single cell level) that is still unobtainable via neuroimaging with humans [Passingham, 2009].
Pharmacology
As discussed above, NHP social behavior can serve as a dependent variable in examining the effects of neuropathology associated with human psychiatric diseases and the drugs used to treat them. In addition, the position in the social hierarchy that is occupied by a monkey can serve as an independent variable. That is, the social rank of a monkey can affect physiology, behavior and the effects of drugs. In the wild and when housed in groups in captivity, monkeys establish clear dominance hierarchies. In the laboratory setting, which often involves relatively small groups, these hierarchies are linear and transitive. Occupying the lower, subordinate positions in the hierarchy is unequivocally stressful. Compared to dominant monkeys, subordinates display suppressed ovarian function, heavier adrenal glands and greater release of cortisol in response to stressors, which indicated a hyper-sensitive HPA axis (e.g., [Shively and Kaplan, 1984]; Kaplan, Adams et al. 1986; Czoty, Gould et al. 2009). Whereas subordinates are exposed to chronic social stress, dominant monkeys live in a chronically enriched environment. Top-ranked monkeys move about the pen as they please, receive more grooming and have primary access to food and other resources. Importantly, position in the social hierarchy can influence the brain as well as effects of drugs. Thus, socially housed NHPs represent an excellent example of the ability of environmental factors to influence drug effects.
One research area in which such drug×environment interactions have been extensively documented is the study of the effects of abused drugs (Nader, Czoty et al. 2012). For example, Miczek and collaborators have shown that the behavioral effects of d-amphetamine and alcohol differ in dominant and subordinate monkeys (e.g. Miczek & Gold 1983; Winslow & Miczek 1985). In dominant but not subordinate cynomolgus monkeys (Macaca fascicularis), the transition from individual to social housing was associated with an increase in the binding availability of D2/D3 dopamine receptors, as measured with positron emission tomography (PET imaging), and lower sensitivity to the abuse-related effects of cocaine (Morgan, Grant et al., 2002). Although several years of cocaine self-administration experience resulted in a dissipation of this social rank-related difference, the significant difference re-emerged once cocaine exposure was discontinued (Czoty, Morgan et al. 2004; Czoty, Gage et al. 2010). Because brain dopamine and D2/D3 receptors in particular have been strongly linked to the behavioral effects of cocaine (e.g., Koob & Volkow 2010), these studies provide a clue to the mechanisms that underlie the ability of the environment to modulate the behavioral effects of drugs. Although rodents will establish dominance hierarchies in the laboratory under some conditions (e.g. Blanchard, Sakai et al. 1993), the vast majority of rodent research uses individually or pair-housed animals. NHPs afford the opportunity to study ethologically relevant sources of environmental stress and enrichment over long periods of time.
Whereas the sophisticated social and behavioral repertoire of monkeys proves advantageous for studying complicated interactions between the environment, the brain and behavior, NHPs have advantages as subjects in more direct pharmacological studies as well. Beyond the closeness between monkeys and humans in phylogeny, neuroanatomy and neurochemistry, it is also apparent that monkeys are the most predictive animal model of the pharmacokinetics of various drugs (see Weerts, Fantegrossi et al. 2007). Furthermore, human drug addicts typically abuse multiple substances over a period of several years before seeking treatment. It is questionable whether a few days or weeks of drug exposure in laboratory animal adequately models the complex pharmacological history observed in humans. Only in species with a lifespan as long as monkeys is it possible to generate subjects with long and varied pharmacological histories. For example, Nader, Morgan, et al. (2006) used PET imaging to study brain changes in monkeys self-administering cocaine for one year. A progressive decrease in the binding availability of D2/D3 receptors was observed in all monkeys, but the time course of this effect differed. Had the analysis terminated after one week or even one month of cocaine self-administration, it would have appeared that only some monkeys were affected. Moreover, when access to cocaine was removed, the time course and extent of recovery of D2/D3 receptor availability to baseline levels also differed across subjects. Thus, monkeys are ideal research subjects not only for the ability to track changes in the brain and sensitivity to drugs over time, but also to be able to study individual differences in these effects—both of which are omnipresent of clinical medicine (see reviews by Howell and Murnane, 2011; Murnane and Howell, 2011; Gould et al., 2012, 2013; Nader and Banks, 2014).
Reproduction
Despite many basic similarities in the endocrine regulation of reproduction that are common among mammals [Ferin, 1983; Karsch et al., 1984; Plant and Witchel, 2006], primates exhibit characteristics not common among other taxa [Weinbauer et al., 2008] and historically have been valuable in elucidating reproductive biology of specific relevance to humans [Dettmer, 2013]. For instance, negative feedback by estradiol inhibits the release of luteinizing hormone (LH) more potently in female than male primates and rodents [Steiner et al., 1976]. In contrast, the sexually differentiated positive feedback effect of high estradiol, that induces an LH surge in female but not male rats [Neill, 1972; Neill et al., 1971], is not sexually differentiated in primates [Karsch et al., 1973], and estradiol can elicit surge release of LH in both males and females [Steiner et al., 1976]. The effects of administered neuropeptides may also differ between species. GnRH can induce testicular damage in rats but not monkeys [Weinbauer and Nieschlag, 1989].
There are fundamental differences in the sources of sex steroids in primates compared with other mammals. The major source of circulating androgens in higher primates is the adrenal cortex [Conley et al., 2004; Nguyen and Conley, 2008], and higher primates experience a pre-pubertal increase in adrenal androgen secretion (adrenarche), the regulation of which is similar in many ways to the human phenomenon [Conley et al., 2012]. Ovarian steroid secretion during the non-pregnant cycle is also notably different in primates from that in most other mammals. It has been known for some time that, uniquely perhaps among higher species, the primate corpus luteum expresses, in addition to progesterone, high levels of aromatase [Doody et al., 1990], and secretes estradiol in concentrations that are measurable in serum [Bosu et al., 1972; Bosu et al., 1973], as well as in urine [Hopper and Tullner, 1970]. Secretion of estradiol during the luteal phase maintains vaginal cornification in primates at levels not vastly different from those seen in the follicular phase before ovulation [Patton et al., 2000]; there is far less cyclic change than is seen in rodents [Eckstein and Zuckerman, 1956]. As a result, the vaginal epithelium remains thick, ensuring protection against infection and trauma during copulation throughout all stages of the cycle. This is an important physiological adaptation because many higher primates [Dixson, 1998], unlike most mammals, engage in copulation throughout their reproductive cycle.
Similarly, uterine physiology differs in primates, experiencing events that are uncommon among other mammalian taxa, if not unique. Primates menstruate [Butler, 1974], and only certain chiropteran species share this phenomenon to any similar degree [Rasweiler Iv and Badwaik, 2000]. Menstruation in higher primates follows luteolysis in non-conceptive cycles [Brenner and Slayden, 2012; Jabbour et al., 2006]. Luteolysis in primates occurs by mechanisms independent of the uterus as in women [Davis and Rueda, 2002]. Rodents have spontaneous ovulation, but an induced luteal phase and do not experience luteolysis under normal circumstances [Melampy and Anderson, 1968]. If pregnancy is established in primates, luteal function is rescued by the embryonic secretion of chorionic gonadotropin [Banerjee and Fazleabas, 2010; Hearn, 1986]. Equine species are the only other mammals that are known to secrete a chorionic gonadotropin, although secretion is initiated at a much later stage in pregnancy, and therefore the functional significance differs from that of primates [Allen and Stewart, 2001].
Pregnancy in primates is associated with quite variable profiles of estrogens and progesterone. Even though no two mammals of any species are exactly alike [Conley et al., 2004], estrogen secretion is still dependent on fetal adrenal androgens [Mapes et al., 2002] among the majority of primate species investigated [Conley et al., 2004; Nguyen and Conley, 2008]. This is again unusual among mammals and provides unique insights into possible mechanisms [Pattison et al., 2007]. Furthermore, progesterone remains elevated until parturition in primates [Casey and MacDonald, 1997; Challis et al., 2000; MacDonald et al., 1982; Mendelson, 2009] unlike many other mammalian species. Human birth occurs predominantly at night [Jolly, 1972], and melatonin likely plays a prominent role in both maternal and fetal compartments during pregnancy [Tamura et al., 2008]. In fact, maternal hormone secretion patterns have a distinct diurnal rhythm that correlates with myometrial activity [Wilson et al., 1991]. Consequently, non-human primates are very valuable models for studies into the initiation of labor and preterm birth [Challis et al., 2000; Nathanielsz, 1998]. They have proven equally valuable in studies of fetal development, placental function [Albrecht and Pepe, 1990] and the post-natal effects of in utero hormonal exposure [Abbott et al., 2008]. Mammary development and lactational physiology does not exhibit features that could be considered unique to primates, but NHP physiology and development will always resemble that of humans more closely than non-primate species. As expected therefore, morphological development [Wood et al., 2007a], differentiation [Stute et al., 2012], response to exogenous hormones and development of disease [Cline, 2007; Wood et al., 2007b] are more similar to the human than other traditional model species. Moreover, recent studies suggest that this is reflected even in the mammary epithelial transcriptome [Lemay et al., 2013] and metabolome [O'Sullivan et al., 2013]. Consequently, NHP may also prove to be more valuable and appropriate models to address critical questions in mammary gland disease, lactation, and neonatal nutrition [Neville et al., 2012].
The rhesus macaque has long been recognized to be a good model of human menopause [Hodgen et al., 1977; Johnson and Kapsalis, 1998; Walker, 1995; Walker and Herndon, 2008]. As in women, the peri-menopause in macaques is characterized by an increase in FSH [Downs and Urbanski, 2006; Hodgen et al., 1977; Kavanagh et al., 2005; Shideler et al., 2001] and LH [Hodgen et al., 1977; Walker, 1995; Woller et al., 2002] and decreasing inhibin [Downs and Urbanski, 2006; Shideler et al., 2001] as follicle reserve declines [Nichols et al., 2005]. This appears true of other primates [Jones et al., 2007; Walker et al., 2009], even if cycles continue [Lacreuse et al., 2008]. Although gonadotropins are elevated in perimenopausal rhesus females, estradiol may not be decreased significantly [Walker, 1995], and in longitudinal studies were numerically (145%) higher [Downs and Urbanski, 2006]. As in women [Burger et al., 2002], estradiol concentrations and cycle length becomes irregular [Downs and Urbanski, 2006; Gilardi et al., 1997; Gore et al., 2004; Hodgen et al., 1977; Shideler et al., 2001] with extended follicular phases [Gilardi et al., 1997]. Eventually there is complete ovarian senescence with low estradiol [Gilardi et al., 1997; Gore et al., 2004; Hodgen et al., 1977]. GnRH pulses [Gore et al., 2004] are elevated in aged female rhesus, as are transcripts for GnRH, KiSS-1 and its receptor in medial basal hypothalamus [Kim et al., 2009]. Like women [Crawford et al., 2009; Lasley et al., 2002], cross-sectional data from a small number of subjects suggests that peri-menopausal rhesus may also have variably elevated DHEAS concentrations [Shideler et al., 2001]. Aged rhesus females suffer cognitive deficits [Roberts et al., 1997] that respond to estradiol therapy [Rapp et al., 2003] as do women [Paganini-Hill and Henderson, 1996] (see Cognitive Aging, above). In summary, primate reproduction is regulated in ways that are fundamentally different from rodent and other mammalian species, making it imperative to use primate models when investigating reproductive development and associated diseases.
Respiratory Diseases
According to the American Lung Association, more than 25 million Americans are living with a chronic lung disease such as chronic obstructive pulmonary disease (COPD) or asthma. In 2010, COPD alone was the fourth most common cause of premature mortality in the United States, resulting in over 150,000 deaths [Collaborators et al., 2013]. According to the Centers for Disease Control and Prevention, 10% of children under the age of 18 in the United States have asthma. Given these statistics, it is imperative for the scientific community to study both the initiating events and subsequent intervention of chronic lung disease throughout the lifespan. Despite these efforts, years of life lost due to premature mortality by lung cancer and chronic obstructive disease death are increasing, whereas rates of death from other common causes such as ischemic heart disease, and stroke are declining [Collaborators et al., 2013]. Translational research is hampered not only by limited funding, but also disappointing drug trials that have not led to interventions or have demonstrated considerable side effects. Although controversial, it has been speculated that the limited success of compounds such as those selected to treat asthma is due to the original observations collected from genetically modified mice, which are currently the most prevalent laboratory animal model used to study human lung disease [Wenzel and Holgate, 2006]. While we have obtained a significant amount of information regarding immunological mechanisms from the rodent, it remains unclear how relevant such findings are to human subjects [Seok et al., 2013b].
