Abstract
Introduction
This study compared survival after initial treatment with esophagectomy as primary therapy to induction therapy followed by esophagectomy for patients with clinical T2N0 (cT2N0) esophageal cancer in the National Cancer Database (NCDB).
Methods
Predictors of therapy selection for patients with cT2N0 esophageal cancer in the NCDB from 1998–2011 were identified with multivariable logistic regression. Survival was evaluated using Kaplan-Meier and Cox proportional-hazards methods.
Results
Surgery was used in 42.9% (2057/4799) of cT2N0 patients. Of 1599 esophagectomy patients for whom treatment timing was recorded, induction therapy was used in 44.1% (688). Pre-treatment staging was proven accurate in only 26.7% of patients (210/786) who underwent initial surgery without induction treatment and had complete pathologic data available: 41.6% (n=327) were upstaged and 31.7% (n=249) were downstaged. Adjuvant therapy (chemotherapy or radiation therapy) was given to 50.2% of patients treated initially with surgery who were found after resection to have nodal disease. There was no significant difference in long-term survival between strategies of primary surgery and induction therapy followed by surgery (median 41.1 versus 41.9 months, p=.51). In multivariable analysis, induction therapy was not independently associated with risk of death (HR 1.16, p=.32).
Conclusions
Current clinical staging for early-stage esophageal cancer is highly inaccurate, with only a quarter of surgically resected cT2N0 patients found to have had accurate pre-treatment staging. Induction therapy for patients with cT2N0 esophageal cancer in the NCDB is not associated with improved survival.
Keywords: Esophageal cancer, esophageal surgery, induction therapy, outcomes
Introduction
The optimal management of patients with esophageal cancer clinically staged as T2N0M0 (cT2N0) has not been definitively established.1 National Comprehensive Cancer Network (NCCN) guidelines for medically fit patients reflect the lack of definitive evidence and allow a wide spectrum of treatment possibilities that include definitive chemoradiation and surgery with or without induction or adjuvant therapy, with some relatively minor differences between adenocarcinoma and squamous cell carcinoma histologies.2 Clinical staging modalities for this subset are known to be unreliable, with significant percentages of patients being under-staged or over staged.3–7 Perhaps because clinical staging inaccuracies lead to a relatively high incidence of patients actually having nodal disease present at the time of surgical resection, induction therapy use in this setting has been increasing and was shown recently to exceed 50% for cases that were reported to the Society of Thoracic Surgeons General Thoracic Database in 2011.3 However, induction therapy has not been definitively shown to improve outcomes in node-negative patients. The purpose of this study was to use one of the largest nationwide cancer databases to test the hypothesis that induction therapy followed by surgical resection is associated with improved survival compared to initial treatment with surgical resection for patients with cT2N0 esophageal cancer.
Patients and Methods
The Duke University Institutional Review Board approved this retrospective review of patients diagnosed with clinical T2N0M0 (cT2N0) esophageal cancer from 1998 to 2011 in the National Cancer Database (NCDB). The NCDB is maintained by the American College of Surgeons and the American Cancer Society and collects data from more than 1,500 Commission on Cancer approved centers across the United States. The NCDB captures approximately 70% of all newly diagnosed cases of cancer annually, and currently contains more than 30 million patient records. Clinical stage is coded in the NCDB using the Facility Oncology Registry Data Standards and is defined as the clinical stage documented by the managing physician based on the best available information, but specific staging modality data are not available. The American Joint Committee on Cancer (AJCC) staging criteria for each patient are recorded in concordance with the year of diagnosis, and the AJCC 5th through 7th editions were therefore used to identify patients for inclusion in this study.8–10 As there were no changes in the definition of T2 or N0 disease across these editions, no attempts to re-code the clinical staging were necessary. Since the recommended treatment for cervical tumors is definitive chemoradiation, only patients with tumors located in the middle- and distal-third of the esophagus were included for analysis. All survival analyses were limited to patients diagnosed through 2006, as long-term survival data is not currently available for patients diagnosed in 2007 or later in the NCDB
Patients were initially stratified based on the use of esophagectomy as part of their treatment (surgical management versus non-operative management). Independent predictors of patients undergoing esophagectomy were then estimated using a logistic regression model that included patient age, sex, race, Charlson/Deyo comorbidity index, tumor histology (squamous cell versus adenocarcinoma), patient census tract education and income levels, patient travel distance to treatment facility, and facility type (academic versus community program). The primary analysis was then focused on patients who underwent surgical management with esophagectomy, with the primary predictor variable being the use of induction therapy versus an approach of surgery as the initial treatment. For the purposes of this analysis, induction therapy was defined as the administration of any chemotherapy or radiation therapy (RT) prior to undergoing esophagectomy, and patients lacking data on the timing of therapy were excluded.
