Abstract
Reactive aldehydes such as 4-hydroxy-2-nonenal (4HNE) are generated in the myocardium in cardiac disease. 4HNE and other toxic aldehydes form adducts with proteins, leading to cell damage and organ dysfunction. Aldehyde dehydrogenases (ALDHs) metabolize toxic aldehydes such as 4HNE into nontoxic metabolites. Both ALDH levels and activity are reduced in cardiac disease. We examined whether reduced ALDH2 activity contributes to cardiomyocyte hypertrophy in mice fed a high-fat diet and injected with low-dose streptozotocin (STZ). These mice exhibited most of the characteristics of metabolic syndrome/type-2 diabetes mellitus (DM): increased blood glucose levels depicting hyperglycemia (415.2 ± 18.7 mg/dL vs. 265.2 ± 7.6 mg/dL; P < 0.05), glucose intolerance with normal plasma insulin levels, suggesting insulin resistance and obesity as evident from increased weight (44 ± 3.1 vs. 34.50 ± 1.32 g; P < 0.05) and body fat. Myocardial ALDH2 activity was 60% lower in these mice (0.1 ± 0.012 vs. 0.04 ± 0.015 mmol/min/mg protein; P < 0.05). Myocardial 4HNE levels were also elevated in the hyperglycemic hearts. Co-immunoprecipitation study showed that 4HNE formed adducts on myocardial ALDH2 protein in the mice exhibiting metabolic syndrome/type-2 DM, and they had obvious cardiac hypertrophy compared with controls as evident from increased heart weight (HW), HW to tibial length ratio, left ventricular (LV) mass and cardiomyocyte hypertrophy. Cardiomyocyte hypertrophy was correlated inversely with ALDH2 activity (R2 = 0.7; P < 0.05). Finally, cardiac dysfunction was observed in mice with metabolic syndrome/type-2 DM. Therefore, we conclude that reduced ALDH2 activity may contribute to cardiac hypertrophy and dysfunction in mice presenting with some of the characteristics of metabolic syndrome/type-2 DM when on a high-fat diet and low-dose STZ injection.
Keywords: Aldehyde dehydrogenase 2, 4-hydroxy-2-nonenal, metabolic syndrome, cardiomyocyte hypertrophy, diabetic cardiac complications, type-2 diabetes mellitus
Introduction
Hyperglycemia leads to production of reactive oxygen species (ROS), which results in formation of advanced glycated-end products (AGE).1 AGE-induced microvascular and macrovascular complications of diabetes mellitus (DM) are very well documented in experimental and clinical studies.2,3 In addition to AGE production, ROS lead to production of toxic reactive aldehydes such as malondialdehyde (MDA), acetaldehyde, and 4-hydroxy-2-nonenal (4HNE). Similar to AGE, 4HNE, and other toxic aldehydes form adducts with proteins, leading to cell damage and organ dysfunction.4–9 In particular, a link between 4HNE adducts and diabetes-induced cardiovascular, neurological, ocular, and renal complications has been shown.10–12 Aldehyde dehydrogenases (ALDHs) are a group of enzymes that metabolize toxic aldehydes into nontoxic acids.13 In the myocardium, ALDH2, the mitochondrial isoform of ALDH, plays an important role in removal of toxic aldehydes and protects the heart from oxidative stress/injury.14 Many studies found ALDH2 plays a protective role in cardiovascular disease models.15–18
A point mutation (E487K) at the interface of the tetrameric complex of ALDH2 (ALDH2*2) decreases catalytic activity to 5–40% of the wild-type form ALDH2*1. ALDH2*2 is associated with many metabolic disorders such as hypertension,19 metabolic syndrome,20 type-2 DM,21 and diabetic complications.22 For example, ALDH2*2 increased maternal inheritance of diabetes in Japanese patients,21 and patients with ALDH2*2 have a higher risk of developing diabetic complications such as vasculopathy and nephropathy.22 This indicates that ALDH2 activity is critical in ameliorating diabetic complications. In previous reports, reduced myocardial ALDH2 levels and activity were found in high-dose streptozotocin (STZ)-induced insulin-deficient type-1 DM models.23,24 It was also reported that an increase in 4HNE adduct formation in the type-1 diabetic heart leads to mitochondrial dysfunction25,26 and cardiac damage.24 However, we could find no study implicating ALDH2 and 4HNE in a type-2 DM model.
Despite having multiple models of type-2 DM/metabolic syndrome with each has its own onset and magnitude of symptoms due to heterogeneous etiology,27 it is challenging to create exact human disease in animals. In this study, we fed a high-fat diet to C57BL/6 mice, a genetically prone strain for type-2 DM28,29 and then injected low-dose STZ, 40 mg/kg three times starting 2 weeks after beginning the high-fat diet. These mice had some of the characteristics of metabolic syndrome/type-2 DM such as obesity, increase in body weight and fat content; hyperglycemia, increased blood glucose levels and glucose intolerance despite having normal insulin levels suggesting insulin resistance. Previous studies reported that rats30–32 and mice33 fed a high-fat diet and injected with low-dose STZ recapitulate the characteristics of type-2 DM. In those studies, both rats and mice were shown to exhibit hyperglycemia, dyslipidemia, insulin resistance, and obesity.
Metabolic syndrome is a risk factor for type-2 DM and often precedes diabetes. The symptoms of metabolic syndrome and type-2 DM overlap. The few characteristics recapitulated in both genetic and nongenetic models of type-2 DM are (a) insulin resistance, (b) hyperglycemia, (c) dyslipidemia, and (d) obesity. Our mice had insulin resistance, hyperglycemia, and obesity. Most importantly, the major components of creating a diabetic cardiovascular disease model including diabetic cardiomyopathy have been outlined by a panel from Animal Models of Diabetic Complications Consortium.34 Our model presents some of the recommended components such as cardiac dysfunction, oxidative stress in the heart, and pathological cardiac remodeling along with some features of type-2 DM/metabolic syndrome as mentioned above. Therefore, our model is valid to study diabetic cardiovascular disease. In this study, we investigated whether both a high-fat diet and multiple low doses of STZ injections in mice would lead to impaired ALDH2 function and cardiac hypertrophy.
Methods
Animal model
8-week-old male C57BL/6 mice were fed a high-fat diet (60% of calories from fat, D12492, Research Diets) for 2 weeks. Then they were injected three times with STZ 40 mg/kg and fed a high-fat diet continuously as described in several studies.30–32 Mice with sustained elevated blood glucose levels at fasting (>400 mg/dL) after 4 weeks of STZ were selected for further studies. Blood was collected from the tail vein and glucose measured with a glucometer. The animal protocol was approved by the Henry Ford Health System Institutional Animal Care and Use Committee. It adheres to the guiding principles of the care and use of experimental animals in accordance with the NIH guidelines. Henry Ford Hospital operates an AAALAC certified animal facility.