Chronic lung disease in both adult and pediatric patients is highly complex, often an interaction of immunity gone awry and alterations in the structure of the lung. Ultimately, a secondary model system that can effectively be used to recapitulate human disease must accurately reflect cellular form and function for both immune and pulmonary compartments. Of all laboratory animals, the nonhuman primate is most similar to humans with regard to developmental maturation of the immune system. For example, thymectomy of neonatal mice results in the development of autoimmune disease, indicating that T cell selection (self versus non-self; see Immunology, above) is not complete at birth, and could potentially become modulated by environmental exposures [Suri-Payer et al., 1999]. In contrast, thymectomy of human infants results in no adverse clinical outcomes, indicating that selection of the T cell repertoire is mostly complete at birth [Wells et al., 1998]. Comparative studies in the infant rhesus macaque suggest that postnatal development of systemic immunity closely parallels that which is observed in human infants [DeMaria et al., 2000]. There are also important similarities in lung development between human and nonhuman primates that are not found in rodents. Humans and other primates share a mixture of cell phenotypes within the conducting airways not found in non-primate species [Plopper et al., 1992]. The overall pattern of conducting airway epithelial differentiation [Jeffery and Reid, 1977; Plopper et al., 1986] and its maturation during the postnatal period are also similar in rhesus monkeys and humans [Bucher and Reid, 1961; Plopper et al., 1986; Thurlbeck et al., 1961]. Collectively, the nonhuman primate exhibits features of lung architecture and immunity that make it highly appropriate for elucidating novel therapeutic approaches to treat chronic lung disease in humans[Plopper and Hyde, 2008].
What have nonhuman primates taught us about chronic lung disease in humans? One important characteristic of normal lung growth in children is that alveolar growth is continuous from birth through school age, a finding that was originally reported in a limited number of postmortem samples [reviewed in [Burri, 2006]]. Yet, when Hyde and colleagues evaluated rhesus macaque monkeys, the trajectory of alveolar growth was found to be continuous through early adulthood; this observation has significant implications with regards to prolonged susceptibility of younger individuals to lung damage from environmental pollutants [Hyde et al., 2007]. Indeed, studies over 20 years ago in bonnet and rhesus monkeys have provided compelling histological data on the destructive nature of ambient air pollutants such as ozone on the conducting airways, and lent critical scientific support to establishment of National Ambient Air Quality Standards by the Environmental Protection Agency [Harkema et al., 1993; Mellick et al., 1977]. While all age groups are susceptible to the inflammatory effects of environmental air pollutants, epidemiology suggests that young children are more vulnerable to detrimental long-term health outcomes such as asthma. Because it is considered unethical to conduct experimental trials in healthy pediatric subjects, studies have relied on infant rhesus monkeys to provide data on long term health effects of environmental exposures such as ozone, tobacco smoke, and allergens. For example, perinatal environmental tobacco smoke exposure in infant monkeys results in altered immune cytokine profiles and airway innervation [Yu et al., 2008]. The health effects of environmental exposures can persist long after the exposure has ended, as evidenced in a study by Maniar-Hew, et. al., in which early life ozone exposures resulted in attenuation of innate immune responses in mature monkeys [Maniar-Hew et al., 2011]. Nonhuman primate models of allergic airways disease have been in existence for over 40 years, exploiting both a naturally occurring parasitic infection in the wild (Ascaris spp.) as well as experimental sensitization with the common human allergen, house dust mite (reviewed in [Coffman and Hessel, 2005]). While both ascaris and house dust mite monkey models have been used to test a number of compounds over the past 5 years, including an anti-IL-13 inhibitor and an inhibitor of OX40L [Bree et al., 2007; Seshasayee et al., 2007], a NHP study of steroid use in childhood asthma has provided important data on the disruptive impact on lung development of this common therapeutic [Plopper et al., 2012]. Overall, what we have learned from the nonhuman primate has had a significant impact on our understanding of the origins and treatment of chronic lung disease in multiple age groups, but it is clear that there is still much more work to be completed before chronic lung disease can be prevented or cured.
Conclusion
NHPs provide highly valuable animal models that have significantly advanced our understanding of numerous behavioral and biological phenomena in humans and other primates. Their value as models of human biological and behavioral processes derives from their common ancestry, and is evident in the unique characteristics that they possess in comparison to non-primate mammals. However, we are at a critical crossroads. Unless NHP research is given the philosophical, emotional, and financial support and infrastructure that is needed to sustain it and grow, we are in danger of losing irreplaceable unique models and thus, our ability to continue to explore and understand, and develop preventions and treatments for numerous conditions that inflict great suffering on humans.
Acknowledgements
We thank Michael Stebbins of the Foundation for Biomedical Research; Dr. Walter I. Horne of Northeast Ohio Medical University; John Harding of the Office of Research Infrastructure Programs/Office of the NIH Director; and Christopher Machado of University of California, Davis.
Footnotes
Office of Laboratory Animal Welfare [http://grants.nih.gov/grants/olaw/olaw.htm] and the Public Health Policy on Humane Care and Use of Laboratory Animals [http://grants.nih.gov/grants/olaw/references/phspol.htm].
Data retrieved from 2010 Annual Reports to the USDA Animal and Plant Health Inspection Service.
The requirements of the Animal Welfare Act (AWA) are set forth under the Regulations and Standards in the Code of Federal Regulations (CFR). These requirements are found in Title 9 CFR, Chapter 1, Subchapter A - Animal Welfare, Parts 1, 2, and 3. The requirement for the psychological well-being of primates is set forth under section 13(a)(2)(B) of the AWA (7 USC, 2143). The standards for environmental enhancement to promote psychological well-being in primates are set forth under 9 CFR, Chapter 1, Subchapter A - Animal Welfare, Part 3, Section 3.81.
The experiment should be so designed and based on the results of animal experimentation and a knowledge of the natural history of the disease or other problem under study, that the anticipated results will justify the performance of the experiment. http://www.hhs.gov/ohrp/archive/nurcode.html
References
- 't Hart BA, Gran B, Weissert R. EAE: imperfect but useful models of multiple sclerosis. Trends Mol Med. 2011;17(3):119–125. doi: 10.1016/j.molmed.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Abbott DH, Nicol LE, Levine JE, Xu N, Goodarzi MO, Dumesic DA. Nonhuman primate models of polycystic ovary syndrome. Mol Cell Endocrinol. 2013;373(1–2):21–28. doi: 10.1016/j.mce.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott DH, Zhou R, Bird IM, Dumesic DA, Conley AJ. Fetal programming of adrenal androgen excess: lessons from a nonhuman primate model of polycystic ovary syndrome. EndocrDev. 2008;13:145–158. doi: 10.1159/000134831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany ABM, Sibeko S, Mlisana KP, Omar Z, et al. Effectiveness and Safety of Tenofovir Gel, an Antiretroviral Microbicide, for the Prevention of HIV Infection in Women. Science. 2010;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MR, Kaplan JR, Clarkson TB, Koritnik DR. Ovariectomy, social status, and atherosclerosis in cynomolgus monkeys. Arteriosclerosis. 1985;5:192–200. doi: 10.1161/01.atv.5.2.192. [DOI] [PubMed] [Google Scholar]
- Adams MR, Kaplan JR, Manuck SB, Koritnik D, Parks JS, Wolfe MS, Clarkson TB. Inhibition of coronary artery atherosclerosis by 17-beta estradiol in ovariectomized monkeys. Lack of an effect of added progesterone. Arteriosclerosis. 1990a;10(6):1051–1057. doi: 10.1161/01.atv.10.6.1051. [DOI] [PubMed] [Google Scholar]
- Adams MR, Kaplan JR, Manuck SB, Koritnik DR, Parks JS, Wolfe MS, Clarkson TB. Inhibition of coronary artery atherosclerosis by 17-beta estradiol in ovariectomized monkeys. Arteriosclerosis. 1990b;10:1051–1057. doi: 10.1161/01.atv.10.6.1051. [DOI] [PubMed] [Google Scholar]
- Ainge H, Thompson C, Ozanne SE, Rooney KB. A systematic review on animal models of maternal high fat feeding and offspring glycaemic control. Int J Obes. 2011;35:325–335. doi: 10.1038/ijo.2010.149. [DOI] [PubMed] [Google Scholar]
- Albrecht ED, Pepe GJ. Placental steroid hormone biosynthesis in primate pregnancy. EndocrRev. 1990;11(1):124–150. doi: 10.1210/edrv-11-1-124. [DOI] [PubMed] [Google Scholar]
- Allen WR, Stewart F. Equine placentation. Reproduction, Fertility and Development. 2001;13(7–8):623–634. doi: 10.1071/rd01063. [DOI] [PubMed] [Google Scholar]
- Allman JM, Tetreault NA, Hakeem AY, Manaye KF, Semendeferi K, Erwin JE, Park S, Goubert V, Hof PR. The von Economo neurons in fronto-insular and anterior cingulate cortex. Annals of the New York Academy of Sciences. 2011;1225:59–71. doi: 10.1111/j.1749-6632.2011.06011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter HJ, Eichberg JW, Masur H, Saxinger WC, Gallo R, Macher AM, Lane HC, Fauci AS. Transmission of HTLV-III infection from human plasma to chimpanzees: an animal model for AIDS. Science. 1984;226:549–552. doi: 10.1126/science.6093251. [DOI] [PubMed] [Google Scholar]
- Amaral D, Lavenex P. Hippocampal neuroanatomy. In: Anderson P, Morris R, Amaral D, Bliss T, O'Keefe J, editors. The hippocampus book. New York: Oxford University Press; 2007. [Google Scholar]
- Ambrose Z, KewalRamani VN, Bieniasz PD, Hatziioannou T. HIV/AIDS: in search of an animal model. Trends in Biotechnology. 2007;25(8):333–337. doi: 10.1016/j.tibtech.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Antoniadis EA, Winslow JT, Davis M, Amaral DG. Role of the primate amygdala in fear-potentiated startle: effects of chronic lesions in the rhesus monkey. Journal of Neuroscience. 2007;27:7386–7396. doi: 10.1523/JNEUROSCI.5643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonow-Schlorke I, Schwab M, Cox LA, Li C, Stuchlik K, Witte OW, Nathanielsz PW, McDonald TJ. Vulnerability of the fetal primate brain to moderate reduction in maternal global nutrient availability. Proc Natl Acad Sci U S A. 2011;108(7):3011–3016. doi: 10.1073/pnas.1009838108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. American Psychiatric Association; 2013. [Google Scholar]
- Apetrei C, Kaur A, Lerche NW, Metzger M, Pandrea I, Hardcastle J, Falkenstein S, Bohm R, Koehler J, Traina-Dorge V, et al. Molecular Epidemiology of Simian Immunodeficiency Virus SIVsm in U.S. Primate Centers Unravels the Origin of SIVmac and SIVstm. Journal Of Virology. 2005;79(14):8991–9005. doi: 10.1128/JVI.79.14.8991-9005.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquinas ST. In: Summa contra Gentiles. Kenny j., editor. New York: Oxford University Press; 1955–1957. [Google Scholar]
- Armitage JA, Khan IY, Taylor PD, Nathanielsz PW, Poston L. Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals? J Physiol. 2004;561(Pt 2):355–377. doi: 10.1113/jphysiol.2004.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage JA, Poston L, Taylor PD. Developmental origins of obesity and the metabolic syndrome: the role of maternal obesity. Front Horm Res. 2008;36:73–84. doi: 10.1159/000115355. [DOI] [PubMed] [Google Scholar]
- Armitage JA, Taylor PD, Poston L. Experimental models of developmental programming: consequences of exposure to an energy rich diet during development. J Physiol. 2005;565(Pt 1):3–8. doi: 10.1113/jphysiol.2004.079756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba TW, Jeong YS, Pennick D, Bronson R, Greene MF, Ruprecht RM. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science. 1995;267(5205):1820–1825. doi: 10.1126/science.7892606. [DOI] [PubMed] [Google Scholar]