Baseline univariable comparisons of patient characteristics as well as unadjusted outcomes between the cohort of patients who had surgery as the initial treatment and the cohort of patients who had induction therapy before esophagectomy were made using Student’s t-test for continuous variables and Pearson’s chi-square test for categorical variables. To estimate the accuracy of clinical staging among the cT2N0 patient population, pathologic staging data were then used to calculate the respective rates of T- and N- upstaging and downstaging across the two groups following resection. To investigate the impact of pathologic N stage on use of adjuvant therapy, rates of postoperative chemotherapy and radiation therapy (RT) were assessed, stratified by both the initial treatment approach (surgery versus induction) and pathologic nodal status. Unadjusted survival analyses were performed using the Kaplan-Meier method comparing survival curves with the log-rank test. All analyses evaluated overall survival, defined as time from date of diagnosis to date of death or censoring. Predictors of long term survival for patients who had esophagectomy were estimated using a multivariable Cox proportional hazards model that included age, sex, race, Charlson/Deyo comorbidity index, final pathologic stage, tumor histology (squamous versus adenocarcinoma), patient census tract education and income levels, treatment facility volume, and use of induction therapy.
Type I error was controlled at the level of the comparison, and a p-value of <.05 was considered statistically significant. Data are presented as median (IQR), counts (%), odds ratios or hazard ratios (95% confidence intervals) where applicable. All analyses were performed using R version 3.0.2, Vienna, Austria.
Results
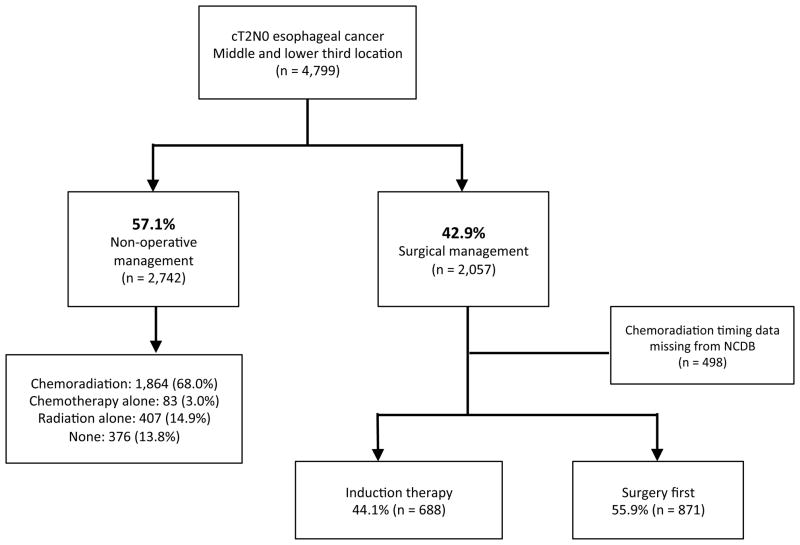
Of 93,164 patients with mid- or lower-third esophageal cancer in the database, 4,799 patients were identified as cT2N0 adenocarcinoma or squamous cell carcinoma with known treatment characteristics (Figure 1). Among these cT2N0 patients, 2,057 (42.9%) underwent esophagectomy as part of their treatment, and 2,742 (57.1%) were treated with non-operative management. Of the non-operatively managed patients, 1,864 (68.0%) received chemoradiation, 407 (14.9%) received RT alone, 83 (3.0%) received chemotherapy alone, and 376 (13.8%) received no definitive therapy. Living in a census tract with higher median education (adjusted odds ratio [AOR] 1.30, p=0.013) and treatment at an academic medical center (AOR 2.61, p<0.001) were independent predictors of undergoing esophagectomy among cT2N0 patients, while increasing patient age (AOR 0.50, p<0.001), female sex (AOR 0.64, p<0.001), and squamous cell histology (AOR 0.40, p<0.001), were associated with non-operative management. On unadjusted comparison of surgery to non-surgical management, patients treated with esophagectomy had markedly better five-year survival compared to their non-operative counterparts who received combined chemoradiation therapy (39.2% [95% CI: 36.4–42.2%] versus 16.6% [95% CI: 14.7–18.9%], p<.001).
Figure 1.
Nationwide management trends in cT2N0 esophageal cancer, 1998–2011
After excluding the 498 (24.2%) esophagectomy patients who were missing complete data regarding the specific timing of chemotherapy and RT, 688 (44.1%) patients were treated with induction therapy while the remaining 871 (55.9 %) were managed with surgery as their initial treatment. Among the 688 patients who were given induction treatment, 605 (87.9%) were treated with chemoradiation, 15 (2.2%) were treated with chemotherapy alone, and 68 (9.9%) were treated with RT alone. Comparison of the surgery-first versus induction therapy groups revealed fairly similar patient cohorts, though the induction therapy patients were younger, had lower Charlson comorbidity scores, more often had private insurance, and had a higher proportion of tumors measuring 5 cm or greater (Table 1). As also shown in table 1, patients treated with induction therapy had a longer interval from diagnosis to surgery, had fewer lymph nodes retrieved, and were less likely to have positive margins following resection. There were no statistically significant differences between the two treatment approaches with respect to perioperative outcomes, including 30-day mortality, unplanned readmissions within 30 days, or hospital length of stay.