Intraperitoneal glucose tolerance test
The intraperitoneal glucose tolerance test (IPGTT) was performed in mice from both groups as explained elsewhere.32 After fasting for 6 h, the mice were injected with 2 g/kg d-glucose. Then blood glucose levels were measured at 0, 60, 90, and 120 min after d-glucose injection using a glucometer.
Determination of blood plasma insulin levels
Blood samples were spun and the separated plasma was used to measure insulin levels using an ELISA kit (Crystal Chem Inc.) as per the manufacturer’s instructions. Each value represents duplicate measurements of each sample.
ALDH activity assay
ALDH2 activity was measured as described elsewhere.15 In brief, enzymatic activity of ALDH2 from cardiac tissue homogenates was determined spectrophotometrically by reductive reaction of NAD+ to NADH at λ340 nm. All assays were carried out at 25° C in 0.1 M sodium pyrophosphate buffer, pH = 9.5 with 2.4 mM NAD+ as a cofactor and 10 mM acetaldehyde as the substrate.
Western blotting of 4HNE- protein adducts
Western blot was performed as described earlier.35,36 In brief, cardiac protein samples were separated on sodium dodecyl sulphate (SDS)-polyacrylamide gels by electrophoresis and the proteins transferred to immobilon-P membranes (Millipore, Billerica, MA). Levels of 4HNE-protein adducts in heart samples were determined using an antibody of 4HNE-Cys/His/Lys (Calbiochem) at a concentration of 1: 1000. Anti-alpha-tubulin mouse monoclonal antibody at a concentration of 1: 1000 (Santa Cruz Biotechnology, Santa Cruz) was used as a housekeeping marker. The bound antibody was visualized with horseradish peroxidase (HRP)-coupled secondary antibody.
Immunoprecipitation of 4HNE-modified ALDH2
Cardiac tissue homogenates were used for co-immunoprecipitation (IP) studies. An antibody against 4HNE-Cys/His/Lys (Calbiochem) was added to tissue protein (150 μg) in a final volume of 200 μL and incubated for 2 h. Then protein-A/G agarose beads (Santa Cruz) were added to each sample and rocked at 4° C overnight. The beads were washed several times and then resuspended in IP buffer. The samples were run by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS PAGE) and probed against ALDH2 antibody (Santa Cruz).
Histopathology
After 4 months of induction of the disease, the hearts were isolated, weighed, and stored appropriately for biochemical studies at −80° C. The middle portions were fixed with 10% formalin in PBS, embedded in paraffin as blocks, and several transverse sections cut.
Immunohistochemistry of 4HNE- protein adducts
Formalin-fixed, paraffin-embedded cardiac tissue sections were used for immunohistochemical staining. After depar-affinization and hydration, the slides were washed in Tris-buffered saline (TBS; 10 mmol/L Tris–HCl, 0.85% NaCl, pH 7.5) containing 0.1% bovine serum albumin. Endogenous peroxidase activity was quenched by incubating the slides in 0.6% H2O2/methanol. A pressure cooker method was used to retrieve the antigen. In the following steps, reagents from an immunoperoxidase staining kit (Millipore) were used as directed. A solution from the kit was used to block non-specific reactions. After overnight incubation with polyclonal rabbit 4HNE-Cys/His/Lys antibody at a concentration of 1: 100 and 4° C, the slides were washed in TBS. Secondary antibody solution and streptavidin peroxidase solution were added and incubated at room temperature. Immunostaining was visualized with chromogen, diaminobenzidine tetrahydrochloride. Finally, the sections were counterstained with hematoxylin.
Measurement of cardiomyocyte hypertrophy
Myocardial sections were stained with hematoxylin–eosin to measure cardiomyocyte hypertrophy by quantifying the myocyte cross-sectional area using MicroSuite software (Olympus America). Relatively circular cardiomyocytes with the nucleus in the center were included for quantification of each high power field. We scored at least 15 photomicrographs for each sample. N > 5 mice.
Cardiac function assessment by echocardiography
Left ventricular (LV) dimension and function were assessed in conscious mice to avoid the effects of anesthesia, using an echocardiograph equipped with a 15-MHz linear transducer (Acuson c256) as described previously.37
Cardiac function assessment by hemodynamic measurements
Cardiac dysfunction was assessed with a Millar Mikro-Tip SPR-1000 pressure catheter (ADInstruments, Australia). In brief, mice were anesthetized by Inactin (100 mg/kg i.p.). The catheter was inserted into the left ventricle via the right carotid artery to assess systolic blood pressure (SBP), diastolic blood pressure, left ventricular pressure, and the peak and minimum values of LV dP/dt (LV dP/dtmax and LV dP/dtmin, respectively). After 30 min of stabilization, hemodynamic parameters were recorded by an eightchannel lab recorder (ADInstruments, Australia) with LABCHART-7 software.
Statistical analysis
Data are presented as mean ± standard error (SEM). For the biochemical and histopathological analysis, we used n = 3–5 from each group unless otherwise mentioned. Student’s t test was applied to compare 2 groups using graphpad Prism 5. For the correlation analysis, linear regression was performed. Statistical significance was achieved when P < 0.05.
Results
High-fat diet and low-dose STZ-induced biometric changes in mice
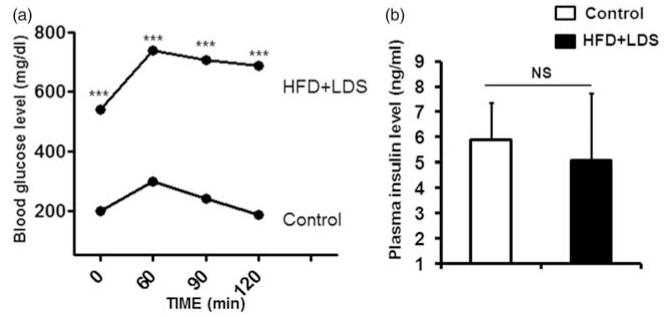
A significant increase in body weight and fat content denoting obesity, heart weight (HW), and HW to tibial length ratio was observed in mice fed a high-fat diet fed and given low-dose STZ (Table 1). Hyperglycemia (Table 1) and glucose intolerance (Figure 1a) with normal insulin levels (Figure 1b) were observed in mice on a high-fat diet and low-dose STZ. The heart rate (Table 1) was significantly decreased, but there was no significant change in systolic and diastolic blood pressure in mice with a high-fat diet and low-dose STZ.