- Baba TW, Liska V, Khimani AH, Ray NB, Dailey PJ, Penninck D, Bronson R, Greene MF, McClure HM, Martin LN, et al. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat Med. 1999;5(2):194–203. doi: 10.1038/5557. [DOI] [PubMed] [Google Scholar]
- Baird G, Cass H, Slonims V. Diagnosis of autism. BMJ. 2003;327:488–493. doi: 10.1136/bmj.327.7413.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales K, Dietz J, Baker A, Miller K, Tardif SD. Effects of allocare-givers on fitness of infants and parents in callitrichid primates. Folia Primatologica. 2000;71(1–2):27–38. doi: 10.1159/000021728. [DOI] [PubMed] [Google Scholar]
- Bales KL, Kitzmann CD. Animal models for computing and communications: Past approaches and future challenges. In: Xiao Y, Hu F, editors. Bio-inspired Communicating and Computing Networks: Auerbach Publications. CRC Press; 2011. pp. 3–18. [Google Scholar]
- Banerjee P, Fazleabas AT. Endometrial responses to embryonic signals in the primate. Int J Dev Biol. 2010;54(2–3):295–302. doi: 10.1387/ijdb.082829pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Becker ML, Parker CC, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. The utility of the non-human primate; model for studying gene by environment interactions in behavioral research. Genes Brain Behav. 2003;2(6):336–340. doi: 10.1046/j.1601-1848.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- Beall MH, Haddad M, Gayle D, Desai M, Ross MG. Adult obesity as a consequence of in utero programming. Clin Obstet Gynecol. 2005;47:957–966. doi: 10.1097/01.grf.0000135668.61661.9c. [DOI] [PubMed] [Google Scholar]
- Bentham J. [1823]. An Introduction to the Principles of Morals and Legislation. Oxford: Clarendon Press; 1907. [Google Scholar]
- Binder EB, Nemeroff CB. The CRF system, stress, depression and anxiety-insights from human genetic studies. Mol Psychiatry. 2010;15(6):574–588. doi: 10.1038/mp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosu WTK, Holmdahl TH, Johansson EDB, Gemzell C. Peripheral plasma levels of oestrogens, progesterone and 17∝-hydroxyprogesterone during the menstrual cycle of the rhesus monkey. Acta Endocrinology. 1972;71:755–762. doi: 10.1530/acta.0.0710755. [DOI] [PubMed] [Google Scholar]
- Bosu WTK, Johansson EDB, Gemzell C. Peripheral plasma levels of oestrone, oestradiol-17β and progesterone during ovulatory mesntrual cycles in the rhesus monkey with special reference to the onset of menstruation. Acta Endocrinology. 1973;74:732–742. doi: 10.1530/acta.0.0740732. [DOI] [PubMed] [Google Scholar]
- Bree A, Schlerman FJ, Wadanoli M, Tchistiakova L, Marquette K, Tan XY, Jacobson BA, Widom A, Cook TA, Wood N, et al. IL-13 blockade reduces lung inflammation after Ascaris suum challenge in cynomolgus monkeys. J Allergy Clin Immunol. 2007;119(5):1251–1257. doi: 10.1016/j.jaci.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2007;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Schacker TW, Ruff LE. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner RM, Slayden OD. Molecular and functional aspects of menstruation in the macaque. Reviews in Endocrine and Metabolic Disorders. 2012;13(4):309–318. doi: 10.1007/s11154-012-9225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brus M, Keller M, Levy F. Temporal features of adult neurogenesis: differences and similarities across mammalian species. Front Neurosci. 2013;7:135. doi: 10.3389/fnins.2013.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson JJ. Agent-based modelling as scientific method: a case study analysing primate social behavior. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 2007;362:1685–1698. doi: 10.1098/rstb.2007.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher U, Reid LM. Development of the mucus-secreting elements in human lung. Thorax. 1961;16:219–225. doi: 10.1136/thx.16.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger HG, Dudley EC, Robertson DM, Dennerstein L. Hormonal changes in the menopause transition. Recent ProgHormRes. 2002;57:257–275. doi: 10.1210/rp.57.1.257. [DOI] [PubMed] [Google Scholar]
- Burri PH. Structural aspects of postnatal lung development - alveolar formation and growth. Biol Neonate. 2006;9(4):313–322. doi: 10.1159/000092868. [DOI] [PubMed] [Google Scholar]
- Butler H. Evolutionary trends in primate sex cycles. ContribPrimatol. 1974;3:2–35. [PubMed] [Google Scholar]
- Call J, Tomasello M. The gestural communication of monkeys and apes. Oxford: Psychology Press; 2007. [Google Scholar]
- Capitanio JP. Personality dimensions in adult male rhesus macaques: prediction of behaviors across time and situation. AmJPrimatol. 1999;47(4):299–320. doi: 10.1002/(SICI)1098-2345(1999)47:4<299::AID-AJP3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Emborg ME. Contributions of non-human primates to neuroscience research. Lancet. 2008;371(9618):1126–1135. doi: 10.1016/S0140-6736(08)60489-4. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Kyes RC, Fairbanks LA. Considerations in the selection and conditioning of Old World monkeys for laboratory research: animals from domestic sources. ILAR J. 2006;47(4):294–306. doi: 10.1093/ilar.47.4.294. [DOI] [PubMed] [Google Scholar]
- Casey ML, MacDonald PC. The endocrinology of human parturition. Ann N Y Acad Sci. 1997;828:273–284. doi: 10.1111/j.1749-6632.1997.tb48548.x. [DOI] [PubMed] [Google Scholar]
- Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev. 2000;21(5):514–550. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- Charrier C, Joshi K, Coutinho-Budd J, Kim JE, Lambert N, de Marchena J, Jin WL, Vanderhaeghen P, Ghosh A, Sassa T, et al. Inhibition of SRGAP2 function by its human-specific paralogs induces neoteny during spine maturation. Cell. 2012;149(4):923–935. doi: 10.1016/j.cell.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Li C, McDonald TJ, Comuzzie AG, Mattern V, Nathanielsz PW. Emergence of insulin resistance in juvenile baboon offspring of mothers exposed to moderate maternal nutrient reduction. Am J Physiol Regul Integr Comp Physiol. 2011;301(3):R757–R762. doi: 10.1152/ajpregu.00051.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christel MI. Catarrhine primates grasping smal objects: techniques and hand preferences. In: Anderson JR, Roeder JJ, Thierry B, Herrenschmidt N, editors. Current primatology vol III: Behavioral neuroscience, physiology and reproduction. Strasbourg: Universite Louis Pasteur; 1994. pp. 37–49. [Google Scholar]
- Clarkson TB, Mehaffey MH. Coronary heart disease of females: lessons learned from nonhuman primates. American Journal of Primatology. 2009;71(9):785–793. doi: 10.1002/ajp.20693. [DOI] [PubMed] [Google Scholar]
- Clements JE, Gama L, Graham DR, Mankowski JL, Zink MC. A simian immunodeficiency virus macaque model of highly active antiretroviral treatment: viral latency in the periphery and the central nervous system. [Miscellaneous Article] Current Opinion in HIV & AIDS. 2011;6(1):37–42. doi: 10.1097/COH.0b013e3283412413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline JM. Assessing the mammary gland of nonhuman primates: effects of endogenus hormones and exogenous hormonal agents and growth factors. Birth Defects Research Part B. Developmental and Reproductive Toxicology. 2007;80:126–146. doi: 10.1002/bdrb.20112. [DOI] [PubMed] [Google Scholar]
- Coffman RL, Hessel EM. Nonhuman primate models of asthma. J Exp Med. 2005;201(12):1875–1879. doi: 10.1084/jem.20050901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborators UBoD. Murray CL, Abraham J, et al. The state of us health, 1990–2010: Burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley AJ, Bernstein RM, Nguyen AD. Adrenarche in Non-human Primates: the evidence for it and the need to re-define it. J Endocrinol. 2012 doi: 10.1530/JOE-11-0467. [DOI] [PubMed] [Google Scholar]
- Conley AJ, Pattison JC, Bird IM. Variations in adrenal androgen production among (nonhuman) primates. Semin Reprod Med. 2004;22(4):311–326. doi: 10.1055/s-2004-861548. [DOI] [PubMed] [Google Scholar]
- Cook DJ, Tymianski M. Nonhuman primate models of stroke for translational neuroprotection research. Neurotherapeutics. 2012;9(2):371–379. doi: 10.1007/s13311-012-0115-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Bunge MB, Fawcett JW, Grossman RG, Kaas JH, Lemon R, Maier I, Martin J, Nudo RJ, Ramon-Cueto A, et al. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat Med. 2007;13(5):561–566. doi: 10.1038/nm1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LA, Li C, Glenn JP, Lange K, Spradling KD, Nathanielsz PW, Jansson T. Expression of the Placental Transcriptome in Maternal Nutrient Reduction in Baboons Is Dependent on Fetal Sex. J Nutr. 2013 doi: 10.3945/jn.112.172148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LA, Nijland MJ, Gilbert JS, Schlabritz-Loutsevitch NE, Hubbard GB, McDonald TJ, Shade RE, Nathanielsz PW. Effect of 30 per cent maternal nutrient restriction from 0.16 to 0.5 gestation on fetal baboon kidney gene expression. J Physiol. 2006a;572(Pt 1):67–85. doi: 10.1113/jphysiol.2006.106872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LA, Nijland MJ, Gilbert JS, Schlabritz-Loutsevitch NE, Hubbard GB, McDonald TJ, Shade RE, Nathanielsz PW. Effect of 30 per cent maternal nutrient restriction from 0.16 to 0.5 gestation on fetal baboon kidney gene expression. J Physiol. 2006b;572(Pt 1):67–85. doi: 10.1113/jphysiol.2006.106872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LA, Schlabritz-Loutsevitch N, Hubbard GB, Nijland MJ, McDonald TJ, Nathanielsz PW. Gene expression profile differences in left and right liver lobes from mid-gestation fetal baboons: a cautionary tale. The Journal of Physiology Online. 2006c;572:59–66. doi: 10.1113/jphysiol.2006.105726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford S, Santoro N, Laughlin GA, Sowers MF, McConnell D, Sutton-Tyrrell K, Weiss G, Vuga M, Randolph J, Lasley B. Circulating dehydroepiandrosterone sulfate concentrations during the menopausal transition. J Clin Endocrinol Metab. 2009;94(8):2945–2951. doi: 10.1210/jc.2009-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel MD, Kirchhoff F, Czajak SC, Sehgal PK, Desrosiers RC. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258(5090):1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- Daniel MD, Letvin NL, King NW, Kannagi M, Sehgal PK, Hunt RD, Kanki PJ, Essex M, Desrosiers RC. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985;228:1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- Davis JS, Rueda BR. The corpus luteum: an ovarian structure with maternal instincts and suicidal tendencies. Front Biosci. 2002;7:d1949–d1978. doi: 10.2741/davis1. [DOI] [PubMed] [Google Scholar]
- Davis M, Antoniadis EA, Amaral DG, Winslow JT. Acoustic startle reflex in rhesus monkeys: a review. Reviews in the Neurosciences. 2008;19:171–185. doi: 10.1515/revneuro.2008.19.2-3.171. [DOI] [PubMed] [Google Scholar]
- Davis MM. A prescription for human immunology. Immunity. 2008;29(6):835–838. doi: 10.1016/j.immuni.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete GQ, Lifson JD. Considerations in the development of nonhuman primate models of combination antiretroviral therapy for studies of AIDS virus suppression, residual virus, and curative strategies. [Miscellaneous Article] Current Opinion in HIV & AIDS. 2013;8(4):262–272. doi: 10.1097/COH.0b013e328361cf40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaria MA, Casto M, O'Connell M, Johnson RP, Rosenzweig M. Characterization of lymphocyte subsets in rhesus macaques during the first year of life. Eur J Haematol. 2000;65(4):245–257. doi: 10.1034/j.1600-0609.2000.065004245.x. [DOI] [PubMed] [Google Scholar]
- Desai M, Gayle D, Babu J, Ross MG. Permanent reduction in heart and kidney organ growth in offspring of undernourished rat dams. American Journal of Obstetrics and Gynecology. 2005;193:1224–1232. doi: 10.1016/j.ajog.2005.05.041. [DOI] [PubMed] [Google Scholar]
- Dettmer AM. The integrative biology of reproductive function in nonhuman primates. American Journal of Primatology. 2013;75:197–201. doi: 10.1002/ajp.22054. [DOI] [PubMed] [Google Scholar]
- Dixson AF. Primate Sexuality. Oxford: Oxford University Press; 1998. Sexual behaviour and sexual response; pp. 93–145. [Google Scholar]
- Dobard C, Sharma S, Martin A, Pau CP, Holder A, Kuklenyik Z, Lipscomb J, Hanson DL, Smith J, Novembre FJ, et al. Durable Protection from Vaginal Simian-Human Immunodeficiency Virus Infection in Macaques by Tenofovir Gel and Its Relationship to Drug Levels in Tissue. Journal Of Virology. 2012;86(2):718–725. doi: 10.1128/JVI.05842-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doody KJ, Lorence MC, Mason JI, Simpson ER. Expression of messenger ribonucleic acid species encoding steroidogenic enzymes in human follicles and corpora lutea throughout the menstrual cycle. J Clin Endocrinol Metab. 1990;70(4):1041–1045. doi: 10.1210/jcem-70-4-1041. [DOI] [PubMed] [Google Scholar]