Table 1.
Baseline characteristics and outcomes among patients treated with esophagectomy, stratified by treatment approach
| Variable | Surgery first (n = 871) | Induction therapy (n = 688) | P-value |
|---|---|---|---|
| Patient characteristics | |||
| Age, yrs (IQR) | 66 (58, 73) | 61 (54, 68) | < 0.001 |
| Female | 152 (17.5%) | 148 (14.2%) | 0.061 |
| Race | 0.892 | ||
| White | 800 (92.9%) | 956 (92.7%) | |
| Black | 46 (5.3%) | 59 (5.7%) | |
| Other | 15 (1.7%) | 16 (1.6%) | |
| Charlson Comorbidity Score | 0.001 | ||
| 0 | 489 (68.4%) | 540 (77%) | |
| 1 | 174 (24.3%) | 127 (18.1%) | |
| ≥2 | 52 (7.3%) | 34 (4.9%) | |
| Census tract education above median | 527 (63.7%) | 610 (61.7%) | 0.412 |
| Census tract income above median | 571 (69%) | 661 (66.9%) | 0.356 |
| Insurance | < 0.001 | ||
| Private | 339 (40.2%) | 576 (56.6%) | |
| Medicare | 451 (53.5%) | 366 (36%) | |
| Medicaid | 33 (3.9%) | 47 (4.6%) | |
| Government | 7 (0.8%) | 9 (0.9%) | |
| Uninsured | 13 (1.5%) | 20 (2%) | |
| Tumor size | < 0.001 | ||
| < 1 cm | 49 (6.3%) | 49 (7.8%) | |
| 1–1.9 cm | 110 (14.1%) | 46 (7.3%) | |
| 2–4.9 cm | 475 (61.1%) | 337 (53.8%) | |
| > 4.9 cm | 144 (18.5%) | 194 (31%) | |
| Facility volume (cases/yr) | 12 (6, 24) | 8 (5, 17) | < 0.001 |
| Days to definitive surgery (IQR) | 43 (29, 64) | 131 (112, 157) | < 0.001 |
| Outcomes and endpoints | |||
| Nodes removed (IQR) | 12 (6, 19) | 7 (2, 13) | < 0.001 |
| Surgical margins | 0.001 | ||
| Negative | 763 (91.1%) | 922 (95.5%) | |
| Positive margin –microscopic | 54 (6.4%) | 29 (3%) | |
| Positive margin -macroscopic | 21 (2.5%) | 14 (1.5%) | |
| 30-day mortality | 37 (4.2%) | 36 (3.5%) | 0.437 |
| 30-day readmission | 53 (7.6%) | 37 (5.6%) | 0.162 |
| Hospital LOS (IQR) | 11 (8, 17) | 10 (8, 15) | 0.069 |
Table 2 summarizes the pathological tumor stage data for both treatment groups. Among patients who had surgery as the initial treatment and had pathologic TNM staging data available (n=786), only 210 (26.7%) were found to have had accurate pre-treatment staging; 41.6% (327 patients) were upstaged based on either pathologic T or N stage and 31.7% (249 patients) were downstaged. Although the true pre-treatment pathologic stage is not known for the patients who were given induction therapy, the induction treatment patients had somewhat lower rates of upstaging and higher rates of downstaging. While patients treated with induction therapy had slightly higher rates of stage 0 disease (5.9% versus 0.1%), comparison between groups revealed a highly similar distribution of overall pathologic stage. Patients receiving induction therapy had slightly lower rates of nodal upstaging, with 30.2% of the surgery-first group and 23.6% of the induction therapy group being pathologically node-positive (p=.01). Of 109 patients with microscopic or macroscopic positive margins after resection, 72.4% were upstaged to pT3 or pT4; 76.6% of the surgery-first group with positive margins was upstaged, while 64.5% of induction therapy group was upstaged (p=0.28).
Table 2.