Table 1.
Biometric changes in control and high fat diet fed and low dose streptozotocin-injected (HFD + LDS) mice
| Parameters | Control | HFD + LDS |
|---|---|---|
| Body weight (g) | 34.50 ± 1.32 | 42.33 ± 3.1* |
| HW (mg) | 138 ± 2.9 | 171 ± 8.3* |
| HW/tibial length ratio (mg/mm) | 7.7 ± 0.1 | 9.5 ± 0.3† |
| Blood glucose (mg/dL) | 265.2 ± 7.6 | 540 ± 18.7† |
| Heart rate (beats per minutes) | 503 ± 11 | 437 ± 9‡ |
| Systolic blood pressure (mm Hg) | 105.3 ± 5.8 | 100.5 ± 2.1 |
| Diastolic blood pressure (mm Hg) | 75.25 ± 1.1 | 74.00 ± 1.7 |
The data expressed are mean ± SEM. N = 5–8
P < 0.05 vs. control;
P < 0.0001 vs. control;
P < 0.01 vs. control
Figure 1.
Insulin resistance in mice given a high-fat diet and low-dose streptozotocin injection: (a) IPGTT data: glucose tolerance test data from control and high-fat/low-dose STZ (HFD + LDS) groups. Blood glucose was measured after 60, 90, and 120 min of 2 g/kg d-glucose intraperitoneal injection. ***P < 0.0001; n = 4 mice from each group. (b) Plasma insulin levels: plasma was isolated from blood collected at the end of the protocol. NS, non significant; n = 5 mice from each group
Increase in 4HNE-protein adduct formation in the hearts of mice fed a high-fat diet and injected with low-dose STZ exhibiting some of the characteristics of metabolic syndrome/type-2 DM
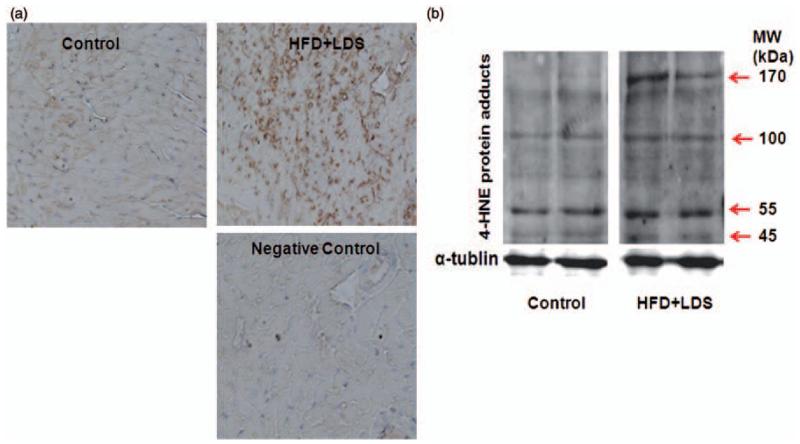
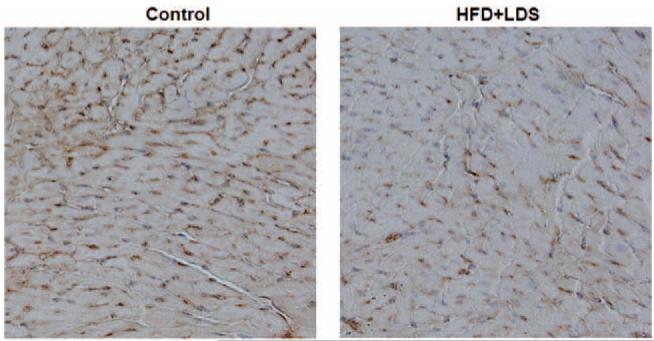
4HNE-protein adduct formation was increased in the hearts of mice fed a high-fat diet and injected with low-dose STZ as seen with both immunohistochemical staining (Figure 2a) and immunoblotting (Figure 2b).
Figure 2.
Increased myocardial 4HNE adducts levels in mice receiving a high-fat diet and low-dose streptozotocin injection (HFD + LDS). (a) immunohistochemistry of 4HNE adducts: representative micrographs of cardiac sections stained with 4HNE adduct antibody are shown. The brown spots indicate immunopositive reaction to 4HNE adduct antibody. A negative control (where the primary antibody was omitted) is also shown (n = 3–5). (b) Immunoblotting bands of 4HNE protein adducts: representative Western blots of 4HNE protein adducts are shown from control and high-fat/low-dose streptozotocin (HFD + LDS) mouse heart homogenates. α-tubulin was used as a loading control. The red arrows show the increase in 4HNE protein adducts (n = 3–5). (A color version of this figure is available in the online journal.)
Decreased myocardial ALDH2 activity as well as increased 4HNE adduct formation on ALDH2 were observed in mice fed a high-fat diet and injected with low-dose STZ
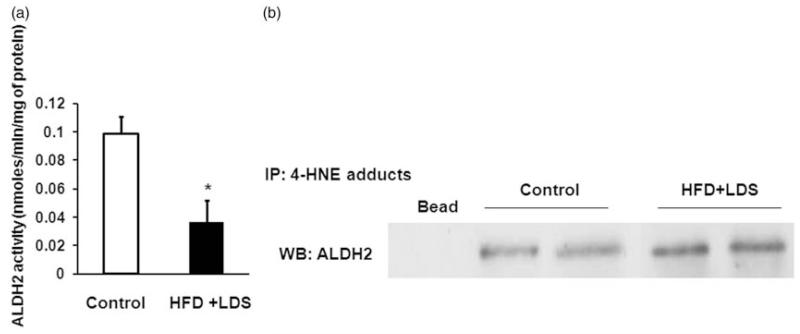
ALDH2 activity was decreased by 60% in the hearts of high-fat fed and low-dose STZ-injected mice (P < 0.05) (Figure 3a). This decrease was due to formation of 4HNE adducts on ALDH2 as found by increased co-IP (Figure 3b).
Figure 3.