- Downs JL, Urbanski HF. Neuroendocrine changes in the aging reproductive axis of female rhesus macaques (Macaca mulatta) BiolReprod. 2006;75(4):539–546. doi: 10.1095/biolreprod.106.051839. [DOI] [PubMed] [Google Scholar]
- Eckstein P, Zuckerman S. Changes in the accessory reproductive organs of the non-pregnant female. In: Parkes AS, Parkes, editors. Marshall's Physiology of Reproduction. London: Longmans; 1956. pp. 543–654. [Google Scholar]
- Emerald BS, Chng K, Masuda S, Sloboda DM, Vickers MH, Kambadur R, Gluckman PD. Gene expression profiling in the Cynomolgus macaque Macaca fascicularis shows variation within the normal birth range. BMC Genomics. 2011;12:509. doi: 10.1186/1471-2164-12-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery Thompson M. Comparative reproductive energetics of human and nonhuman primates. Annual Review of Anthropology. 2013;42:287–304. [Google Scholar]
- Engle JR, Tinling S, Recanzone GH. Age-related hearing loss in rhesus monkeys is correlated with cochlear histopathologies. PLoS One. 2013;8(2):e55092. doi: 10.1371/journal.pone.0055092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DT, Silvestri G. Nonhuman primate models in AIDS research. [Miscellaneous Article] Current Opinion in HIV & AIDS. 2013;8(4):255–261. doi: 10.1097/COH.0b013e328361cee8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedurek P, Slocombe KE. Primate vocal communication: a useful tool for understanding human speech and language evolution? Human Biology. 2011;83(2):153–173. doi: 10.3378/027.083.0202. [DOI] [PubMed] [Google Scholar]
- Ferin M. Neuroendocrine control of ovarian function in the primate. Journal of Reproduction and Fertility. 1983;69(1):369. doi: 10.1530/jrf.0.0690369. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque E, Huck M. Till death (or an intruder) do us part: intrasexual competition in a monogamous primate. PLoS One. 2013;8:e53724. doi: 10.1371/journal.pone.0053724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Duque E, Mason WA, Mendoza SP. Effects of separation on responses to mates and strangers in the monogamous titi monkey. American Journal of Primatology. 1997;43:225–237. doi: 10.1002/(SICI)1098-2345(1997)43:3<225::AID-AJP3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Fernandez-Twinn DS, Ozanne SE. Early life nutrition and metabolic programming. Ann N Y Acad Sci. 2010;1212:78–96. doi: 10.1111/j.1749-6632.2010.05798.x. [DOI] [PubMed] [Google Scholar]
- Finch CE, Austad SN. Primate aging in the mammalian scheme: the puzzle of extreme variation in brain aging. Age. 2012;34:1075–1091. doi: 10.1007/s11357-011-9355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowden AL, Giussani DA, Forhead AJ. Intrauterine programming of physiological systems: causes and consequences. Physiology (Bethesda) 2006;21:29–37. doi: 10.1152/physiol.00050.2005. [DOI] [PubMed] [Google Scholar]
- Fuentes A. Re-evaluating primate monogamy. American Anthropologist. 1999;100:890–907. [Google Scholar]
- Gallup GG. Chimpanzees: Self-recognition. Science. 1970;167(3914):86–87. doi: 10.1126/science.167.3914.86. [DOI] [PubMed] [Google Scholar]
- Gao F, Bailes E, Robertson DL. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature. 1999;397:436. doi: 10.1038/17130. [DOI] [PubMed] [Google Scholar]
- Garcia-Lerma JG, Heneine W. Animal models of antiretroviral prophylaxis for HIV prevention. [Miscellaneous Article] Current Opinion in HIV & AIDS. 2012;7(6):505–513. doi: 10.1097/COH.0b013e328358e484. [DOI] [PubMed] [Google Scholar]
- Getz GS, Reardon CA. Animal models of atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(5):1104–1115. doi: 10.1161/ATVBAHA.111.237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilardi KVK, Shideler SE, Valverde CR, Roberts JA, Lasley BL. Characterization of the onset of menopause in the rhesus macaque. Biology of Reproduction. 1997;57(2):335–340. doi: 10.1095/biolreprod57.2.335. [DOI] [PubMed] [Google Scholar]
- Goodman AL, Descalzi CD, Johnson DK, Hodgen GD. Composite pattern of circulating LH, FSH, estradiol, and progesterone during the menstrual cycle in cynomolgus monkeys. Proceedings of the Society for Experimental Biology and Medicine. 1977;155:479–481. doi: 10.3181/00379727-155-39834. [DOI] [PubMed] [Google Scholar]
- Gore AC, Windsor-Engnell BM, Terasawa E. Menopausal increases in pulsatile gonadotropin-releasing hormone release in a nonhuman primate (Macaca mulatta) Endocrinology. 2004;145(10):4653–4659. doi: 10.1210/en.2004-0379. [DOI] [PubMed] [Google Scholar]
- Grant RM. Antiretroviral Agents Used by HIV-Uninfected Persons for Prevention: Pre- and Postexposure Prophylaxis. Clinical Infectious Diseases. 2010;50(Supplement 3):S96–S101. doi: 10.1086/651479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapía Mn, Guanira-Carranza JV, Ramirez-Cardich ME, et al. Preexposure Chemoprophylaxis for HIV Prevention in Men Who Have Sex with Men. New England Journal of Medicine. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson BE, Allen SE, Billes SK, Williams SM, Smith MS, Grove KL. Prenatal development of hypothalamic neuropeptide systems in the nonhuman primate. Neuroscience. 2006;143:975–986. doi: 10.1016/j.neuroscience.2006.08.055. [DOI] [PubMed] [Google Scholar]
- Grayson BE, Levasseur PR, Williams SM, Smith MS, Marks DL, Grove KL. Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet. Endocrinology. 2010;151(4):1622–1632. doi: 10.1210/en.2009-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield PM, Savage-Rumbaugh ES. Grammatical combination in Pan paniscus: processes of learning and invention in the evolution and development of language. In: Parker ST, Gibson KR, editors. "Language" and intelligence in monkeys and apes: Comparative developmental perspectives. New York: Cambridge University Press; 1990. pp. 540–578. [Google Scholar]
- Grodstein F, Manson JE, Colditz GA, Willet WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Annals of Internal Medicine. 2000;133:933–941. doi: 10.7326/0003-4819-133-12-200012190-00008. [DOI] [PubMed] [Google Scholar]
- Group B. The Boyd Group papers on the use of non-human primates in research and testing. Leicester: The British Psychological Society. 2002 [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biological Psychiatry. 2008;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Guergnon J, Theodorou I. What did we learn on host's genetics by studying large cohorts of HIV-1-infected patients in the genome-wide association era? Curr Opin HIV AIDS. 2011;6(4):290–296. doi: 10.1097/COH.0b013e3283478449. [DOI] [PubMed] [Google Scholar]
- Haase AT. Early Events in Sexual Transmission of HIV and SIV and Opportunities for Interventions. Annual Review of Medicine. 2011;62(1):127–139. doi: 10.1146/annurev-med-080709-124959. [DOI] [PubMed] [Google Scholar]
- Habbershon HM, Ahmed SZ, Cohen YE. Rhesus macaques recognize unique multimodal face-voice relations of familiar individuals and not of unfamiliar ones. Brain, Behavior, and Evolution. 2013 doi: 10.1159/000351203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. American Journal of Psychiatry. 2012;169(7):693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PubMed] [Google Scholar]
- Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473(7348):523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Jr, M P, Ventura AB, Hughes CM, Gilbride RM, Ford JC, Oswald K, Shoemaker R, Li Y, Lewis MS, et al. Immune clearance of highly pathogenic SIV infection. Nature. 2013;502(7469):100–104. doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkema JR, Plopper CG, Hyde DM, St George JA, Wilson DW, Dungworth DL. Response of macaque bronchiolar epithelium to ambient concentrations of ozone. Am J Pathol. 1993;143(3):857–866. [PMC free article] [PubMed] [Google Scholar]
- Harlow H. The nature of learning sets. Psychological Review. 1949;56:51–65. doi: 10.1037/h0062474. [DOI] [PubMed] [Google Scholar]
- Hass CA, Horwitz GD. V1 mechanisms underlying chromatic contrast detection. J Neurophysiol. 2013;109(10):2483–2494. doi: 10.1152/jn.00671.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatziioannou T, Ambrose Z, Chung NPY, Piatak M, Yuan F, Trubey CM, Coalter V, Kiser R, Schneider D, Smedley J, et al. A macaque model of HIV-1 infection. Proceedings of the National Academy of Sciences. 2009;106(11):4425–4429. doi: 10.1073/pnas.0812587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearn JP. The embryo-maternal dialogue during early pregnancy in primates. J Reprod Fertil. 1986;76(2):809–819. doi: 10.1530/jrf.0.0760809. [DOI] [PubMed] [Google Scholar]
- Heffron R, Donnell D, Rees H, Celum C, Mugo N, Were E, de Bruyn G, Nakku-Joloba E, Ngure K, Kiarie J, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. The Lancet Infectious Diseases. 2012;12(1):19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise C, Miller CJ, Lackner A, Dandekar S. Primary acute simian immunodeficiency virus infection of intestinal lymphoid tissue is associated with gastrointestinal dysfunction. JInfectDis. 1994;169:1116. doi: 10.1093/infdis/169.5.1116. [DOI] [PubMed] [Google Scholar]
- Hillier SLP, Moench TMD, Shattock RP, Black RP, Reichelderfer PP, Veronese FP. In Vitro and In Vivo: The Story of Nonoxynol 9. [Review] JAIDS Journal of Acquired Immune Deficiency Syndromes. 2005;39(1):1–8. doi: 10.1097/01.qai.0000159671.25950.74. [DOI] [PubMed] [Google Scholar]
- Hirsch VM, Johnson PR. Pathogenesis of experimental SIV infection of macaques. SeminVirol. 1992;3:175–183. [Google Scholar]
- Hirsch VM, Olmsted RA, Murphey-Corb M, Purcell RH, Johnson PR. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature. 1989;339(6223):389–392. doi: 10.1038/339389a0. [DOI] [PubMed] [Google Scholar]
- Hodgen GD, Goodman AL, O'Connor A, Johnson DK. Menopause in rhesus monkeys: model for study of disorders in the human climacteric. AmJObstetGynecol. 1977;127(6):581–584. doi: 10.1016/0002-9378(77)90352-0. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Behavioral and brain asymmetries in chimpanzees: A case for continuity. Annals of the New York Academy of Sciences. 2013;1288:27–35. doi: 10.1111/nyas.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper B, Tullner WW. Urinary estrone and plasma progesterone levels during the menstrual cycle of the rhesus monkey. Endocrinology. 1970;86(6):1225–1230. doi: 10.1210/endo-86-6-1225. [DOI] [PubMed] [Google Scholar]
- Hu SL, Abrams K, Barber GN, Moran P, Zarling JM, Langlois AJ, Kuller L, Morton WR, Benveniste RE. Protection of macaques against SIV infection by subunit vaccines of SIV envelope glycoprotein gp160. Science. 1992;255(5043):456–459. doi: 10.1126/science.1531159. [DOI] [PubMed] [Google Scholar]
- Hulley S, Grady D, Bush TL, Furberg C, Herrington D, Riggs BL, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- Hutchison RM, Everling S. Monkey in the middle: why non-human primates are needed to bridge the gap in resting-state investigations. Frontiers in Neuroanatomy. 2012;6:1–19. doi: 10.3389/fnana.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde DM, Blozis SA, Avdalovic MV, Putney LF, Dettorre R, Quesenberry NJ, Singh P, Tyler NK. Alveoli increase in number but not size from birth to adulthood in rhesus monkeys. Am J Physiol Lung Cell Mol Physiol. 2007;293(3):L570–L579. doi: 10.1152/ajplung.00467.2006. [DOI] [PubMed] [Google Scholar]
- Insausti R. Comparative neuroanatomical parcellation of the human and nonhuman primate temporal pole. J Comp Neurol. 2013 doi: 10.1002/cne.23431. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327(5963):291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbour HN, Kelly RW, Fraser HM, Critchley HO. Endocrine regulation of menstruation. EndocrRev. 2006;27(1):17–46. doi: 10.1210/er.2004-0021. [DOI] [PubMed] [Google Scholar]
- Jarcho MR, Mendoza SP, Mason WA, Yang X, Bales KL. Intranasal vasopressin affects pair bonding and peripheral gene expression in male Callicebus cupreus. Genes Brain Behav. 2011;10(3):375–383. doi: 10.1111/j.1601-183X.2010.00677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayo MJ, Register TC, Carlson CS. Effects on bone of oral hormone replacement therapy initiated 2 years after ovariectomy in young adult monkeys. Bone. 1998;23:361–366. doi: 10.1016/s8756-3282(98)00106-9. [DOI] [PubMed] [Google Scholar]