Summary of pathologic tumor stage changes from initial cT2N0, among patients with pathologic staging data available in NCDB
| Pathologic staging | Surgery first (n = 786) | Induction therapy (n = 477) | P-value |
|---|---|---|---|
| Accurate staging (pT2N0) | 210 (26.7%) | 116 (24.3%) | 0.34 |
| Upstaged (T or N) | 327 (41.6%) | 163 (34.2%) | 0.01 |
| Downstaged (T or N) | 249 (31.7%) | 198 (41.6%) | <0.001 |
| pT-stage | <0.001 | ||
| T0/IS | 6 (0.7%) | 110 (22.5%) | |
| T1 | 243 (30.1%) | 88 (18.0%) | |
| T2 | 334 (41.4%) | 187 (38.2%) | |
| T3 | 210 (26.1%) | 100 (20.4%) | |
| T4 | 13 (1.6%) | 4 (0.8%) | |
| pN-stage | 0.013 | ||
| Node negative | 549 (69.8%) | 381 (76.4%) | |
| Node positive | 237 (30.2%) | 118 (23.6%) | |
| Pathologic stage | <0.001 | ||
| pStage 0 | 1 (0.1%) | 23 (5.9%) | |
| pStage I | 220 (28.4%) | 95 (24.4%) | |
| pStage II | 409 (52.8%) | 209 (53.6%) | |
| pStage III | 128 (16.5%) | 58 (14.9%) | |
| pStage IV | 16 (2.1%) | 5 (1.3%) |
Table 3 shows the use of adjuvant treatment after surgery for patients who had not been given induction treatment. Overall, 22.8% (179/786) of patients who were treated with initial surgery and had data available regarding adjuvant treatment were subsequently given some form of adjuvant therapy (either chemotherapy, RT, or both). Patients who were upstaged due to having positive nodal disease were much more likely to receive adjuvant therapy than patients who had pathologic node-negative disease (50.2% [119/237] versus 10.9% [60/549], p<.001). Of patients treated with surgery first who were subsequently upstaged due to node-positive disease, 15.6% (37/237) went on to receive adjuvant chemotherapy alone, 5.1% (12/237) were given adjuvant RT alone, and 29.5% (70/237) were given combined adjuvant chemoradiation.
Table 3.
Utilization of adjuvant therapy, by treatment approach and pathologic N stage
| Adjuvant therapy | Total (n = 786) | Node negative (n = 549) | Node positive (n = 237) | p-value |
|---|---|---|---|---|
| Any therapy (either) | 179 (22.8%) | 60 (10.9%) | 119 (50.2%) | < 0.001 |
| Chemotherapy alone | 61 (7.8%) | 24 (4.4%) | 37 (15.6%) | < 0.001 |
| Radiation alone | 23 (2.9%) | 11 (2%) | 12 (5.1%) | 0.04 |
| Chemoradiation | 95 (12.1%) | 25 (4.6%) | 70 (29.5%) | < 0.001 |
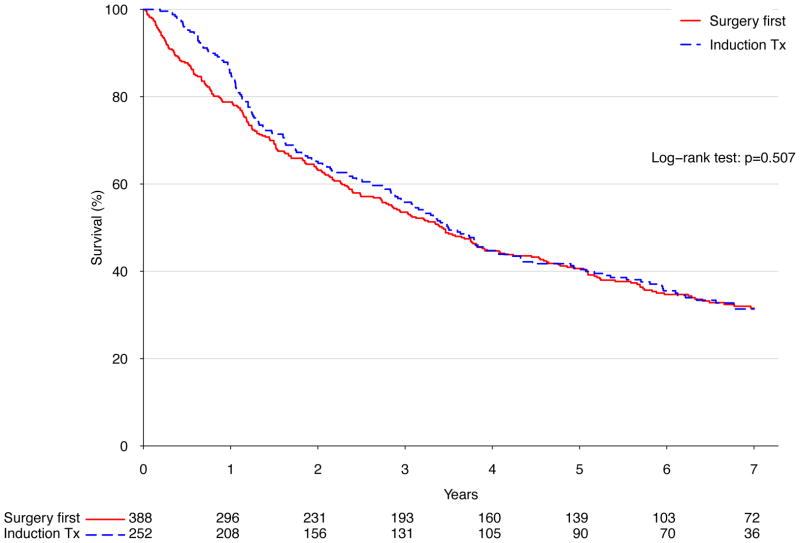
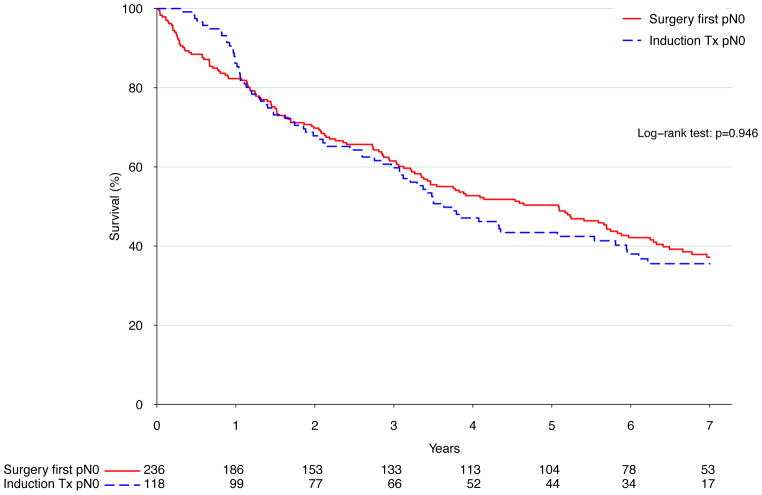
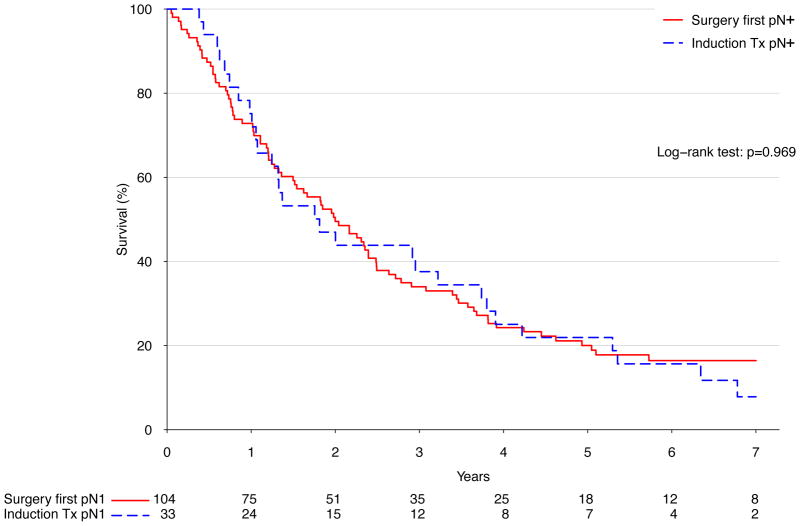
There was no significant difference in long term survival between an approach of surgery first (median survival 41.1 months) versus induction therapy (median survival 41.9 months, p=.51) (Figure 2A). Similarly, after stratifying patients treated with surgery into those who were node negative and those who were node positive, induction therapy was not associated with significantly different long-term survival in either case (p=.95 and p=.97 for node-negative and positive, respectively) (Figure 2B and C). Following Cox proportional hazards adjustment among patients treated with esophagectomy, induction therapy was not found to be independently associated with risk of death (HR 1.16, 95% CI 0.87 to 1.56, p=.32). The only factors significantly associated with death were patient age (HR 1.22 per decade of life, p=.005), pathologic stage (HR 1.61 per stage group, p<.001), and facility volume (HR 0.89 per 10 cases annually, p=.002) (Table 4).