Impaired ALDH2 by 4-HNE adducts in mice receiving a high-fat diet and low-dose streptozotocin. (a) ALDH2 activity: decrease in myocardial ALDH2 activity in mice given a high-fat diet/low-dose streptozotocin (HFD + LDS) relative to controls. The ALDH2 activity was quantified by a spectrophotometer using acetaldehyde as substrate (*P < 0.05) (n = 3–5). (b) Co-immunoprecipitation of 4HNE protein adducts and ALDH2 protein: immunoblots of ALDH2 were immunoprecipitated with 4HNE protein adduct antibody, implicating 4HNE adduct formation on ALDH2 in control and HFD + LDS groups (n = 3)
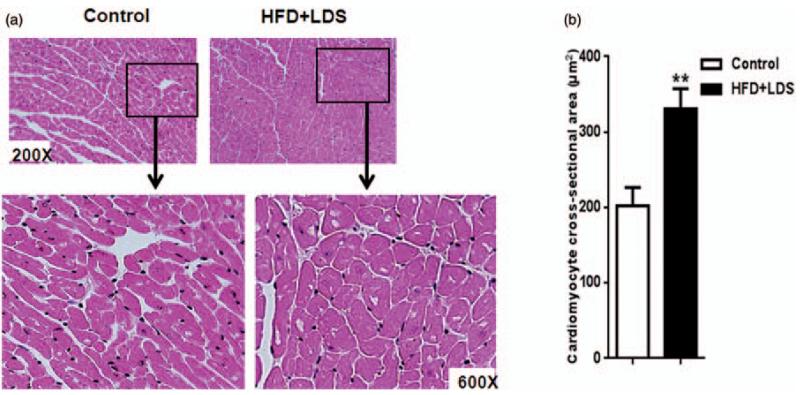
Pathological cardiac remodeling was observed in high-fat fed and low-dose STZ-injected mice as evident from increased cardiomyocyte hypertrophy and decreased capillaries. Morphological analysis revealed increased cardiomyocyte cross-sectional area, a hallmark of hypertrophy, in mice with some of the characteristics of metabolic syndrome/type-2 DM (Figure 4a and b). The number of endothelial cells/capillaries in the cardiac sections was reduced in these mice PECAM-1 staining (Figure 5).
Figure 4.
Increase in cardiomyocyte hypertrophy in mice receiving a high-fat diet and low-dose streptozotocin. (a) Micrographs of cardiac sections: representative photomicrographs of hematoxylin-eosin stained cardiac sections from control and HFD + LDS groups depicting cardiomyocyte size. The insets are magnified to distinguish the difference in cardiomyocyte size. (b) Quantification data of cardiomyocyte cross-sectional area from control and HFD + LDS groups: At least 10 relatively circular cardiomyocytes with the nucleus in the center were included for quantification of each high power field. We scored at least 15 photomicrographs for each sample (n = 5–8; **P < 0.01). (A color version of this figure is available in the online journal.)
Figure 5.
Decrease in capillaries in myocardial tissue sections from mice fed a high-fat diet and given low-dose streptozotocin: immunostaining of PECAM-1: Representative photomicrographs of PECAM-1 stained cardiac sections are shown from control and HFD + LDS groups. The brown staining in capillaries and micro vessels implicate endothelial cells (n = 3–5). (A color version of this figure is available in the online journal.)
Cardiac dysfunction in mice challenged with a high-fat diet and low-dose STZ injection
Echocardiography revealed significantly decreased% fractional shortening (FS) and% ejection fraction (EF), with significant increases in LV mass, LV dimension during diastole (LVDd) and systole (LVDs), LV end-diastolic posterior wall thickness (PWTd), and LV end-systolic posterior wall thickness (PWTs) compared with controls (Table 2).
Table 2.
Echocardiographic data from control and high fat diet fed and low dose streptozotocin-injected (HFD + LDS) mice
| Parameters | Control | HFD + LDS |
|---|---|---|
| FS (%) | 54 ± 1.3 | 47 ± 2* |
| EF (%) | 76 ± 2 | 65 ± 1.9* |
| LVDd (mm) | 2.7 ± 0.04 | 3 ± 0.07* |
| LVDs (mm) | 1.2 ± 0.03 | 1.54 ± 0.08* |
| PWTd (mm) | 0.85 ± 0.03 | 1.08 ± 0.04† |
| PWTs (mm) | 1.29 ± 0.09 | 1.54 ± 0.08 |
| LV mass (mg) | 73.22 ± 6.5 | 98.07 ± 3.5* |
| SV (mL) | 47.78 ± 6.13 | 59.38 ± 9.2 |
| CO (mL/min) | 30.77 ± 3.02 | 36.62 ± 5.8 |
SV, stroke volume; CO, cardiac output. The data expressed are mean ± SEM. N = 4
P < 0.05 vs. control
P < 0.01 vs. control
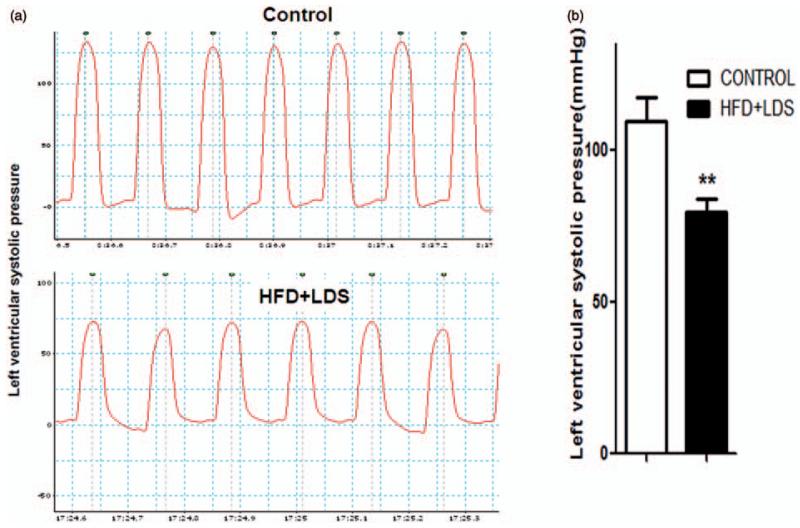
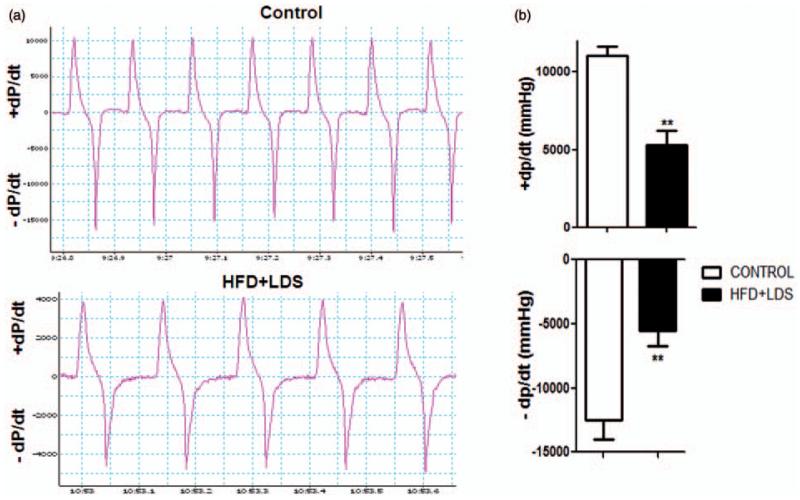
LV systolic pressure (Figure 6a and b) and peak and minimum LV dP/dt (+dP/dt and −dP/dtmin, respectively) (Figure 7a and b) were decreased in mice fed a high-fat diet and injected with low-dose STZ using the LV pressure catheter.