- Jeffery P, Reid L. Ultrastructure of airway epithelium and submucosal glands during development. Marcel Dekker, Inc.; 1977. pp. 87–134. [Google Scholar]
- Jerome CP, Carlson CS, Register TC, Bain FT, Jayo MJ, Weaver DS, Adams MR. Bone functional changes in intact, ovariectomized, and overiectomized hormone-supplemented adult cynomolgus monkeys (Macaca fascicularis) evaluated by serum markers and dynamic histomorphometry. Journal of Bone and Mineral Research. 1994;9:527–540. doi: 10.1002/jbmr.5650090413. [DOI] [PubMed] [Google Scholar]
- Jewitt DA, Dukelow WR. Cyclicity and gestation length of Macaca fascicularis. Primates. 1972;13:327–330. [Google Scholar]
- Johnson RL, Kapsalis E. Ageing, infecundity, and reproductive senescence in free-ranging female rhesus monkeys. Journal of Reproduction and Fertility. 1995;105:271–278. doi: 10.1530/jrf.0.1050271. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Kapsalis E. Menopause in free-ranging rhesus macaques: Estimated incidence, relation to body condition, and adaptive significance. International Journal of Primatology. 1998;19(4):751–765. [Google Scholar]
- Jokinen MP, Clarkson TB, Prichard RW. Animal models in atherosclerosis research. Experimental and Molecular Pathology. 1985;42(1):1–28. doi: 10.1016/0014-4800(85)90015-2. [DOI] [PubMed] [Google Scholar]
- Jolly A. Hour of birth in primates and man. Folia Primatologica. 1972;18:108–121. doi: 10.1159/000155472. [DOI] [PubMed] [Google Scholar]
- Jolly CJ, Burrell AS, Phillips-Conroy JE, Bergey C, Rogers J. Kinda baboons (Papio kindae) and grayfoot chacma baboons (P. ursinus griseipes) hybridize in the Kafue river valley, Zambia. Am J Primatol. 2011;73(3):291–303. doi: 10.1002/ajp.20896. [DOI] [PubMed] [Google Scholar]
- Jones KP, Walker LC, Anderson D, Lacreuse A, Robson SL, Hawkes K. Depletion of ovarian follicles with age in chimpanzees: similarities to humans. BiolReprod. 2007;77(2):247–251. doi: 10.1095/biolreprod.106.059634. [DOI] [PubMed] [Google Scholar]
- Kant I. The Metaphysics of Morals. Cambridge: Cambridge University Press; 1996 [1797]. [Google Scholar]
- Kaplan JR. Origins and health consequences of stress-induced ovarian dysfunction. Interdisciplinary Topics in Gerontology. 2008;36:162–185. doi: 10.1159/000137709. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Chen H, Manusck SB. The relationship between social status and atherosclerosis in male and female monkeys as revealed by meta-analysis. American Journal of Primatology. 2009;71:732–741. doi: 10.1002/ajp.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr JF, Kantor JA, Hand PH, Eggensperger DL, Schlom J. The presence of prostate-specific antigen-related genes in primates and the expression of recombinant human prostate-specific antigen in a transfected murine cell line. Cancer Res. 1995;55(11):2455–2462. [PubMed] [Google Scholar]
- Karsch FJ, Bittman EL, Foster DL, Goodman RL, Legan SJ, Robinson JE. Neuroendocrine basis of seasonal reproduction. Recent ProgHormRes. 1984;40:185–232. doi: 10.1016/b978-0-12-571140-1.50010-4. [DOI] [PubMed] [Google Scholar]
- Karsch FJ, Dierschke DJ, Knobil E. Sexual differentiation of pituitary function: apparent difference bewteen primates and rodents. Science. 1973;179(4072):484–486. doi: 10.1126/science.179.4072.484. [DOI] [PubMed] [Google Scholar]
- Kavanagh K, Williams JK, Wagner JD. Naturally occurring menopause in cynomolgus monkeys: changes in hormone, lipid, and carbohydrate measures with hormonal status. Journal of Medical Primatology. 2005;34(4):171–177. doi: 10.1111/j.1600-0684.2005.00114.x. [DOI] [PubMed] [Google Scholar]
- Keele BF, Li H, Learn GH, Hraber P, Giorgi EE, Grayson T, Sun C, Chen Y, Yeh WW, Letvin NL, et al. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J Exp Med. 2009;206(5):1117–1134. doi: 10.1084/jem.20082831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan K, Bartlett TQ, Nijland M, Rodriguez JS, Nathanielsz PW, Zürcher NR. Poor nutrition during pregnancy and lactation negatively affects neurodevelopment of the offspring: evidence from a translational primate model. Am J Clin Nutr. 2013 doi: 10.3945/ajcn.112.040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Jessen HM, Auger AP, Terasawa E. Postmenopausal increase in KiSS-1, GPR54, and luteinizing hormone releasing hormone (LHRH-1) mRNA in the basal hypothalamus of female rhesus monkeys. Peptides. 2009;30(1):103–110. doi: 10.1016/j.peptides.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmelman J, London AJ, Ravina B, Ramsay T, Bernstein M, Fine A, Stahnisch FW, Emborg ME. Launching invasive, first-in-human trials against Parkinson's disease: ethical considerations. Mov Disord. 2009;24(13):1893–1901. doi: 10.1002/mds.22712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt NR, Canary LA, Sun X, Vinton CL, Funderburg NT, Morcock DR, ones M, Deming CB, Perkins M, Hazuda DJ, et al. Probiotic/prebiotic supplementation of antiretrovirals improves gastrointestinal immunity in SIV-infected macaques. The Journal of Clinical Investigation. 2013;123(2):903–907. doi: 10.1172/JCI66227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt NR, Harris LD, Vinton CL, Sung H, Briant JA, Tabb B, Morcock D, McGinty JW, Lifson JD, Lafont BA, et al. Compromised gastrointestinal integrity in pigtail macaques is associated with increased microbial translocation, immune activation, and IL-17 production in the absence of SIV infection. MucosalImmunol. 2010;3(4):387–398. doi: 10.1038/mi.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler W. The Mentality of Apes. New York: Harcourt, Brace & World; 1925. [Google Scholar]
- Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3(8):711–715. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- Koo BB, Oblak AL, Zhao Y, Farris CW, Bowley B, Rosene DL, Killiany RJ. Hippocampal network connections account for differences in memory performance in the middle-aged rhesus monkey. Hippocampus. 2013 doi: 10.1002/hipo.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosova G, Urbanek M. Genetics of the polycystic ovary syndrome. Mol Cell Endocrinol. 2013;373(1–2):29–38. doi: 10.1016/j.mce.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke CD. Guest editor's introduction. Methods. 2010;50(3):123–124. doi: 10.1016/j.ymeth.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Krubitzer L. The magnificent compromise: cortical field evolution in mammals. Neuron. 2007;56(2):201–208. doi: 10.1016/j.neuron.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Kumar S, Hedges SB. A molecular timescale for vertebrate evolution. Nature. 1998;392:917–920. doi: 10.1038/31927. [DOI] [PubMed] [Google Scholar]
- Lacreuse A. Effects of ovarian hormones on cognitive function in nonhuman primates. Neuroscience. 2006;138:859–867. doi: 10.1016/j.neuroscience.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Chennareddi L, Gould KG, Hawkes K, Wijayawardana SR, Chen J, Easley KA, Herndon JG. Menstrual cycles continue into advanced old age in the common chimpanzee (Pan troglodytes) BiolReprod. 2008;79(3):407–412. doi: 10.1095/biolreprod.108.068494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasley BL, Santoro N, Randolf JF, Gold EB, Crawford S, Weiss G, McConnell DS, Sowers MF. The relationship of circulating dehydroepiandrosterone, testosterone, and estradiol to stages of the menopausal transition and ethnicity. JClinEndocrinol Metab. 2002;87(8):3760–3767. doi: 10.1210/jcem.87.8.8741. [DOI] [PubMed] [Google Scholar]
- Lemay DG, Hovey RC, Hartono SR, Hinde K, Smilowitz JT, Ventimiglia F, Schmidt KA, Lee JW, Islas-Trejo A, Silva PI, et al. Sequencing the transcriptome of milk production: milk trumps mammary tissue. BMC Genomics. 2013;14:872. doi: 10.1186/1471-2164-14-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Meyer J, Glatz K, Flugge G, Hinney A, Hebebrand J, Klauck SM, Poustka A, Poustka F, Bengel D, et al. The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: alternative biallelic variation in rhesus monkeys. Rapid communication. J Neural Transm. 1997;104(11–12):1259–1266. doi: 10.1007/BF01294726. [DOI] [PubMed] [Google Scholar]
- Leung YW, Flora DB, Gravely S, Irvine J, Carney RM, Grace SL. The impact of premorbid and postmorbid depression onset on mortality and cardiac morbidity among patients with coronary heart disease: meta-analysis. Psychosomatic Medicine. 2012;74(8):786–801. doi: 10.1097/PSY.0b013e31826ddbed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Schlabritz-Loutsevitch NE, Hubbard GB, Han V, Nygard K, Cox LA, McDonald TJ, Nathanielsz PW. Effects of maternal global nutrient restriction on fetal baboon hepatic insulin-like growth factor system genes and gene products. Endocrinology. 2009;150(10):4634–4642. doi: 10.1210/en.2008-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Sloboda DM, Vickers MH. Maternal obesity and developmental programming of metabolic disorders in offspring: evidence from animal models. Exp Diabetes Res. 2011;2011:592408. doi: 10.1155/2011/592408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Xiao Y. A survey of biological collaboration models. Journal of Ambient Intelligence and Humanized Computing. 2012 [Google Scholar]
- Lifson JD, Haigwood NL. Lessons in Nonhuman Primate Models for AIDS Vaccine Research: From Minefields to Milestones. Cold Spring Harbor Perspectives in Medicine. 2012;2(6) doi: 10.1101/cshperspect.a007310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhu F, Wang G, Xiao Z, Wang H, Tang J, Wang X, Qiu D, Liu W, Cao Z, et al. Association of corticotropin-releasing hormone receptor1 gene SNP and haplotype with major depression. Neurosci Lett. 2006;404(3):358–362. doi: 10.1016/j.neulet.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Loffredo JT, Maxwell J, Qi Y, Glidden CE, Borchardt GJ, Soma T, Bean AT, Beal DR, Wilson NA, Rehrauer WM, et al. Mamu-B*08-positive macaques control simian immunodeficiency virus replication. J Virol. 2007;81(16):8827–8832. doi: 10.1128/JVI.00895-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald PC, Cutrer S, MacDonald SC, Casey ML, Parker CR., Jr Regulation of extraadrenal steroid 21-hydroxylase activity. Increased conversion of plasma progesterone to deoxycorticosterone during estrogen treatment of women pregnant with a dead fetus. J ClinInvest. 1982;69(2):469–478. doi: 10.1172/JCI110471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Nelson EE. Eye-tracking with nonhuman primates is now more accessible than ever before. American Journal of Primatology. 2011;73:562–569. doi: 10.1002/ajp.20928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloyan A, Muralimanoharan S, Huffman S, Cox LA, Nathanielsz PW, Myatt L, Nijland MJ. Identification and Comparative Analyses of Myocardial miRNAs Involved in the Fetal Response to Maternal Obesity. Physiol Genomics. 2013 doi: 10.1152/physiolgenomics.00050.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniar-Hew K, Postlethwait EM, Fanucchi MV, Ballinger CA, Evans MJ, Harkema JR, Carey SA, McDonald RJ, Bartolucci AA, Miller LA. Postnatal episodic ozone results in persistent attenuation of pulmonary and peripheral blood responses to LPS challenge. Am J Physiol Lung Cell Mol Physiol. 2011;300(3):L462–L471. doi: 10.1152/ajplung.00254.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapes S, Tarantal AF, Parker CR, Moran FM, Bahr JM, Pyter L, Conley AJ. Adrenocortical cytochrome b5 expression during fetal development of the rhesus macaque. Endocrinology. 2002;143(4):1451–1458. doi: 10.1210/endo.143.4.8718. [DOI] [PubMed] [Google Scholar]
- Markham A. Adaptive social hierarchies: from nature to networks. In: Xiao Y, editor. Bio-Inspired Computing and Networking. Boca Raton, FL: CRC Press; 2011. pp. 305–350. [Google Scholar]
- Marx PA, Spira AI, Gettie A. Progesterone implants enhance SIV vaginal transmission and early virus load. NatMed. 1996;2:1084. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- Mason WA, Mendoza SP. Generic aspects of primate attachments: Parents, offspring and mates. Psychoneuroendocrinology. 1998;23(8):765–778. doi: 10.1016/s0306-4530(98)00054-7. [DOI] [PubMed] [Google Scholar]
- Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434(7037):1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- Matzinger P. The danger model: a renewed sense of self. Science. 2002;296(5566):301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- McDonald T, Nijland M, Nathanielsz P. The insulin-like growth factor system and the fetal brain: Effects of poor maternal nutrition. Reviews in Endocrine & Metabolic Disorders. 2007;8:71–84. doi: 10.1007/s11154-007-9044-2. [DOI] [PubMed] [Google Scholar]