Figure 2.
A) Kaplan-Meier survival estimates for cT2N0 patients undergoing esophagectomy, comparing induction therapy vs. surgery first; 1998–2006. B) Kaplan-Meier survival estimates for cT2N0 patients found to be node negative following esophagectomy, comparing induction therapy vs. surgery-first; 1998–2006. C) Kaplan-Meier survival estimates for cT2N0 patients found to be node positive following esophagectomy, comparing induction therapy vs. surgery-first; 1998–2006.
Table 4.
Adjusted predictors of survival among patients treated with esophagectomy for cT2N0 esophageal cancer
| Predictor | Hazard ratio | Lower 95% CI | Upper 95% CI | p-value |
|---|---|---|---|---|
| Induction therapy | 1.16 | 0.87 | 1.56 | 0.32 |
| Age (per decade) | 1.22 | 1.06 | 1.40 | 0.005 |
| Female sex | 1.06 | 0.68 | 1.65 | 0.80 |
| Race (ref = White) | ||||
| Black | 1.13 | 0.52 | 2.48 | 0.76 |
| Other | 1.14 | 0.39 | 3.34 | 0.81 |
| Charlson/Deyo (0,1,2) | 1.07 | 0.84 | 1.36 | 0.57 |
| Squamous histology (vs. adenocarcinoma) | 1.35 | 0.90 | 2.02 | 0.15 |
| Pathologic Stage (per stage group) | 1.61 | 1.33 | 1.94 | <0.001 |
| Education above median | 1.05 | 0.73 | 1.50 | 0.80 |
| Income above median | 0.86 | 0.59 | 1.26 | 0.45 |
| Facility volume (per 10 annual cases) | 0.89 | 0.83 | 0.96 | 0.002 |
Discussion
In this study, we found that management of patients in the NCDB with cT2N0 esophageal cancer included surgery in the minority of patients, inconsistent with treatment guidelines.2 Treatment in the United States was rather variable, with 57% of patients being treated without surgery and slightly less than half of the patients who were treated operatively being first given induction therapy. However, patients whose management included esophagectomy had significantly better long term survival than those treated non-operatively. Factors associated with receiving esophagectomy included younger age, adenocarcinoma histology, demographic factors and facility type. Clinical T and N status was found to be accurate in only 26.7% of patients, with 41.6% being pathologically upstaged and 31.7% being pathologically downstaged. Slightly more than half of node-positive surgery-first patients received adjuvant therapy. Induction therapy was used in nearly half of patients who underwent surgery, but was not associated with improved survival in multivariable analysis, even after stratifying by pathologic nodal status. Following adjustment, only younger patient age, higher facility volume and lower pathologic stage were associated with improved long-term survival.