Figure 6.
Decrease in left ventricular systolic pressure in mice with high-fat/low-dose streptozotocin. (a) Tracings of lab chart measurements of left ventricular systolic pressure of controls and HFD + LDS mice. (b) Quantification data of left ventricular systolic pressure (n = 3–5; ****P < 0.01). (A color version of this figure is available in the online journal.)
Figure 7.
Decrease in left ventricular peak and minimum values of left ventricular dP/dt in mice receiving a high-fat diet and low-dose streptozotocin. (a) Tracings of lab chart measurements of left ventricular +dP/dt and −dP/dt from controls and HFD + LDS mice. (b) Quantification of left ventricular +dP/dt and −dP/dt (n = 3–5; ****P < 0.001). (A color version of this figure is available in the online journal.)
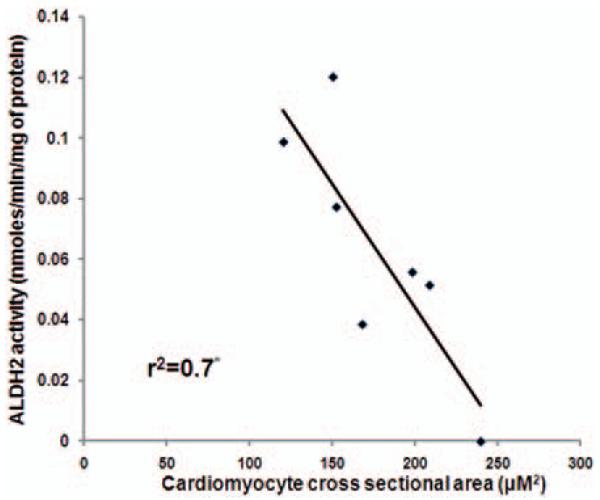
Reduced ALDH activity contributes to cardiomyocyte hypertrophy
ALDH2 activity and cardiomyocyte hypertrophy correlated inversely (R2=0.7; P < 0.05 (Figure 8).
Figure 8.
ALDH2 activity correlates inversely with cardiomyocyte hypertrophy: Graph showing inverse correlation between ALDH2 activity and cardiomyocyte hypertrophy. Data were analyzed by linear regression (*P < 0.05)
Discussion
We found decreased ALDH2 activity and increased 4HNE adducts in the myocardium of mice fed a high-fat diet and injected with low-dose STZ exhibiting important characteristics of metabolic syndrome/type-2 DM. The decrease in ALDH2 activity is due to increased 4HNE adduct formation on the ALDH2 enzyme itself and is associated with increased cardiac hypertrophy.
Several epidemiological studies indicated that ALDH2 activity is critical in cardiovascular diseases; the inactive ALDH2 genotype is associated with myocardial infarction,38,39 angina,40 and hypertension41 in humans. Further, patients with an inactivating point mutation in ALDH2 are more likely to develop diabetic complications.22 The cardioprotective effect of ALDH2 activation has been demonstrated using in vivo and ex vivo models of myocardial ischemia-reperfusion injury.14,42 Transgenic studies explored ALDH-mediated cardioprotection and its mechanism in alcoholic cardiomyopathy. Over-expression of wild-type ALDH2 resulted in cardioprotective effects in alcohol-induced acute myocardial damage in mice43 and reduced hypertrophy and contractile dysfunction compared with wild-type mice following chronic alcohol ingestion.44 Most importantly, ALDH2 over-expression led to reduced protein carbonyl formation in addition to improved calcium handling and reduction in apoptosis.44 Alcoholic cardiomyopathy-induced glucose intolerance, reduced glucose uptake, cardiac hypertrophy, and reduced cell shortening were also alleviated by ALDH2 overexpression.45 Overall, ALDH2 overexpression attenuates alcoholic cardiomyopathy-induced cardiac insulin insensitivity and contractile dysfunction by preserving insulin signaling at the levels of insulin receptors, IRS, Akt, Foxo3a, and JNK.45 Our results are consistent with these reports that reduced ALDH activity can be detrimental to the metabolically abnormal heart.
Reactive aldehydes such as methylglyoxal (MGO), MDA, and 4HNE are important contributors to diabetic tissue damage.46 For instance, MGO accumulation due to hyperglycemia is an important intermediate molecule in adduct formation in mesenchymal cells, smooth muscle cells, fibroblasts, and diabetic atherosclerotic lesions.12 Increased MDA levels in serum47 and MDA adducts in cardiac tissue were found in diabetic animals.48 Elevated 4HNE levels are also found in diabetic patients relative to control groups10 and implicated in diabetic complications.40 Accumulation of abundant 4HNE in diabetic tissue is injurious. In the diabetic heart 4HNE formed adducts with critical mitochondrial protein, succinyl dehydrogenase and impaired complex II activity.26 In this study increase in myocardial 4HNE adduct levels in mice fed a high-fat diet and injected with low-dose STZ was found using immunostaining and Western blot.
Overall, 4HNE adducts can lead to cellular dysfunction as they can result in mitochondrial dysfunction49 and inactivation of proteosomes.50,51 4HNE can induce ROS formation52 and ROS produces 4HNE6,9; thus a vicious cycle sets in. Adduct formation on ALDH itself results in decreased ALDH2 activity and a further rise in 4HNE-induced cell toxicity.53 Similarly, we also found increased 4HNE adducts on ALDH2 protein in the hearts of mice with symptoms of metabolic syndrome/type-2 DM (Figure 3b). It may be the reason for the reduced ALDH2 activity.
