Abstract
Dental fluorosis is characterized by subsurface hypomineralization and retention of enamel matrix proteins. Fluoride (F−) exposure generates reactive oxygen species (ROS) that can cause ER-stress. We therefore screened oxidative stress arrays to identify genes regulated by F− exposure. Vitamin E is an antioxidant so we asked if a diet high in vitamin E would attenuate dental fluorosis. Maturation stage incisor enamel organs (EO) were harvested from F− treated rats and mice were assessed to determine if vitamin E ameliorates dental fluorosis. Uncoupling protein-2 (Ucp2) was significantly up-regulated by F− (~1.5 & 2.0 fold for the 50 or 100 ppm F− treatment groups respectively). Immunohistochemical results on maturation stage rat incisors demonstrated that UCP2 protein levels increased with F− treatment. UCP2 down-regulates mitochondrial production of ROS, which decreases ATP production. Thus, in addition to reduced protein translation caused by ER-stress, a reduction in ATP production by UCP2 may contribute to the inability of ameloblasts to remove protein from the hardening enamel. Fluoride treated mouse enamel had significantly higher quantitative fluorescence (QF) than the untreated controls. No significant QF difference was observed between control and vitamin E enriched diets within a given F− treatment group. Therefore, a diet rich in vitamin E did not attenuate dental fluorosis. We have identified a novel oxidative stress response gene that is up-regulated in vivo by F− and activation of this gene may adversely affect ameloblast function.
Keywords: fluoride, fluorosis, enamel, UCP2, oxidative stress, vitamin E
Introduction
Dental fluorosis is caused by chronic ingestion of excess F− during enamel formation that manifests as mottled, discolored and porous enamel. Previously we reported that F− induces an ER-stress response in ameloblasts that leads to the inhibition of protein synthesis and secretion from ameloblasts during enamel formation [1,2]. ER-stress initiates the unfolded protein response (UPR) and ER chaperones such as Grp78 are up-regulated. Consequently, increased levels of ATP must be delivered to the ER, which requires increased ATP production by the mitochondrial respiratory chain. This enhances reactive oxygen species (ROS) production [3]. High ROS concentrations cause oxidative stress, mitochondrial dysfunction, cell damage, and may cause cell death. F− exposure can increase generation of the superoxide anion (O2−), which causes an oxidative stress response [4]. However the F−-mediated molecular pathways in ameloblasts remain unclear. Uncoupling protein-2 (UCP2) is an antioxidant and belongs to the mitochondrial anion transporter superfamily located in the inner mitochondrial membrane. UCP2 is activated by ROS and negatively regulates mitochondrial membrane potential and ATP synthesis to decrease mitochondrial superoxide emission and therefore ROS production under oxidative stress [5]. Vitamin E is a fat-soluble vitamin with antioxidant properties. It is a tocopherol and has garnered interest for potential beneficial health effects including promotion of plasma membrane repair and prevention of disease [6]. Vitamin E scavenges active oxygen radicals thereby preventing potentially harmful oxidation reactions. Previously, vitamin E was demonstrated to prevent excessive accumulation of F− in bones and teeth [7]. Herein we report that F− treatment increases Ucp2 mRNA levels and increases UCP2 protein levels in rat enamel organs (EO). However, a diet high in vitamin E did not ameliorate dental fluorosis in mice.
Materials and Methods
Animals
All animals were treated humanely. Our protocols were approved by the Institutional Animal Care Use Committee (IACUC) at The Forsyth Institute. Sprague-Dawley rats or C57BL/6 mice (6-week-old) (Charles River Laboratories, Wilmington, MA) were provided water ad libitum containing 0–125 ppm F− as NaF. After 6 weeks, animals were euthanized and tissues were obtained for qPCR arrays, immunohistochemistry, quantitative fluorescence (QF) and for measurement of F− concentration in bone and plasma.
RNA Extraction, cDNA Preparation and qPCR Data Analysis
Total RNA was extracted from rat maturation stage mandibular incisor EO using TRIzol® (Invitrogen, Grand Island, NY). Equal amounts of RNA (1 μg) were reverse transcribed into cDNA using RT2 First Strand Kit (Qiagen, Valencia, CA). The Rat Oxidative Stress RT2 Profiler™ PCR Array (Qiagen) was performed in triplicate using a Roche LightCycler 480 (Roche Diagnostics, Minneapolis, MN) and any gene with an average cycle threshold of >30 for any treatment group was eliminated. All genes up-regulated more than Ucp2 were eliminated because of this cutoff. Relative expression of the target gene was determined by the 2−ΔΔCT method [8].