Previously published studies have shown conflicting results on the use of induction therapy for T2N0 esophageal cancer. Studies in support of induction therapy include a report of 49 patients from MD Anderson Cancer Center who had an excellent 10-year actuarial survival of greater than 60% associated with induction chemoradiation therapy followed by surgical resection.1 In addition, the T2N0M0 esophageal cancer substage was included in a randomized trial that demonstrated a survival benefit to induction chemoradiotherapy followed by surgery compared to surgery alone for esophageal or esophagogastric junction cancer.11 However, patients with T2N0M0 disease represented a minority of the study participants; the number of clinical T2N0 patients included in this study was not specifically reported, but only 51 of 366 (17%) patients overall clinically had T2 disease with or without nodal disease.11 Other small studies have advocated for the use of induction therapy due to a high incidence of nodal disease detected after resection.6,7 However, analyses from at least two retrospective series have questioned whether induction therapy improves survival. In a study of the Surveillance, Epidemiology, and End Results (SEER) database, patients treated with both RT and surgery, with RT being used prior to surgery in the majority of cases, did not have improved survival compared to patients treated with surgery alone.12 In addition, another small single center series found no survival advantage associated with induction therapy, and advocated for initial treatment with surgery with adjuvant therapy given if after resection the tumor was found to be initially under-staged.5 The results of our present study support a treatment strategy that does not routinely use induction therapy.
Interestingly, comparison of the survival curves for the two groups shows an apparent initial superior survival followed by a delayed and sharp decrease among the induction therapy group in the first year following diagnosis. This finding is probably at least partially due to the timing of surgery in relation to the time of diagnosis in the two groups. While the observed early survival difference may be partly related to the induction therapy itself, it is much more likely that this is the result of a treatment time-lag bias given that survival was assessed from date of diagnosis. Even though the two groups experienced similar rates of perioperative morbidity and mortality, the induction therapy group had a substantially longer time interval between diagnosis and complications due to esophagectomy. The delay before surgery in the induction therapy group is likely partially responsible for the early survival appearing to be better in this group compared to the patients that went straight to surgery early after their diagnosis.
Based on the rates of nodal upstaging and actual rates of adjuvant therapy delivery demonstrated in this study, 85% of surgery-first patients are treated according to NCCN guidelines. On the other hand, while considering that 69.8% of cT2N0 patients are correctly staged as node negative, a strategy of induction therapy for cT2N0 disease may lead to administration of chemoradiation that may not have been necessary in over two-thirds of cases. Although induction therapy has been shown to be safe and does not increase risk of surgery3,13,14, overtreatment can lead to unnecessary morbidity and costs. In addition, our study may overestimate the benefits of induction therapy, because the initial treatment intent for the patients who were treated with chemotherapy and/or RT and did not get surgery is unknown. Some of those patients may have been started on chemotherapy or RT with the plan to ultimately undergo esophagectomy, but never made it to surgery. We are unable to identify instances of patient death following induction therapy but prior to definitive resection. However, previous studies have suggested that only a small number of patients do not proceed to resection, with disease progression the most common reason that surgery is ultimately not utilized.15
Node positive disease is a strong predictor of systemic disease and poor long-term survival.16,17 The benefit of induction therapy for patients who truly have N0 disease prior to therapy is likely to be minimal given their better prognosis, and may needlessly subject these patients to the risks associated with chemotherapy and radiotherapy.5 However, in case of upstaging in the pathological report to node-positive disease, multimodality therapy could still be delivered using adjuvant radiotherapy and chemotherapy. This strategy is supported by others based on gastroesophageal adenocarcinomas.18 In our analysis of patients in the NCDB, over half of the node-positive surgery-first patients received some amount of adjuvant therapy. Although one potential downside of using esophagectomy as the initial treatment is that morbidity or debilitation after surgery may prevent patients from receiving adjuvant chemotherapy, our analysis does not suggest that giving treatment prior to surgery in this clinical setting ultimately improves survival. Interestingly, although the rates of nodal upstaging between patients who were given induction therapy and the patients who went straight to surgery were significantly different statistically (23.6% versus 30.2%, p=0.01), based on the observed survival in each group, this difference did not appear to be associated with improved survival.
The use of the NCDB has a significant strength of being able investigate uncommon tumor stages with enough power to even perform subgroup analysis, due to its population based nature, especially considering that previously published reports on induction therapy for T2N0 esophageal cancer have been small and highly selected single-center experiences.1,5,6 Although a randomized trial would be ideal to definitely evaluate treatment strategies, such a trial is unlikely to ever happen considering the relative uncommon nature of this esophageal cancer substage. Even if such a trial were performed, it is extremely unlikely that any clinical trial will be able to assemble the number of patients that were considered in this study. However, clinicians should strongly consider including patients with this stage of disease in multi-institutional registries to allow further evaluation of different treatment strategies and outcomes in a prospective fashion.