It is well known that hyperglycemia induces cardiomyocyte hypertrophy in vitro54 and in vivo.23 We show here a significant increase in cardiac hypertrophy with an increase in HW, HW/tibial length, LV mass, and cardiomyocyte cross-sectional area in mice with signs of metabolic syndrome/type-2 DM. Further, PECAM-1 staining indicates reduced capillary density. An earlier study described reduced myocardial capillary density in alloxan-induced type-1 diabetic mice.55 Ren and co-workers23 induced type-1 DM by high-dose STZ (200 mg/kg) in mice with ALDH2 overexpression and found reduced cardiac hypertrophy and contractile dysfunction compared with wild-type diabetic mice. In our study, we injected low-dose STZ (40 mg/kg) in high-fat fed mice and found that ALDH activity inversely correlated with cardiomyocyte hypertrophy.
We observed systolic and diastolic dysfunction in mice with high fat and STZ compared with normal mice as evident from hemodynamic and echocardiographic measurements. STZ has been broadly used to induce DM in experimental animals; however, few acute studies using isolated cardiomyocytes found it could be toxic per se.56,57 Therefore, it is possible that this acute damage may lead to compensatory changes in the hearts of mice fed a high-fat diet and injected with low-dose STZ. Nevertheless, based on current data and previous reports from type-1 and type-2 DM models and other models of cardiometabolic challenges, it can be suggested that reduced ALDH activity plays a role in developing cardiac hypertrophy in the metabolically abnormal heart.
In conclusion, we believe this is the first demonstration that decreased myocardial ALDH2 activity due to 4HNE adduct formation may contribute to cardiac hypertrophy in mice exhibiting some of the characteristics of metabolic syndrome/type-2 DM when fed a high-fat diet and injected with multiple low doses of STZ.
ACKNOWLEDGMENTS
The authors thank Prof. William Beierwaltes for his critical comments. This work was supported by the National Institute on Aging Grants AG031811 (J.C.), 1R41NS080329 (J.C.), and an internal grant from Henry Ford Health System (S.S.P.).
Footnotes
Author contributions: V.R.M. performed histopathology, hemodynamic measurements, and biochemistry as well as prepared figures. R.N. contributed in the induction of diabetic model and took care of the animals routinely, measured blood glucose, and sacrificed animals. X.-P.Y. and J.X. performed echocardiographic measurements. J.C. set up the experimental design and mentored the animal protocol. S.S.P. planned the study, performed biochemical studies, and wrote the manuscript.
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–46. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 2.Yamagishi S, Nakamura K, Matsui T. Regulation of advanced glycation end product (AGE)-receptor (RAGE) system by PPAR-gamma agonists and its implication in cardiovascular disease. Pharmacol Res. 2009;60:174–8. doi: 10.1016/j.phrs.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Sourris KC, Harcourt BE, Forbes JM. A new perspective on therapeutic inhibition of advanced glycation in diabetic microvascular complications: common downstream endpoints achieved through disparate therapeutic approaches? Am J Nephrol. 2009;30:323–35. doi: 10.1159/000226586. [DOI] [PubMed] [Google Scholar]
- 4.Cohn JA, Tsai L, Friguet B, Szweda LI. Chemical characterization of a protein-4-hydroxy-2-nonenal cross-link: immunochemical detection in mitochondria exposed to oxidative stress. Arch Biochem Biophys. 1996;328:158–64. doi: 10.1006/abbi.1996.0156. [DOI] [PubMed] [Google Scholar]
- 5.Eaton P, Li JM, Hearse DJ, Shattock MJ. Formation of 4-hydroxy-2-nonenal-modified proteins in ischemic rat heart. Am J Physiol. 1999;276(3 Pt 2):H935–43. doi: 10.1152/ajpheart.1999.276.3.H935. [DOI] [PubMed] [Google Scholar]
- 6.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 7.Forbes SP, Druhan LJ, Guzman JE, Parinandi N, Zhang L, Green-Church KB, Cardounel AJ. Mechanism of 4-HNE mediated inhibition of hDDAH-1: implications in no regulation. Biochemistry. 2008;47:1819–26. doi: 10.1021/bi701659n. [DOI] [PubMed] [Google Scholar]
- 8.Isom AL, Barnes S, Wilson L, Kirk M, Coward L, Darley-Usmar V. Modification of Cytochrome c by 4-hydroxy-2-nonenal: evidence for histidine, lysine, and arginine-aldehyde adducts. J Am Soc Mass Spectrom. 2004;15:1136–47. doi: 10.1016/j.jasms.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Roede JR, Jones DP. Reactive species and mitochondrial dysfunction: mechanistic significance of 4-hydroxynonenal. Environ Mol Mutagen. 2010;51:380–90. doi: 10.1002/em.20553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calabrese V, Mancuso C, Sapienza M, Puleo E, Calafato S, Cornelius C, Finocchiaro M, Mangiameli A, Di Mauro M, Stella AM, Castellino P. Oxidative stress and cellular stress response in diabetic nephropathy. Cell Stress Chaperones. 2007;12:299–306. doi: 10.1379/CSC-270.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uchida K. Role of reactive aldehyde in cardiovascular diseases. Free Radic Biol Med. 2000;28:1685–96. doi: 10.1016/s0891-5849(00)00226-4. [DOI] [PubMed] [Google Scholar]
- 12.Cantero AV, Portero-Otin M, Ayala V, Auge N, Sanson M, Elbaz M, Thiers JC, Pamplona R, Salvayre R, Negre-Salvayre A. Methylglyoxal induces advanced glycation end product (AGEs) formation and dysfunction of PDGF receptor-beta: implications for diabetic atherosclerosis. FASEB J. 2007;21:3096–106. doi: 10.1096/fj.06-7536com. [DOI] [PubMed] [Google Scholar]
- 13.Vasiliou V, Pappa A, Petersen DR. Role of aldehyde dehydrogenases in endogenous and xenobiotic metabolism. Chem Biol Interact. 2000;129:1–19. doi: 10.1016/s0009-2797(00)00211-8. [DOI] [PubMed] [Google Scholar]