- Mehandru S, Poles MA, Tenner-Racz K. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melampy RM, Anderson LL. Role of the uterus in corpus luteum function. JAnim Sci. 1968;27(Suppl 1):77–96. [PubMed] [Google Scholar]
- Mellick PW, Dungworth DL, Schwartz LW, Tyler WS. Short term morphologic effects of high ambient levels of ozone on lungs of rhesus monkeys. Lab Invest. 1977;36(1):82–90. [PubMed] [Google Scholar]
- Mendelson CR. Minireview: fetal-maternal hormonal signaling in pregnancy and labor. MolEndocrinol. 2009;23(7):947–954. doi: 10.1210/me.2009-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza SP, Mason WA. Autonomic balance in Saimiri sciureus and Callicebus moloch: Relation to life-style. Folia Primatologica. 1997;68:307–318. doi: 10.1159/000157256. [DOI] [PubMed] [Google Scholar]
- Menzel EW, menzel CR. Do primates plan routes? Simple detour problems reconsidered. In: Washburn DA, editor. Primate perspectives on behavior and cognition. Washington, DC: Americal Psychological Association; 2007. [Google Scholar]
- Mestas J, Hughes CC. Of Mice and Not Men: Differences between Mouse and Human Immunology. J Immunol. 2004;172(5):2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Agid Y, Brune M, Bullmore ET, Carter CS, Clayton NS, Connor R, Davis S, Deakin B, DeRubeis RJ, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes, and the quest for improved therapy. Nature Reviews Drug Discovery. 2012;11:141–168. doi: 10.1038/nrd3628. [DOI] [PubMed] [Google Scholar]
- Millan MJ, bales KL. Towards improved models for evaluating social cognition and its disruption in schizophrenia: the CNTRICS initiative. Neuroscience and Biobehavioral Reviews epub ahead of print. 2013 doi: 10.1016/j.neubiorev.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Mills KH. TLR-dependent T cell activation in autoimmunity. Nat Rev Immunol. 2011;11(12):807–822. doi: 10.1038/nri3095. [DOI] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB. Impairment in abstraction and set shifting in aged rhesus monkeys. Neurobiology of Aging. 2003;24:125–134. doi: 10.1016/s0197-4580(02)00054-4. [DOI] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB. A non-human primate test of abstraction and set shifting: an automated adaptation of the Wisconsin Card Sorting Test. Journal of Neurocience Methods. 2005;146:165–173. doi: 10.1016/j.jneumeth.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB. Executive system dysfunction occurs as early as middle-age in the rhesus monkey. Neurobiology of Aging. 2006;27:1484–1493. doi: 10.1016/j.neurobiolaging.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Morimoto S, Calzada L, Sosa TC, Reyes-Castro LA, Rodriguez-González GL, Morales A, Nathanielsz PW, Zambrano E. Emergence of ageing-related changes in insulin secretion by pancreatic islets of male rat offspring of mothers fed a low-protein diet. Br J Nutr. 2011:1–4. doi: 10.1017/S0007114511004855. [DOI] [PubMed] [Google Scholar]
- Morris CW. The idea of moral standing. In: Beauchamp TL, Frey RG, editors. The Oxford Book of Animal Ethics. Oxford University Press; 2011. pp. 225–275. [Google Scholar]
- Morris RW, Weickert CS, Loughland CM. Emotional face processing in schizophrenia. Current Opinion in Psychiatry. 2009;22:140–146. doi: 10.1097/YCO.0b013e328324f895. [DOI] [PubMed] [Google Scholar]
- Mubiru JN, Hubbard GB, Dick EJ, Jr, Furman J, Troyer DA, Rogers J. Nonhuman primates as models for studies of prostate specific antigen and prostatic diseases. Prostate. 2008;68(14):1546–1554. doi: 10.1002/pros.20814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara AH, Bernot T, Tuszynski MH. Age-related cognitive deficits in rhesus monkeys mirroe human deficits on an automated test battery. Neurobiology of Aging. 2010;31:1020–1031. doi: 10.1016/j.neurobiolaging.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanielsz PW. Comparative studies on the initiation of labor. Eur J Obstet Gynecol Reprod Biol. 1998;78(2):127–132. doi: 10.1016/s0301-2115(98)00058-x. [DOI] [PubMed] [Google Scholar]
- Neill JD. Sexual differences in the hypothalamic regulation of prolactin secretion. Endocrinology. 1972;90(5):1154–1159. doi: 10.1210/endo-90-5-1154. [DOI] [PubMed] [Google Scholar]
- Neill JD, Freeman ME, Tillson SA. Control of the proestrus surge of prolactin and luteinizing hormone secretion by estrogens in the rat. Endocrinology. 1971;89(6):1448–1453. doi: 10.1210/endo-89-6-1448. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Matthews P, Poston L. Maternal metabolism and obesity: modifiable determinants of pregnancy outcome. Hum Reprod Update. 2010;16(3):255–275. doi: 10.1093/humupd/dmp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville MC, Anderson SM, McManaman JL, Badger TM, Bunik M, Contractor N, Crume T, Dabelea D, Donovan SM, Forman N, et al. Lactation and neonatal nutrition: defining and refining the critical questions. Journal of Mammary Gland Biology and Neoplasia. 2012;17:167–188. doi: 10.1007/s10911-012-9261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AD, Conley AJ. Adrenal androgens in humans and nonhuman primates: production, zonation and regulation. EndocrDev. 2008;13:33–54. doi: 10.1159/000134765. [DOI] [PubMed] [Google Scholar]
- Nichols SM, Bavister BD, Brenner CA, Didier PJ, Harrison RM, Kubisch HM. Ovarian senescence in the rhesus monkey (Macaca mulatta) Hum Reprod. 2005;20(1):79–83. doi: 10.1093/humrep/deh576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijland MJ, Ford SP, Nathanielsz PW. Prenatal origins of adult disease. Curr Opin Obstet Gynecol. 2008;20(2):132–138. doi: 10.1097/GCO.0b013e3282f76753. [DOI] [PubMed] [Google Scholar]
- Nijland MJ, Mitsuya K, Li C, Ford S, McDonald TJ, Nathanielsz PW, Cox LA. Epigenetic modification of fetal baboon hepatic phosphoenolpyruvate carboxykinase following exposure to moderately reduced nutrient availability. The Journal of Physiology. 2010;588(Pt 8):1349–1359. doi: 10.1113/jphysiol.2009.184168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijland MJ, Schlabritz-Loutsevitch NE, Hubbard GB, Nathanielsz PW, Cox LA. Non-human primate fetal kidney transcriptome analysis indicates mammalian target of rapamycin (mTOR) is a central nutrient-responsive pathway. J Physiol. 2007;579(Pt 3):643–656. doi: 10.1113/jphysiol.2006.122101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak MA, Hamel AF, Kelly BJ, Dettmer AM, Meyer JS. Stress, the HPA axis, and nonhuman primate well-being: a review. Applied Animal Behaviour Science. 2013;143:135–149. doi: 10.1016/j.applanim.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Bleness M, Searles VB, Varki A, Gagneux P, Sikela JM. Evolution of genetic and genomic features unique to the human lineage. Nat Rev Genet. 2012;13(12):853–866. doi: 10.1038/nrg3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty JE, Lebedev MA, Ifft PJ, Zhuang KZ, Shokur S, Bleuler H, Nicolelis MA. Active tactile exploration using a brain-machine-brain interface. Nature. 2011;479(7372):228–231. doi: 10.1038/nature10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil SP, Novembre FJ, Hill AB, Suwyn C, Hart CE, Evans-Strickfaden T, Anderson DC, deRosayro J, Herndon JG, Saucier M, et al. Progressive Infection in a Subset of HIV-1,ÄîPositive Chimpanzees. Journal of Infectious Diseases. 2000;182(4):1051–1062. doi: 10.1086/315823. [DOI] [PubMed] [Google Scholar]
- O'Sullivan A, He X, McNiven EM, Hinde K, Haggarty NW, Lonnerdal B, Slupsky CM. Metabolomic phenotyping validates the infant rhesus monkey as a model of human infant metabolism. Journal of Pediatric Endocrinology and Nutrition. 2013;56:355–363. doi: 10.1097/MPG.0b013e31827e1f07. [DOI] [PubMed] [Google Scholar]
- Okuyama S, Iwata J, Tanji J, Mushiake H. Goal-oriented, flexible use of numerical operations by monkeys. Animal Cognition. 2013;16:509–518. doi: 10.1007/s10071-012-0592-9. [DOI] [PubMed] [Google Scholar]
- Paganini-Hill A, Henderson VW. Estrogen replacement therapy and risk of Alzheimer disease. Arch Intern Med. 1996;156(19):2213–2217. [PubMed] [Google Scholar]
- Papadopoulou H, Taylor PR, Poston RN. Evidence for a role of MCP-1 and GRO-chemokines in the adhesion of monocytes to human atherosclerotic plaques. Atherosclerosis. 2003;(Suppl) 147-. [Google Scholar]
- Parr LA, Modi ME, Siebert E, Young LJ. Intranasal oxytocin selectively attenuates rhesus monkeys' attention to negative facial expressions. Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham R. How good is the macaque monkey model of the human brain? Current Opinion in Neurobiology. 2009;19:6–11. doi: 10.1016/j.conb.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattison JC, Saltzman W, Abbott DH, Hogan BK, Nguyen AD, Husen B, Einspanier A, Conley AJ, Bird IM. Gender and gonadal status differences in zona reticularis expression in marmoset monkey adrenals: Cytochrome b5 localization with respect to cytochrome P450 17,20-lyase activity. Molecular and Cellular Endocrinology. 2007;265–266:93–101. doi: 10.1016/j.mce.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton DL, Thwin SS, Meier A, Hooton TM, Stapleton AE, Eschenbach DA. Epithelial cell layer thickness and immune cell populations in the normal human vagina at different stages of the menstrual cycle. Am J Obstet Gynecol. 2000;183(4):967–973. doi: 10.1067/mob.2000.108857. [DOI] [PubMed] [Google Scholar]
- Petrides M, Tomaiuolo F, Yeterian EH, Pandya DN. The prefrontal cortex: comparative architectonic organization in the human and the macaque monkey brains. Cortex. 2012;48:46–57. doi: 10.1016/j.cortex.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Plant TM, Witchel SF. Puberty in Nonhuman Primates and Humans. In: Neill JD, Challis JR, De Kretser DM, Pfaff D, Richards JS, Plant TM, Wassarman PM, editors. Knobil and Neill's Physiology of Reproduction. St. Louis, MO: Raven Press; 2006. pp. 2177–2230. [Google Scholar]
- Plopper C, George JS, Cardoso W, Wu R, Pinkerton K, Buckpitt A. Development of airway epithelium: patterns of expression for markers of differentiation. Chest. 1992;101:2S–5S. [PubMed] [Google Scholar]
- Plopper CG, Alley JL, Weir AJ. Differentiation of tracheal epithelium during fetal lung maturation in the rhesus monkey Macaca mulatta. Am J Anatomy. 1986;175:59–71. doi: 10.1002/aja.1001750107. [DOI] [PubMed] [Google Scholar]
- Plopper CG, Hyde DM. The non-human primate as a model for studying COPD and asthma. Pulm Pharmacol Ther. 2008;21(5):755–766. doi: 10.1016/j.pupt.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Plopper CG, Joad JP, Miller LA, Schelegle ES, Fanucchi MV, Van Winkle LS, Tyler NK, Avdalovic MV, Evans MJ, Lasley WL, et al. Lung effects of inhaled corticosteroids in a rhesus monkey model of childhood asthma. Clin Exp Allergy. 2012;42(7):1104–1118. doi: 10.1111/j.1365-2222.2012.04005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povinelli DJ. Monkeys, apes, mirrors and minds: The evolution of self-awareness in primates. Human Evolution. 1987;2(6):493–509. [Google Scholar]
- Povinelli DJ, Gallup JGG, Eddy TJ, Bierschwale DT, Engstrom MC, Perilloux HK, Toxopeus IB. Chimpanzees recognize themselves in mirrors. Animal Behaviour. 1997;53(5):1083–1088. [Google Scholar]
- Prabhakar S, Visel A, Akiyama JA, Shoukry M, Lewis KD, Holt A, Plajzer-Frick I, Morrison H, Fitzpatrick DR, Afzal V, et al. Human-specific gain of function in a developmental enhancer. Science. 2008;321(5894):1346–1350. doi: 10.1126/science.1159974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado-Martinez J, Sudmant PH, Kidd JM, Li H, Kelley JL, Lorente-Galdos B, Veeramah KR, Woerner AE, O'Connor TD, Santpere G, et al. Great ape genetic diversity and population history. Nature. 2013 doi: 10.1038/nature12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss TM. Do rats have prefrontal cortex? The rose-woolsey-akert program reconsidered. Journal of Cognitive Neuroscience. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23(13):5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasweiler IvJJ, Badwaik NK. Anatomy and Physiology of the Female Reproductive Tract. In: Crichton EG, Krutzsch PH, editors. Reproductive Biology of Bats. San Diego: Academic Press; 2000. pp. 157–219. [Google Scholar]
- Refinement JWGo. Refinements in husbandry, care and common procedures for non-human primates: Ninth report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. In: Jennings M, Prescott MJ, editors. Lab Animal. S1. Vol. 43. 2009. pp. 1–47. [DOI] [PubMed] [Google Scholar]