The NCDB does also have some inherent limitations. First, there is relatively limited information regarding patient comorbidities and functional status, which may be important in predicting both survival and treatment, particularly multimodality therapy. Even though multivariable adjusted analysis can correct for measured covariates, unmeasured confounding cannot be excluded. Additionally, specific details on chemotherapy agents and doses are not available. The NCDB also presents limitations in assessing overall stage, as patients are staged using the AJCC edition used at the time of diagnosis. While this did not impact our primary analysis as the definition of clinical T2N0 did not change over time, this may have biased our results somewhat, particularly with respect to the inclusion of overall pathologic stage in the multivariable survival models. Also, details regarding the staging modalities used to establish the clinical T2N0 diagnosis for the patients in this study are not available. Modalities generally used to establish clinical esophageal cancer stage prior to treatment include computed tomography (CT) scanning, positron emission tomography (PET) scanning, endoscopic ultrasound (EUS), EUS with fine-needle aspiration (FNA), and laparoscopy. Similarly, it is likely that variability in staging modalities over the course of the study time period may have led to different rates of stage migration over time, particularly as PET has become more accessible in recent years. EUS has also gained popularity and is a valuable tool in clinical staging, but is known to be less accurate for early-stage lesions such as T1 or T2 compared to more advanced tumors.5,19–21 Most incidences of understaging are due to missed nodal disease.4 As many as 55% of patients with clinically staged T2N0 esophageal cancers have previously been reported as having nodal disease after resection.5,6 The specificity and the sensitivity for identifying lymph node disease is better for EUS-FNA compared to EUS alone.22 If the use of staging modalities was not balanced between the groups, then the groups may have had different likelihoods of over- or under-staging clinically. However, the rates of under and over staging in this study are similar to previously published reports.3–7 Lastly, while we attempted to control for imbalance statistically, due to the retrospective nature of this study there is potential for selection bias, particularly regarding the somewhat larger tumor size and smaller number of lymph nodes retrieved among the patients treated with induction therapy compared to their surgery-first counterparts.
In conclusion, treatment of cT2N0 esophageal cancer is variable. In the NCDB, less than half the patients had esophagectomy as part of their treatment. For the patients that did have surgery, slightly less than half of patients had induction therapy prior to surgery. In this study, we did not find that induction therapy improved outcomes compared to surgery alone. We found that more than half of patients who had node-positive disease after esophagectomy without induction treatment received adjuvant therapy. The lack of benefit seen in this population-based analysis does not support the clinical practice of treating this stage of esophageal cancer with induction therapy, particularly when the cost and potential morbidity of induction therapy are considered. Although prospective studies in the form of either a randomized trial or via creation of a prospective registry would be ideal to more definitively establish optimal treatment, this current study suggests that the most efficient treatment strategy for cT2N0 esophageal cancer is initial surgery. Subsequent administration of adjuvant therapy can be considered for patients who are pathologically upstaged postoperatively, however further study is necessary to determine if this strategy improves survival.
Acknowledgments
The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigators.
Footnotes
Sources of support and disclosures: This work was supported by the NIH funded Cardiothoracic Surgery Trials Network (M.F.B). One of the authors (T.A.D.) serves as a consultant for Scanlan International, Inc.