- 14.Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–5. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma H, Guo R, Yu L, Zhang Y, Ren J. Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischaemia/reperfusion injury: role of autophagy paradox and toxic aldehyde. Eur Heart J. 2011;32:1025–38. doi: 10.1093/eurheartj/ehq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palaniyandi SS, Marie-Helene D, Sun L, Vishnumangalam JJ, Xia X, Pavlovic A, Bhalla V, Ashley E, Mochly-Rosen D. Aldehyde dehydrogenase activator attenuates diabetic cardiomyopathy; a role in improving the quality of resident cardiac stem cells? FASEB J. 2010;24:572.3. meeting abstract supplements. [Google Scholar]
- 17.Zhang Y, Ren J. Autophagy in ALDH2-elicited cardioprotection against ischemic heart disease: slayer or savior? Autophagy. 2010;6:1212–3. doi: 10.4161/auto.6.8.13652. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Ren J. ALDH2 in alcoholic heart diseases: molecular mechanism and clinical implications. Pharmacol Ther. 2011;132:86–95. doi: 10.1016/j.pharmthera.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang YC, Chiu YF, Lee IT, Ho LT, Hung YJ, Hsiung CA, Quertermous T, Donlon T, Lee WJ, Lee PC, Chen CH, Mochly-Rosen D, Chuang LM. Common ALDH2 genetic variants predict development of hypertension in the SAPPHIRe prospective cohort: gene-environmental interaction with alcohol consumption. BMC Cardiovasc Disord. 2012;12:58. doi: 10.1186/1471-2261-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murata C, Watanabe T, Furuya H, Sugioka Y, Mikurube H, Yokoyama A, Atsumi Y, Matsuoka K, Okazaki I. Aldehyde dehydrogenase 2 and beta3-adrenergic receptor gene polymorphisms: their association with elevated liver enzymes and metabolic syndrome. Metabolism. 2003;52:1096–101. doi: 10.1016/s0026-0495(03)00183-5. [DOI] [PubMed] [Google Scholar]
- 21.Murata C, Taniyama M, Kuriyama S, Muramatsu T, Atsumi Y, Matsuoka K, Suzuki Y. Meta-analysis of three diabetes population studies: association of inactive ALDH2 genotype with maternal inheritance of diabetes. Diabetes Res Clin Pract. 2004;66(Suppl 1):S145–7. doi: 10.1016/j.diabres.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki Y, Taniyama M, Muramatsu T, Higuchi S, Ohta S, Atsumi Y, Matsuoka K. ALDH2/ADH2 polymorphism associated with vasculopathy and neuropathy in type 2 diabetes. Alcohol Clin Exp Res. 2004;28(8 Suppl Proceedings):111S–6S. doi: 10.1097/01.alc.0000133583.44581.99. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Babcock SA, Hu N, Maris JR, Wang H, Ren J. Mitochondrial aldehyde dehydrogenase (ALDH2) protects against streptozotocin-induced diabetic cardiomyopathy: role of GSK3beta and mitochondrial function. BMC Med. 2012;10:40. doi: 10.1186/1741-7015-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Wang J, Wang H, Hao P, Xue L, Wei S, Zhang Y, Chen Y. Inhibition of aldehyde dehydrogenase 2 by oxidative stress is associated with cardiac dysfunction in diabetic rats. Mol Med. 2011;17:172–9. doi: 10.2119/molmed.2010.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamblin M, Friedman DB, Hill S, Caprioli RM, Smith HM, Hill MF. Alterations in the diabetic myocardial proteome coupled with increased myocardial oxidative stress underlies diabetic cardiomyopathy. J Mol Cell Cardiol. 2007;42:884–95. doi: 10.1016/j.yjmcc.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lashin OM, Szweda PA, Szweda LI, Romani AM. Decreased complex II respiration and HNE-modified SDH subunit in diabetic heart. Free Radic Biol Med. 2006;40:886–96. doi: 10.1016/j.freeradbiomed.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 27.Panchal SK, Brown L. Rodent models for metabolic syndrome research. J Biomed Biotechnol. 2011;2011:351982. doi: 10.1155/2011/351982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37:1163–7. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- 29.Winzell MS, Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53(Suppl 3):S215–9. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]
- 30.Reed MJ, Meszaros K, Entes LJ, Claypool MD, Pinkett JG, Gadbois TM, Reaven GM. A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metabolism. 2000;49:1390–4. doi: 10.1053/meta.2000.17721. [DOI] [PubMed] [Google Scholar]
- 31.Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52:313–20. doi: 10.1016/j.phrs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Zhang M, Lv XY, Li J, Xu ZG, Chen L. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp Diabetes Res. 2008;2008:704045. doi: 10.1155/2008/704045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arulmozhi DK, Kurian R, Bodhankar SL, Veeranjaneyulu A. Metabolic effects of various antidiabetic and hypolipidaemic agents on a high-fat diet and multiple low-dose streptozocin (MLDS) mouse model of diabetes. J Pharm Pharmacol. 2008;60:1167–73. doi: 10.1211/jpp.60.9.0008. [DOI] [PubMed] [Google Scholar]
- 34.Hsueh W, Abel ED, Breslow JL, Maeda N, Davis RC, Fisher EA, Dansky H, McClain DA, McIndoe R, Wassef MK, Rabadán-Diehl C, Goldberg IJ. Recipes for creating animal models of diabetic cardiovascular disease. Circ Res. 2007;100:1415–27. doi: 10.1161/01.RES.0000266449.37396.1f. [DOI] [PubMed] [Google Scholar]
- 35.Selvakumar D, Drescher MJ, Dowdall JR, Khan KM, Hatfield JS, Ramakrishnan NA, Drescher DG. CNGA3 is expressed in inner ear hair cells and binds to an intracellular C-terminus domain of EMILIN1. Biochem J. 2012;443:463–76. doi: 10.1042/BJ20111255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palaniyandi SS, Nagai Y, Watanabe K, Ma M, Veeraveedu PT, Prakash P, Kamal FA, Abe Y, Yamaguchi K, Tachikawa H, Kodama M, Aizawa Y. Chymase inhibition reduces the progression to heart failure after autoimmune myocarditis in rats. Exp Biol Med (Maywood) 2007;232:1213–221. doi: 10.3181/0703-RM-85. [DOI] [PubMed] [Google Scholar]
- 37.Yang XP, Liu YH, Rhaleb NE, Kurihara N, Kim HE, Carretero OA. Echocardiographic assessment of cardiac function in conscious and anesthetized mice. Am J Physiol. 1999;277(5 Pt 2):H1967–74. doi: 10.1152/ajpheart.1999.277.5.H1967. [DOI] [PubMed] [Google Scholar]