- Register TC, Adams MR, Golden DL, Clarkson TB. Conjugated equine estrogens alone, but not in combination with medroxyprogesterone acetate, inhibit aortic connective tissue remodeling after plasma lipid lowering in female monkeys. Arterioscler Thromb Vasc Biol. 1998;18(7):1164–1171. doi: 10.1161/01.atv.18.7.1164. [DOI] [PubMed] [Google Scholar]
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, et al. Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. The New England Journal of Medicine:NEJMoa0908492. 2009 doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- Roberts DJ, Post MD. The placenta in pre-eclampsia and intrauterine growth restriction. J Clin Pathol. 2008;61(12):1254–1260. doi: 10.1136/jcp.2008.055236. [DOI] [PubMed] [Google Scholar]
- Roberts JA, Gilardi KV, Lasley B, Rapp PR. Reproductive senescence predicts cognitive decline in aged female monkeys. Neuroreport. 1997;8(8):2047–2051. doi: 10.1097/00001756-199705260-00048. [DOI] [PubMed] [Google Scholar]
- Rodriguez JS, Zürcher NR, Keenan KE, Bartlett TQ, Nathanielsz PW, Nijland MJ. Prenatal betamethasone exposure has sex specific effects in reversal learning and attention in juvenile baboons. Am J Obstet Gynecol. 2011;204(6):545.e1–545.e10. doi: 10.1016/j.ajog.2011.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Raveendran M, Fawcett GL, Fox AS, Shelton SE, Oler JA, Cheverud J, Muzny DM, Gibbs RA, Davidson RJ, et al. CRHR1 genotypes, neural circuits and the diathesis for anxiety and depression. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Raveendran M, Fawcett GL, Fox AS, Shelton SE, Oler JA, Cheverud J, Muzny DM, Gibbs RA, Davidson RJ, et al. CRHR1 genotypes, neural circuits and the diathesis for anxiety and depression. Mol Psychiatry. 2013;18(6):700–707. doi: 10.1038/mp.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos C, Zinner D, Kubatko LS, Schwarz C, Yang M, Meyer D, Nash SD, Xing J, Batzer MA, Brameier M, et al. Nuclear versus mitochondrial DNA: evidence for hybridization in colobine monkeys. BMC Evol Biol. 2011;11:77. doi: 10.1186/1471-2148-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati AG, Stevens JR, Hare B, Hauser MD. The evolutionary origins of human patience: temporal preferences in chimpanzees, bonobos and human adults. Current Biology. 2007;17:1663–1668. doi: 10.1016/j.cub.2007.08.033. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford BA, Howard BV, Johnson KC, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Roth G, Dicke U. Evolution of the brain and intelligence. Trends Cogn Sci. 2005;9(5):250–257. doi: 10.1016/j.tics.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Rumbaugh DM. Language learning by a chimpanzee: The Lana project. New york: Academic Press; 1977. [Google Scholar]
- Russell WMS, Burch RL. The principles of humane experimental technique. Wheathampstead: Universities Federation for Animal Welfare. 1959 [Google Scholar]
- Sachs DH. Tolerance: Of mice and men. J Clin Invest. 2003;111(12):1819–1821. doi: 10.1172/JCI18926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage-Rumbaugh ES, Lewin R. Kanzi: The Ape at the Brink of the Human Mind. New York: John Wiley; 1994. [Google Scholar]
- Savage-Rumbaugh ES, Murphy ES, Sevcik RA, Brakke KE, Williams SL, Rumbaugh DM. Language comprehension in ape and child. Monographs of the Society for Research in Child Development. 1993;58:1–256. [PubMed] [Google Scholar]
- Seelke AM, Dooley JC, Krubitzer LA. The emergence of somatotopic maps of the body in S1 in rats: the correspondence between functional and anatomical organization. PLoS One. 2012;7(2):e32322. doi: 10.1371/journal.pone.0032322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semendeferi K, Lu A, Schenker N, Damasio H. Humans and great apes share a large frontal cortex. Nature Neuroscience. 2002;5:272–276. doi: 10.1038/nn814. [DOI] [PubMed] [Google Scholar]
- Sen S, Simmons RA. Maternal antioxidant supplementation prevents adiposity in the offspring of Western diet-fed rats. Diabetes. 2010;59(12):3058–3065. doi: 10.2337/db10-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013a;110(9):3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proceedings of the National Academy of Sciences. 2013b doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshasayee D, Lee WP, Zhou M, Shu J, Suto E, Zhang J, Diehl L, Austin CD, Meng YG, Tan M, et al. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J Clin Invest. 2007;117(12):3868–3878. doi: 10.1172/JCI33559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PM, Hahn BH. Origins of HIV and the AIDS Pandemic. Cold Spring Harbor Perspectives in Medicine. 2011;1(1) doi: 10.1101/cshperspect.a006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw GM, Hunter E. HIV Transmission. Cold Spring Harbor Perspectives in Medicine. 2012;2(11) doi: 10.1101/cshperspect.a006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata R, Siemon C, Czajak SC, Desrosiers RC, Martin MA. Live, attenuated simian immunodeficiency virus vaccines elicit potent resistance against a challenge with a human immunodeficiency virus type 1 chimeric virus. J Virol. 1997;71(11):8141–8148. doi: 10.1128/jvi.71.11.8141-8148.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shideler SE, Gee NA, Chen J, Lasley BL. Estrogen and progesterone metabolites and follicle-stimulating hormone in the aged macaque female. Biol Reprod. 2001;65(6):1718–1725. doi: 10.1095/biolreprod65.6.1718. [DOI] [PubMed] [Google Scholar]
- Shidler SE, Gee NA, Chen J, Lasley BL. Estrogen and progesterone metabolites and follicle-stimulating hormone in the aged macaque female. Biology of Reproduction. 2001;65:1718–1725. doi: 10.1095/biolreprod65.6.1718. [DOI] [PubMed] [Google Scholar]
- Shively CA, Kaplan JR. Effects of social factors on adrenal weight and related physiology in Macaca fascicularis. Physiology and Behavior. 1984;33:777–782. doi: 10.1016/0031-9384(84)90047-7. [DOI] [PubMed] [Google Scholar]
- Shively CA, Musselman DL, Willard SL. Stress, depression, and coronary artery disease: modeling comorbidity in female primates. Neuroscience and Biobehavioral Reviews. 2009;33(2):133–144. doi: 10.1016/j.neubiorev.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Willard SL. Behavioral and neurobiological characteristics of social stress versus depression in nonhuman primates. Experimental Neurology. 2012;233(1):87–94. doi: 10.1016/j.expneurol.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silwa J, Duhamel JR, Pascalis O, Wirth S. Spontaneous voice-face identity matching by rhesus monkeys for familiar conspecifics and humans. Proceedings of the National Academy of Sciences. 2011;108:1735–1740. doi: 10.1073/pnas.1008169108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan EK, Capitanio JP, Tarara RP, Mendoza SP, Mason WA, Cole SW. Social stress enhances sympathetic innervation of primate lymph nodes: mechanisms and implications for viral pathogenesis. J Neurosci. 2007;27(33):8857–8865. doi: 10.1523/JNEUROSCI.1247-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D, Trennery P, Farningham D, Klapwijk J. The selection of marmoset monkeys (Callithrix jaccus) in pharmaceutical toxicology. Lab Animal. 2001;35:117–130. doi: 10.1258/0023677011911444. [DOI] [PubMed] [Google Scholar]
- Solomon CG, Hu FB, Dunaif A, Rich-Edwards JE, Stampfer MJ, Willet WC, Speizer FE, Manson JE. Menstrual cycle irregularity and risk for future cardiovascular disease. Journal of Clinical Endocrinology & Metabolism. 2002;87(5):2013–2017. doi: 10.1210/jcem.87.5.8471. [DOI] [PubMed] [Google Scholar]
- Springer MS, Murphy WJ, Eizirik E, O'Brien SJ. Placental mammal diversification and the Cretaceous-Tertiary boundary. Proceedings of the National Academy of Sciences. 2003;100:1056–1061. doi: 10.1073/pnas.0334222100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfer MJ, Willet WC, Colditz GA, Rosner B, Speizer FE, Hennekens CH. A prospective study of postmenopausal estrogen therapy and coronary heart disease. New England Journal of Medicine. 1985;313:1044–1049. doi: 10.1056/NEJM198510243131703. [DOI] [PubMed] [Google Scholar]
- Steger RW, Peluso JJ. Sex hormones in the aging female. Endocrinology and Metabolism Clinics. 1987;16:1027–1043. [PubMed] [Google Scholar]
- Steiner RA, Clifton DK, Spies HG, Resko JA. Sexual differentiation and feedback control of luteinizing hormone secretion in the rhesus monkey. BiolReprod. 1976;15(2):206–212. doi: 10.1095/biolreprod15.2.206. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Mellman I. Immunotherapy: bewitched, bothered, and bewildered no more. Science. 2004;305(5681):197–200. doi: 10.1126/science.1099688. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Kivimäki M. Stress and cardiovascular disease. National Review of Cardiology. 2012;9(6):360–370. doi: 10.1038/nrcardio.2012.45. [DOI] [PubMed] [Google Scholar]
- Stute P, Sielker S, Wood CE, Register TC, Lees CJ, Dewi FN, Williams JK, Wagner JD, Stefenelli U, Cline JM. Life stage differences in mammary gland gene expression profile in non-human primates. Breast Cancer Research and Treatment. 2012;133:617–634. doi: 10.1007/s10549-011-1811-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EL, Grayson B, Takahashi D, Robertson N, Maier A, Bethea CL, Smith MS, Coleman K, Grove KL. Chronic Consumption of a High-Fat Diet during Pregnancy Causes Perturbations in the Serotonergic System and Increased Anxiety-Like Behavior in Nonhuman Primate Offspring. Journal of Neuroscience. 2010;30(10):3826–3830. doi: 10.1523/JNEUROSCI.5560-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EL, Grove KL. Metabolic imprinting in obesity. Forum Nutr. 2010;63:186–194. doi: 10.1159/000264406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EL, Smith MS, Grove KL. Perinatal exposure to high-fat diet programs energy balance, metabolism and behavior in adulthood. Neuroendocrinology. 2011;93(1):1–8. doi: 10.1159/000322038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundgren PC, Cao Y. Brain irradiation: effects on normal brain parenchyma and radiation injury. Neuroimaging Clinics of North America. 2009;19:657–668. doi: 10.1016/j.nic.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri-Payer E, Amar AZ, McHugh R, Natarajan K, Margulies DH, Shevach EM. Post-thymectomy autoimmune gastritis: fine specificity and pathogenicity of anti-H/K ATPase-reactive T cells. Eur J Immunol. 1999;29(2):669–677. doi: 10.1002/(SICI)1521-4141(199902)29:02<669::AID-IMMU669>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Suter M, Bocock P, Showalter L, Hu M, Shope C, McKnight R, Grove K, Lane R, agaard-Tillery K. Epigenomics: maternal high-fat diet exposure in utero disrupts peripheral circadian gene expression in nonhuman primates. The FASEB Journal. 2011;25(2):714–726. doi: 10.1096/fj.10-172080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter MA, Sangi-Haghpeykar H, Showalter L, Shope C, Hu M, Brown K, Williams S, Harris RA, Grove KL, Lane RH, et al. Maternal High-Fat Diet Modulates the Fetal Thyroid Axis and Thyroid Gene Expression in a Nonhuman Primate Model. Mol Endocrinol. 2012 doi: 10.1210/me.2012-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter MA, Takahashi D, Grove KL, Aagaard KM. Postweaning exposure to a high-fat diet is associated with alterations to the hepatic histone code in Japanese macaques. Pediatr Res. 2013;74(3):252–258. doi: 10.1038/pr.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura H, Nakamura Y, Terron MP, Flores LJ, Manchester LC, Tan DX, Sugino N, Reiter RJ. Melatonin and pregnancy in the human. Reproductive Toxicology. 2008;25:291–303. doi: 10.1016/j.reprotox.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Tardif S, Bales K, Williams L, Moeller E, Abbott D, Schultz-Darken N, Mendoza S, Mason W, Bourgeois S, Ruiz J. Preparing New World monkeys for laboratory research. ILAR Journal. 2006;47:307–315. doi: 10.1093/ilar.47.4.307. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Carson RL, Gangaware BL. Infant-Care Behavior of Nonreproductive Helpers in A Communal-Care Primate, the Cotton-Top Tamarin (Saguinus-Oedipus) Ethology. 1992;92(2):155–167. [Google Scholar]
- Tchoukalova YD, Krishnapuram R, White UA, Burk D, Fang X, Nijland MJ, Nathanielsz PW. Fetal baboon sex-specific outcomes in adipocyte differentiation at 0.9 gestation in response to moderate maternal nutrient reduction. Int J Obes (Lond) 2013 doi: 10.1038/ijo.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchoukalova YD, Nathanielsz PW, Conover CA, Smith SR, Ravussin E. Regional variation in adipogenesis and IGF regulatory proteins in the fetal baboon. Biochem Biophys Res Commun. 2009;380(3):679–683. doi: 10.1016/j.bbrc.2009.01.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry B, Singh M, Kaumanns W. Macaque Societies: A Model for the Study of Social Organization. Cambridge: Cambridge University Press; 2004. [Google Scholar]