Immunohistochemistry
Rat incisor paraffin sections were incubated with rabbit anti-UCP2 (Abcam, Cambridge, MA) followed by incubation with a peroxidase-conjugated antibody, Vectastain Elite ABC Regent, and ImmPACT™ DAB kit (Vector Labs, Burlingame, CA). Sections were counterstained with 0.1% Fast Green in PBS.
Vitamin E Treatment and Measurement of Fluoride
Mice were provided water containing 0 or 50 ppm F−, with control chow (α-tocopherol; 50 IU/kg) or vitamin E enriched chow (α-tocopherol; 500 IU/kg), (TestDiet, Richmond, IN) for 6 weeks. The level of fluorosis was assessed by use of QF as previously described [9]. Quantification of F− concentration in mouse plasma and femurs were as previously described [10].
Statistical Analysis
QF data and F− concentrations were analyzed by Student’s t-test. All data are presented as mean ± standard error (SE). P < 0.05 was considered significant.
Results
UCP2 Gene Expression and Protein Concentration were Induced by F− Exposure In Vivo
Among several genes (Table 1), qPCR arrays identified Ucp2 as induced by F− exposure (~1.5 fold with 50 ppm and 2.0 fold with 100 ppm F−). We assessed UCP2 protein expression in rat EO by immunohistochemistry. For the 100 ppm and 125 ppm F− treatment groups, the maturation stage ameloblasts stained strongly for UCP2 compared to untreated controls (Fig. 1). These data demonstrate that F− induces both gene and protein expression of UCP2 in rat EO.
Table 1.
Oxidative stress RT2 Profiler PCR Array
| Gene | Fold change (vs 0 ppm)
|
|
|---|---|---|
| F− 50 ppm | F− 100 ppm | |
| Ccl5 | 0.6477* | 0.3392a |
| Fmo2 | 0.0225a | 0.0616a |
| Gpx6 | 0.4698b | 0.4796b |
| Noxa1 | 1.5619b | 2.4284b |
| Slc38a5 | 2.2294b | 2.3295b |
| Sod3 | 1.4473b | 2.3295b |
| Ucp2 | 1.4142* | 2.0139* |
| Ucp3 | 4.297b | 3.8106a |
| Hspa1a | 0.712c | 0.473c |
| Alb | 0.712c | 0.473c |
| Gpx5 | 0.712c | 0.473c |
| Noxo1 | 0.712c | 0.473c |
| Rag2 | 0.712c | 0.473c |
| Tpo | 0.712c | 0.473c |
Ct < 30,
Ct in either the control or the test sample > 30,
Ct > 30,
Ct > 35 (cut-off value)
Figure 1.

F− induces UCP2 expression in rat maturation stage EO. Immunohistochemistry was performed on rat incisor sections stained with anti-UCP2 antibody. UCP2 protein increased in maturation stage ameloblasts (denoted by brackets) treated with F− (100 and 125 ppm). Bar = 10 μm. Lower panels were treated with pre-immune IgG primary antiserum as a negative control.
Absence of an Effect on Dental Fluorosis by the Anti-Oxidant Vitamin E
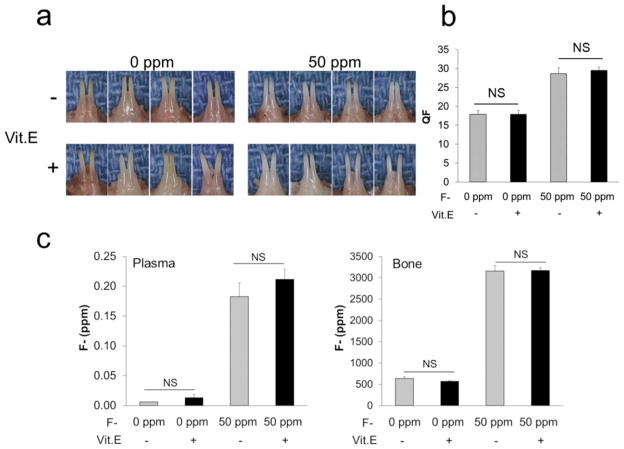
For six weeks mice were supplied with 0 or 50 ppm F− in their drinking water in the presence of a control diet or a vitamin E enriched diet. Mandibular incisors from mice in the 50 ppm F− treatment group appeared chalky white compared to the 0 ppm F− group and this was independent of the amount of dietary vitamin E (Fig. 2A). To assess the level of fluorosis, QF analysis was performed on mouse mandibular incisors. Enamel fluorescence increased in F− treated groups compared to the 0 ppm F− group. No significant differences were observed between the control group and the vitamin E enriched group within a given F− treatment group (Fig. 2B). The same was true for the F− concentrations in plasma and bone (Fig. 2C). Within a F− treatment group, vitamin E had no effect on the severity of fluorosis or on the quantity of F− that accumulated in the plasma or bone. Trace amounts of F− were found in heparin (0.003 ppm), control diet (1.487 ppm), vitamin E enriched diet (2.423 ppm), control water (0.002 ppm) and for F− at 50 ppm in water, we measured a concentration of 51.350 ppm.