- 38.Jo SA, Kim EK, Park MH, Han C, Park HY, Jang Y, Song BJ, Jo I. A Glu487Lys polymorphism in the gene for mitochondrial aldehyde dehydrogenase 2 is associated with myocardial infarction in elderly Korean men. Clin Chim Acta. 2007;382:43–7. doi: 10.1016/j.cca.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 39.Takagi S, Iwai N, Yamauchi R, Kojima S, Yasuno S, Baba T, Terashima M, Tsutsumi Y, Suzuki S, Morii I, Hanai S, Ono K, Baba S, Tomoike H, Kawamura A, Miyazaki S, Nonogi H, Goto Y. Aldehyde dehydrogenase 2 gene is a risk factor for myocardial infarction in Japanese men. Hypertens Res. 2002;25:677–81. doi: 10.1291/hypres.25.677. [DOI] [PubMed] [Google Scholar]
- 40.Furfaro AL, Menini S, Patriarca S, Pesce C, Odetti P, Cottalasso D, Marinari UM, Pronzato MA, Traverso N. HNE-dependent molecular damage in diabetic nephropathy and its possible prevention by N-acetyl-cysteine and oxerutin. Biofactors. 2005;24:291–8. doi: 10.1002/biof.5520240134. [DOI] [PubMed] [Google Scholar]
- 41.Amamoto K, Okamura T, Tamaki S, Kita Y, Tsujita Y, Kadowaki T, Nakamura Y, Ueshima H. Epidemiologic study of the association of low-Km mitochondrial acetaldehyde dehydrogenase genotypes with blood pressure level and the prevalence of hypertension in a general population. Hypertens Res. 2002;25:857–64. doi: 10.1291/hypres.25.857. [DOI] [PubMed] [Google Scholar]
- 42.Lagranha CJ, Deschamps A, Aponte A, Steenbergen C, Murphy E. Sex differences in the phosphorylation of mitochondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circ Res. 2010;106:1681–91. doi: 10.1161/CIRCRESAHA.109.213645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma H, Li J, Gao F, Ren J. Aldehyde dehydrogenase 2 ameliorates acute cardiac toxicity of ethanol: role of protein phosphatase and forkhead transcription factor. J Am Coll Cardiol. 2009;54:2187–96. doi: 10.1016/j.jacc.2009.04.100. [DOI] [PubMed] [Google Scholar]
- 44.Hintz KK, Relling DP, Saari JT, Borgerding AJ, Duan J, Ren BH, Kato K, Epstein PN, Ren J. Cardiac overexpression of alcohol dehydrogenase exacerbates cardiac contractile dysfunction, lipid peroxidation, and protein damage after chronic ethanol ingestion. Alcohol Clin Exp Res. 2003;27:1090–8. doi: 10.1097/01.ALC.0000075823.73536.DD. [DOI] [PubMed] [Google Scholar]
- 45.Li SY, Gilbert SA, Li Q, Ren J. Aldehyde dehydrogenase-2 (ALDH2) ameliorates chronic alcohol ingestion-induced myocardial insulin resistance and endoplasmic reticulum stress. J Mol Cell Cardiol. 2009;47:247–55. doi: 10.1016/j.yjmcc.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horie K, Miyata T, Maeda K, Miyata S, Sugiyama S, Sakai H, van Ypersole de Strihou C, Monnier VM, Witztum JL, Kurokawa K. Immunohistochemical colocalization of glycoxidation products and lipid peroxidation products in diabetic renal glomerular lesions. Implication for glycoxidative stress in the pathogenesis of diabetic nephropathy. J Clin Invest. 1997;100:2995–3004. doi: 10.1172/JCI119853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao J, Lv Y, Lin S, Jin L, Zhang Y, Wang X, Ma J, Hu K, Feng W, Cai L, Li X, Tan Y. Cardiac protection by basic fibroblast growth factor from ischemia/reperfusion-induced injury in diabetic rats. Biol Pharm Bull. 2010;33:444–9. doi: 10.1248/bpb.33.444. [DOI] [PubMed] [Google Scholar]
- 48.Ye G, Metreveli NS, Donthi RV, Xia S, Xu M, Carlson EC, Epstein PN. Catalase protects cardiomyocyte function in models of type 1 and type 2 diabetes. Diabetes. 2004;53:1336–43. doi: 10.2337/diabetes.53.5.1336. [DOI] [PubMed] [Google Scholar]
- 49.Lucas DT, Szweda LI. Cardiac reperfusion injury: aging, lipid peroxidation, and mitochondrial dysfunction. Proc Natl Acad Sci U S A. 1998;95:510–4. doi: 10.1073/pnas.95.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bardag-Gorce F, Li J, French BA, French SW. The effect of ethanol-induced CYP2E1 on proteasome activity: the role of 4-hydroxynonenal. Exp Mol Pathol. 2005;78:109–15. doi: 10.1016/j.yexmp.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 51.Ferrington DA, Kapphahn RJ. Catalytic site-specific inhibition of the 20S proteasome by 4-hydroxynonenal. FEBS Lett. 2004;578:217–23. doi: 10.1016/j.febslet.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 52.Nakamura K, Miura D, Kusano KF, Fujimoto Y, Sumita-Yoshikawa W, Fuke S, Nishii N, Nagase S, Hata Y, Morita H, Matsubara H, Ohe T, Ito H. 4-Hydroxy-2-nonenal induces calcium overload via the generation of reactive oxygen species in isolated rat cardiac myocytes. J Card Fail. 2009;15:709–16. doi: 10.1016/j.cardfail.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 53.Traverso N, Menini S, Odetti P, Pronzato MA, Cottalasso D, Marinari UM. Diabetes impairs the enzymatic disposal of 4-hydroxynonenal in rat liver. Free Radic Biol Med. 2002;32:350–9. doi: 10.1016/s0891-5849(01)00811-5. [DOI] [PubMed] [Google Scholar]
- 54.Feng B, Chen S, Chiu J, George B, Chakrabarti S. Regulation of cardiomyocyte hypertrophy in diabetes at the transcriptional level. Am J Physiol Endocrinol Metabol. 2008;294:E1119–26. doi: 10.1152/ajpendo.00029.2008. [DOI] [PubMed] [Google Scholar]
- 55.Camp TM, Tyagi SC, Senior RM, Hayden MR. Gelatinase B(MMP-9) an apoptotic factor in diabetic transgenic mice. Diabetologia. 2003;46:1438–45. doi: 10.1007/s00125-003-1200-y. [DOI] [PubMed] [Google Scholar]
- 56.Salem KA, Kosanovic M, Qureshi A, Ljubisavljevic M, Howarth FC. The direct effects of streptozotocin and alloxan on contractile function in rat heart. Pharmacol Res. 2009;59:235–41. doi: 10.1016/j.phrs.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 57.Wold LE, Ren J. Streptozotocin directly impairs cardiac contractile function in isolated ventricular myocytes via a p38 map kinase-dependent oxidative stress mechanism. Biochem Biophys Res Commun. 2004;318:1066–71. doi: 10.1016/j.bbrc.2004.04.138. [DOI] [PubMed] [Google Scholar]