- Thoma RJ, Hanlon FM, Huang M-X, Miller GA, Moses SN, Weisend MP, Jones A, Paulson KM, Irwin J, Canive JM. Impaired somatosensory gating in patients with schizophrenia. Psychiatry Research. 2007;151:189–199. doi: 10.1016/j.psychres.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurlbeck W, Benjamin B, Reid L. Development and distribution of mucous glands in the foetal human trachea. Br J Dis Chest. 1961;55:55–64. doi: 10.1016/s0007-0971(61)80002-8. [DOI] [PubMed] [Google Scholar]
- Tosh DN, Fu Q, Callaway CW, McKnight RA, McMillen IC, Ross MG, LANE RH, Desai M. Epigenetics of programmed obesity: alteration in IUGR rat hepatic IGF1 mRNA expression and histone structure in rapid vs. delayed postnatal catch-up growth. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2010;299:G1023–G1029. doi: 10.1152/ajpgi.00052.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CC, Emau P, Follis KE, Beck TW, Benveniste RE, Bischofberger N, Lifson JD, Morton WR. Effectiveness of Postinoculation (R)-9-(2-Phosphonylmethoxypropyl)Adenine Treatment for Prevention of Persistent Simian Immunodeficiency Virus SIVmne Infection Depends Critically on Timing of Initiation and Duration of Treatment. Journal Of Virology. 1998;72(5):4265–4273. doi: 10.1128/jvi.72.5.4265-4273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CC, Follis KE, Sabo A, Beck TW, Grant RF, Bischofberger N, Benveniste RE, Black R. Prevention of SIV Infection in Macaques by (R)-9-(2-Phosphonylmethoxypropyl)adenine. Science. 1995;270(5239):1197–1199. doi: 10.1126/science.270.5239.1197. [DOI] [PubMed] [Google Scholar]
- Tsujimoto S, Genovesio A, Wise SP. Frontal pole cortex: encoding ends at the end of the endbrain. Trends Cogn Sci. 2011;15(4):169–176. doi: 10.1016/j.tics.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Tuersunjiang N, Odhiambo JF, Long NM, Shasa DR, Nathanielsz PW, Ford SP. Diet reduction in obese ewes from early gestation prevents glucose-insulin dysregulation and returns fetal adiposity and organ development to control levels. Am J Physiol Endocrinol Metab. 2013 doi: 10.1152/ajpendo.00117.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung J, Alberts SC, Wray GA. Evolutionary genetics in wild primates: combining genetic approaches with field studies of natural populations. Trends in Genetics. 2010;26:353–362. doi: 10.1016/j.tig.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski MH. Nerve growth factor gene delivery: animal models to clinical trials. Dev Neurobiol. 2007;67(9):1204–1215. doi: 10.1002/dneu.20510. [DOI] [PubMed] [Google Scholar]
- Uberla K, Stahl-Hennig C, Böttiger D, Mätz-Rensing K, Kaup FJ, Li J, Haseltine WA, Fleckenstein B, Hunsmann G, Oberg B. Animal model for the therapy of acquired immunodeficiency syndrome with reverse transcriptase inhibitors. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(18):8210–8214. doi: 10.1073/pnas.92.18.8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behavioral Brain Research. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Van Damme L, Govinden R, Mirembe FM, Guédou F, Solomon S, Becker ML, Pradeep BS, Krishnan AK, Alary M, Pande B, et al. Lack of Effectiveness of Cellulose Sulfate Gel for the Prevention of Vaginal HIV Transmission. New England Journal of Medicine. 2008;359(5):463–472. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- Van Rompay KK. The use of nonhuman primate models of HIV infection for the evaluation of antiviral strategies. AIDS ResHumRetroviruses. 2012;28(1):16–35. doi: 10.1089/aid.2011.0234. [DOI] [PubMed] [Google Scholar]
- Veazey RS. Animal models for microbicide safety and efficacy testing. [Miscellaneous Article] Current Opinion in HIV & AIDS. 2013;8(4):295–303. doi: 10.1097/COH.0b013e328361d096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal Tract as a Major Site of CD4+ T Cell Depletion and Viral Replication in SIV Infection. Science. 1998;280(5362):427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- Vega CC, Reyes-Castro LA, Bautista CJ, Larrea F, Nathanielsz PW, Zambrano E. Exercise in obese female rats has beneficial effects on maternal and male and female offspring metabolism. Int J Obes (Lond) 2013 doi: 10.1038/ijo.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura-Antunes L, Mota B, Herculano-Houzel S. Different scaling of white matter volume, cortical connectivity, and gyrification across rodent and primate brains. Front Neuroanat. 2013;7:3. doi: 10.3389/fnana.2013.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers MH, Sloboda DM. Strategies for reversing the effects of metabolic disorders induced as a consequence of developmental programming. Front Physiol. 2012;3:242. doi: 10.3389/fphys.2012.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytko ML. Bridging the gap between animal and human cognitive testing. In: Iqbal K, Winblad B, editors. Alzheimer's Disease and Related Disorders: "Ana Aslan" International Academy of Aging. 2003. [Google Scholar]
- Voytko ML, Higgs CJ, Murray R. Differential effects on visual and spatial recognition memory of a novel hormone therapy regimen of estrogen alone or combined with progesterone in older surgically menopausal monkeys. Neuroscience. 2008;154(4):1205–1217. doi: 10.1016/j.neuroscience.2008.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytko ML, Mach RH, Gage HD, Ehrenkaufer RL, Efange SM, Tobin JR. Cholinergic activity of aged rhesus monkeys revealed by positron emission tomography. Synapse. 2001;39:95–100. doi: 10.1002/1098-2396(20010101)39:1<95::AID-SYN12>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Voytko ML, Murray R, Higgs CJ. Executive function and attention are preserved in older surgically menopausal monkeys receiving estrogen or estrogen plus progesterone. JNeurosci. 2009;29(33):10362–10370. doi: 10.1523/JNEUROSCI.1591-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytko ML, Murray RS, Bourland JD, Robbins ME. Radiation-induced cognitive impairment in postmenipausal female nonhuman primates; International Cognition and Cancer Task Force Conference; 2012. p. 18. [Google Scholar]
- Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med. 2009;27(5):358–368. doi: 10.1055/s-0029-1237424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker ML. Menopause in female rhesus-monkeys. American Journal of Primatology. 1995;35(1):59–71. doi: 10.1002/ajp.1350350106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker ML, Anderson DC, Herndon JG, Walker LC. Ovarian aging in squirrel monkeys (Saimiri sciureus) Reproduction. 2009;138(5):793–799. doi: 10.1530/REP-08-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker ML, Herndon JG. Menopause in nonhuman primates? BiolReprod. 2008;79(3):398–406. doi: 10.1095/biolreprod.108.068536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JM, Luther JS, Milne JS, Aitken RP, Redmer DA, Reynolds LP, Hay WW. Nutritional modulation of adolescent pregnancy outcome -- a review. Placenta. 2006;27(Suppl A):S61–S68. doi: 10.1016/j.placenta.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Walters JR. Transition to adulthood. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate Societies. Chicago: University of Chicago PRess; 1987. pp. 358–369. [Google Scholar]
- Wang J, Wu Z, Li D, Li N, Dindot SV, Satterfield MC, Bazer FW, Wu G. Nutrition, Epigenetics, and Metabolic Syndrome. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2011.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbauer GF, Niehoff M, Niehaus M, Srivastav S, Fuchs A, Van Esch E, Cline JM. Physiology and endocrinology of the ovarian cycle in macaques. Toxicologic Pathology. 2008;36(7S):7S–23S. doi: 10.1177/0192623308327412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbauer GF, Nieschlag E. Reversibility of GnRH agonist-induced inhibition of testicular function: differences between rats and primates. Progress in Clinical and Biological Research. 1989;303(75):75–87. [PubMed] [Google Scholar]
- Wells WJ, Parkman R, Smogorzewska E, Barr M. Neonatal Thymectomy: Does It Affect Immune Function? J Thorac Cardiovasc Surg. 1998;115:1041–1046. doi: 10.1016/S0022-5223(98)70403-9. [DOI] [PubMed] [Google Scholar]
- Wenzel S, Holgate ST. The Mouse Trap. American Journal of Respiratory and Critical Care Medicine. 2006;174(11):1173–1176. doi: 10.1164/rccm.2609002. [DOI] [PubMed] [Google Scholar]
- Wey HY, Phillips KA, Mckay DR, Laird AR, Kochunov P, Davis MD, Glahn DC, Blangero J, Duong TQ, Fox PT. Multi-region hemispheric specialization differentiates human from nonhuman primate brain function. Brain Structure and Function. 2013 doi: 10.1007/s00429-013-0620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard SL, Shively CA. Modeling depression in adult female cynomolgus monkeys (Macaca fascicularis) American Journal of Primatology. 2012;74(6):528–542. doi: 10.1002/ajp.21013. [DOI] [PubMed] [Google Scholar]
- Williams JK, Anthony MS, Honoré EK, Herrington DM, Morgan TM, Register TC, Clarkson TB. Regression of atherosclerosis in female monkeys. ArteriosclerThrombVascBiol. 1995;15(7):827–836. doi: 10.1161/01.atv.15.7.827. [DOI] [PubMed] [Google Scholar]
- Wilson JL, Parsons MT, Flouret G. Forward shift in the initiation of the nocturnal estradiol surge in the pregnant baboon: is this the genesis of labor? American Journal of Obstetrics and Gynecology. 1991;165(5 Pt 1):1487–1498. doi: 10.1016/0002-9378(91)90396-9. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28(5):910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- Wissler RW, Vesselinovitch D. Atherosclerosis in nonhuman primates. Advances in Veterinary Science and Comparative Medicine. 1977;21:351–420. [PubMed] [Google Scholar]
- Woller MJ, Everson-Binotto G, Nichols E, Acheson A, Keen KL, Bowers CY, Terasawa E. Aging-related changes in release of growth hormone and luteinizing hormone in female rhesus monkeys. The Journal of Clinical Endocrinology & Metabolism. 2002;87(11):5160–5167. doi: 10.1210/jc.2002-020659. [DOI] [PubMed] [Google Scholar]
- Wood CE, Hester JM, Cline JM. Mammary gland development in early pubertal female macaques. Toxicologic Pathology. 2007a;35:795–805. doi: 10.1080/01926230701584213. [DOI] [PubMed] [Google Scholar]
- Wood CE, Sitruk-Ware RL, Tsong YY, Register TC, Lees CJ, Cline JM. Effects of estradiol with oral or intravaginal progesterone on risk markers for breast cancer in a postmenopausal monkey model. Menopause. 2007b;14:639–647. doi: 10.1097/01.gme.0000247017.41007.80. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Zhang Y, Liang X. Primate-inspired communication methods for mobile and static sensors and RFID tags. ACM Transactions on Autonomous and Adaptive Systems. 2011;6(4):26–37. [Google Scholar]
- Xie L, Antonow-Schlorke I, Schwab M, McDonald TJ, Nathanielsz PW, Li C. The frontal cortex IGF system is down regulated in the term, intrauterine growth restricted fetal baboon. Growth Horm IGF Res. 2013;23(5):187–192. doi: 10.1016/j.ghir.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Cheng PH, Banta H, Piotrowska-Nitsche K, Yang JJ, Cheng EC, Snyder B, Larkin K, Liu J, Orkin J, et al. Towards a transgenic model of Huntington's disease in a non-human primate. Nature. 2008;453(7197):921–924. doi: 10.1038/nature06975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Zheng X, Peake J, Joad JP, Pinkerton KE. Perinatal environmental tobacco smoke exposure alters the immune response and airway innervation in infant primates. J Allergy Clin Immunol. 2008;122(3):640 e1–647 e1. doi: 10.1016/j.jaci.2008.04.038. [DOI] [PubMed] [Google Scholar]
- Zambrano E, Martínez-Samayoa PM, Rodríguez-González GL, Nathanielsz PW. Dietary intervention prior to pregnancy reverses metabolic programming in male offspring of obese rats. J Physiol. 2010;588(Pt 10):1791–1799. doi: 10.1113/jphysiol.2010.190033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xiao Y, Bales KL. Primate social systems, scent-marking, and their applications in mobile and static sensor networks. International Journal of Sensory Networks. 2009;5:210–222. [Google Scholar]
- Zinner D, Wertheimer J, Liedigk R, Groeneveld LF, Roos C. Baboon phylogeny as inferred from complete mitochondrial genomes. Am J Phys Anthropol. 2013;150(1):133–140. doi: 10.1002/ajpa.22185. [DOI] [PMC free article] [PubMed] [Google Scholar]