References
- 1.Kountourakis P, Correa AM, Hofstetter WL, et al. Combined modality therapy of cT2N0M0 esophageal cancer: the University of Texas M. D. Anderson Cancer Center experience. Cancer. 2011;117:925–930. doi: 10.1002/cncr.25651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajani JA, Barthel JS, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers. Journal of the National Comprehensive Cancer Network: JNCCN. 2011;9:830–887. doi: 10.6004/jnccn.2011.0072. [DOI] [PubMed] [Google Scholar]
- 3.Crabtree TD, Kosinski AS, Puri V, et al. Evaluation of the reliability of clinical staging of T2 N0 esophageal cancer: a review of the Society of Thoracic Surgeons database. The Annals of thoracic surgery. 2013;96:382–390. doi: 10.1016/j.athoracsur.2013.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crabtree TD, Yacoub WN, Puri V, et al. Endoscopic ultrasound for early stage esophageal adenocarcinoma: implications for staging and survival. The Annals of thoracic surgery. 2011;91:1509–1515. doi: 10.1016/j.athoracsur.2011.01.063. discussion 1515–1506. [DOI] [PubMed] [Google Scholar]
- 5.Rice TW, Mason DP, Murthy SC, et al. T2N0M0 esophageal cancer. The Journal of thoracic and cardiovascular surgery. 2007;133:317–324. doi: 10.1016/j.jtcvs.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Stiles BM, Mirza F, Coppolino A, et al. Clinical T2-T3N0M0 esophageal cancer: the risk of node positive disease. The Annals of thoracic surgery. 2011;92:491–496. doi: 10.1016/j.athoracsur.2011.04.004. discussion 496–498. [DOI] [PubMed] [Google Scholar]
- 7.Zhang JQ, Hooker CM, Brock MV, et al. Neoadjuvant chemoradiation therapy is beneficial for clinical stage T2 N0 esophageal cancer patients due to inaccurate preoperative staging. The Annals of thoracic surgery. 2012;93:429–435. doi: 10.1016/j.athoracsur.2011.10.061. discussion 436–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleming ID, Cooper JS, Henson DE, et al., editors. AJCC Cancer Staging Manual. 5. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- 9.Greene FL, Page DL, Fleming ID, et al., editors. AJCC Cancer Staging Manual. 6. New York: Springer-Verlag; 2002. [Google Scholar]
- 10.Edge SB, Byrd DR, Compton CC, et al., editors. AJCC Cancer Staging Manual. 7. New York: Springer-Verlag; 2009. [Google Scholar]
- 11.van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. The New England journal of medicine. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 12.Martin JT, Worni M, Zwischenberger JB, et al. The role of radiation therapy in resected T2 N0 esophageal cancer: a population-based analysis. The Annals of thoracic surgery. 2013;95:453–458. doi: 10.1016/j.athoracsur.2012.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merritt RE, Whyte RI, D’Arcy NT, Hoang CD, Shrager JB. Morbidity and mortality after esophagectomy following neoadjuvant chemoradiation. The Annals of thoracic surgery. 2011;92:2034–2040. doi: 10.1016/j.athoracsur.2011.05.121. [DOI] [PubMed] [Google Scholar]
- 14.Wright CD, Kucharczuk JC, O’Brien SM, Grab JD, Allen MS. Predictors of major morbidity and mortality after esophagectomy for esophageal cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database risk adjustment model. The Journal of thoracic and cardiovascular surgery. 2009;137:587–595. doi: 10.1016/j.jtcvs.2008.11.042. discussion 596. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert S, Gresham GK, Jonker DJ, et al. Impact of patient selection, disease progression, and adverse events on esophageal cancer outcomes after trimodality therapy. The Annals of thoracic surgery. 2012;94:1659–1666. doi: 10.1016/j.athoracsur.2012.05.044. [DOI] [PubMed] [Google Scholar]
- 16.Rice TW, Rusch VW, Ishwaran H, Blackstone EH. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer. 2010;116:3763–3773. doi: 10.1002/cncr.25146. [DOI] [PubMed] [Google Scholar]
- 17.Rizk NP, Ishwaran H, Rice TW, et al. Optimum lymphadenectomy for esophageal cancer. Annals of surgery. 2010;251:46–50. doi: 10.1097/SLA.0b013e3181b2f6ee. [DOI] [PubMed] [Google Scholar]
- 18.Kofoed SC, Muhic A, Baeksgaard L, et al. Survival after adjuvant chemoradiotherapy or surgery alone in resectable adenocarcinoma at the gastro-esophageal junction. Scandinavian journal of surgery: SJS: official organ for the Finnish Surgical Society and the Scandinavian Surgical Society. 2012;101:26–31. doi: 10.1177/145749691210100106. [DOI] [PubMed] [Google Scholar]
- 19.DeWitt J, Kesler K, Brooks JA, et al. Endoscopic ultrasound for esophageal and gastroesophageal junction cancer: Impact of increased use of primary neoadjuvant therapy on preoperative locoregional staging accuracy. Diseases of the esophagus: official journal of the International Society for Diseases of the Esophagus/ISDE. 2005;18:21–27. doi: 10.1111/j.1442-2050.2005.00444.x. [DOI] [PubMed] [Google Scholar]
- 20.Kutup A, Link B-C, Schurr PG, et al. Quality control of endoscopic ultrasound in preoperative staging of esophageal cancer. Endoscopy. 2007;39:715–719. doi: 10.1055/s-2007-966655. [DOI] [PubMed] [Google Scholar]
- 21.Pech O, Günter E, Dusemund F, Origer J, Lorenz D, Ell C. Accuracy of endoscopic ultrasound in preoperative staging of esophageal cancer: results from a referral center for early esophageal cancer. Endoscopy. 2010;42:456–461. doi: 10.1055/s-0029-1244022. [DOI] [PubMed] [Google Scholar]
- 22.Vazquez-Sequeiros E, Wiersema MJ, Clain JE, et al. Impact of lymph node staging on therapy of esophageal carcinoma. Gastroenterology. 2003;125:1626–1635. doi: 10.1053/j.gastro.2003.08.036. [DOI] [PubMed] [Google Scholar]