Figure 2.
Effect of vitamin E on dental fluorosis. (A) Images of mouse mandibular incisors provided drinking water containing 0 ppm (left panel) or 50 ppm (right panel) of F− for 6 weeks with control diet (upper panel) or vitamin E enriched diet (lower panel). (B) QF analysis of enamel as a function of F− treatment and vitamin E diet. (C) The concentration of F− in mouse plasma and bone was quantified. Assays were performed using four mice per group. Data are expressed as mean ± SE. (NS; not significant).
Discussion
UCP2 is an oxidative stress response gene that was up-regulated by F− treatment in rat maturation stage EO. UCP2 down-regulates mitochondrial production of ROS by decreasing ATP production necessary for such activities as glucose metabolism [5]. Therefore, in addition to reduction of protein translation caused by ER-stress, an attenuation of ATP production by fluoride-induced Ucp2 may contribute to the inability of maturation stage ameloblasts to reabsorb enamel matrix proteins necessary for the formation of hardened mature enamel.
Contrary to our expectation, a diet rich in vitamin E did not ameliorate dental fluorosis (Fig. 2). Since F− exposure generates ROS, it is still worth investigating if other antioxidants alleviate dental fluorosis. Therefore, the fluoride-induced ROS-Ucp2 pathway may play an important role in dental fluorosis and studies targeting the oxidative stress response may someday contribute to fluorosis therapy.
Acknowledgments
We thank Dr. Justine Dobeck for histology expertise. This work was supported by the NIH/NIDCR R01DE018106.
Footnotes
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of this article.
References
- 1.Kubota K, Lee DH, Tsuchiya M, Young CS, Everett ET, Martinez-Mier EA, Snead ML, Nguyen L, Urano F, Bartlett JD. Fluoride induces endoplasmic reticulum stress in ameloblasts responsible for dental enamel formation. The Journal of biological chemistry. 2005;280:23194–23202. doi: 10.1074/jbc.M503288200. [DOI] [PubMed] [Google Scholar]
- 2.Sharma R, Tsuchiya M, Bartlett JD. Fluoride induces endoplasmic reticulum stress and inhibits protein synthesis and secretion. Environmental health perspectives. 2008;116:1142–1146. doi: 10.1289/ehp.11375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxidants & redox signaling. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 4.Izquierdo-Vega JA, Sanchez-Gutierrez M, Del Razo LM. Decreased in vitro fertility in male rats exposed to fluoride-induced oxidative stress damage and mitochondrial transmembrane potential loss. Toxicology and applied pharmacology. 2008;230:352–357. doi: 10.1016/j.taap.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Mailloux RJ, Harper ME. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free radical biology & medicine. 2011;51:1106–1115. doi: 10.1016/j.freeradbiomed.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 6.Howard AC, McNeil AK, McNeil PL. Promotion of plasma membrane repair by vitamin E. Nature communications. 2011;2:597. doi: 10.1038/ncomms1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaszczyk I, Birkner E, Gutowska I, Romuk E, Chlubek D. Influence of methionine and vitamin E on fluoride concentration in bones and teeth of rats exposed to sodium fluoride in drinking water. Biological trace element research. 2012;146:335–339. doi: 10.1007/s12011-011-9251-2. [DOI] [PubMed] [Google Scholar]
- 8.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic acids research. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Everett ET, McHenry MA, Reynolds N, Eggertsson H, Sullivan J, Kantmann C, Martinez-Mier EA, Warrick JM, Stookey GK. Dental fluorosis: variability among different inbred mouse strains. Journal of dental research. 2002;81:794–798. doi: 10.1177/0810794. [DOI] [PubMed] [Google Scholar]
- 10.Sharma R, Tye CE, Arun A, MacDonald D, Chatterjee A, Abrazinski T, Everett ET, Whitford GM, Bartlett JD. Assessment of dental fluorosis in Mmp20 +/− mice. Journal of dental research. 2011;90:788–792. doi: 10.1177/0022034511398868. [DOI] [PMC free article] [PubMed] [Google Scholar]