Abstract
PURPOSE
We hypothesized that the combination of bevacizumab, carboplatin and pemetrexed will be an effective first-line regimen in fit, elderly patients with nonsquamous NSCLC.
PATIENTS AND METHODS
Treatment-naïve, stage IIIB/IV nonsquamous NSCLC patients ≥ 70 years old with good performance status (ECOG PS 0-1) and adequate organ function were eligible. Carboplatin AUC 6, pemetrexed 500 mg/m2 and bevacizumab 15 mg/kg were administered on day 1 of each 21-day cycle (up to 6 cycles) followed by maintenance pemetrexed and bevacizumab. The primary endpoint of 6-month progression-free survival rate (PFS6) of at least 70% was assessed using a one-stage binomial design. Quality of life (QOL) questionnaires were administered. Polymorphisms in genes encoding relevant proteins (drug targets, transport and metabolism proteins) were correlated with treatment outcome.
RESULTS
Fifty-seven eligible patients were enrolled. Median age was 74.5 years. Median treatment cycles received was 6. The most common grade 3 or higher non-hematologic adverse events were fatigue (26%) and hypertension (11%). 16% had grade 4 neutropenia and 6.5% had grade 4 thrombocytopenia. Three patients experienced grade 3/4 hemorrhagic events (one pulmonary, two gastrointestinal). Primary endpoint of PFS6 was 60% (95% CI: 45.9–73%). Median PFS was 7.0 months (95% CI: 5.9–10.1), median overall survival was 13.7 months (95% CI: 9.4–16.8). Polymorphic KDR and VEGFA variants correlated with survival and toxicity, respectively. There was no significant change in overall QOL scores over time.
CONCLUSION
This regimen is feasible and did not decrease the QOL in this study population. However, it did not meet the primary efficacy endpoint.
Keywords: Non-small cell lung cancer, Elderly, Nonsquamous histology, Bevacizumab, Survival
INTRODUCTION
The phase III ECOG4599 study demonstrated the relevance of angiogenesis as a therapeutic target in metastatic non-small cell lung cancer (NSCLC) by showing that addition of bevacizumab to first-line carboplatin and paclitaxel (CbPac) improved survival.1 However, subsequent analysis showed that patients older than 70 years old in the bevacizumab arm had more frequent and severe toxicities and did not have better progression-free survival (PFS) or overall survival (OS).2 One hypothesis is that the baseline toxicity of the CbPac backbone in elderly patients could contribute to this finding.
Pemetrexed is a multi-targeted anti-folate that is indicated for NSCLC patients with nonsquamous histology.3–5 A phase II single-arm study of carboplatin, pemetrexed, bevacizumab (CbPemBev)as front-line therapy for advanced nonsquamous NSCLC showed promising efficacy (overall response rate [ORR] of 55%, PFS of 7.8 months and OS of 14 months).6 Because of the favorable safety profile in a general population, N0821 was designed to evaluate the efficacy and tolerability of CbPemBev specifically in good PS patients ≥ 70 years old using the same regimen described by Patel, et al.6 Gene polymorphisms in the folate and angiogenesis pathways are associated with variations in treatment outcomes. 7–15 We therefore tested for an association of clinical outcomes with variations in eight pathway genes associated with pemetrexed and bevacizumab.
METHODS
Patients and Treatment
Eligible patients were ≥ 70 years old with ECOG PS 0–1, chemonaive stage IIIB (malignant pleural effusion) or IV nonsquamous NSCLC (TNM 6th edition) with measurable disease as defined by RECIST (1.0) and adequate bone marrow (hemoglobin ≥ 9 g/dl; absolute neutrophil count ≥ 1500/ul; platelet count ≥ 100,000/ul), hepatic (total bilirubin ≤ 1.5x upper limit of normal or direct bilirubin < upper limit of normal; AST and ALT ≤ 3x upper limit of normal or ≤ 5x upper limit of normal in the presence of hepatic metastases) and renal function (creatinine clearance ≥ 45 ml/min). Patients with treated and asymptomatic brain metastases were allowed to participate. Exclusions include uncontrolled hypertension, acute cardiovascular events within 6 months of registration, history of abdominal fistulas, gastrointestinal perforation or intra-abdominal perforations within the preceding 12 months, active hemoptysis > 2.5 ml per event, concurrent use of anticoagulants and presence of serious or non-healing ulcers, wounds or bone fractures. Central review of pathologic diagnosis was mandatory. NSCLC tumors were classified as adenocarcinoma, squamous cell carcinoma, carcinoma not otherwise specified based on H&E morphology and available immunohistochemistry. Patients with a NSCLC diagnosis other than squamous cell carcinoma subtype were deemed eligible.
This single-arm, multicenter phase II study consisted of two treatment phases. In the induction phase, patients were treated with up to 6 cycles of carboplatin (AUC6), pemetrexed (500 mg/m2) and bevacizumab (15 mg/kg) administered on day 1 of a 21-day cycle. In the maintenance phase, patients without progression were eligible to continue pemetrexed (500 mg/m2) and bevacizumab (15 mg/kg) administered on day 1 of a 21-day cycle until progression or intolerance. All patients received standard of care vitamin supplementation and dexamethasone premedication. Restaging CT scans were obtained after each two cycles of therapy. Patients who had disease progression or undue toxicity, refused further treatment or were deemed by their treating physicians to be more suitably treated with alternative methods at any treatment phase were taken off study treatment and monitored for adverse events (AE) and survival.
Quality of life (QOL) assessment
Quality of life (QOL) was assessed using the Lung Cancer Symptom Scale (LCSS) and the single-item Linear Analogue Self Assessment (LASA) questionaires. A treatment-specific AEs scale consisting of one item each for fatigue, neuropathy and nausea was also administered. QOL was assessed at baseline, prior to cycle 3 and prior to cycle 5.
Pharmacogenetic studies
Patient DNA samples were analyzed for polymorphisms in genes encoding proteins that affect pemetrexed metabolism or efficacy (FPGS, GGH, SLC19A1 and TYMS) and in genes that represent key mediators of the VEGF signaling pathway (VEFGA, FLT1 (VEGFR1), KDR (VEGFR2) and FLT4 (VEGFR3). Acquisition of tagSNPs and genotyping were as previously described. 7
Statistical Analyses
The primary endpoint of this single stage phase II study was the 6-month progression-free survival (PFS6). A one stage binomial design with an exact two-sided significance level of 0.05 and power of 93% was used to test the hypothesis that the true success rate (eligible patient who started treatment and is progression free at 6 months) was at most 50% versus the alternative hypothesis that the true success rate was at least 70%. The null and alternate rates for the primary endpoint were derived based on an exponential survival model from the 95% CI for the median PFS reported in the Patel phase II study,6 which was 5.2–11.5 months. With a sample size of 55 evaluable patients, the regimen would be declared promising if at least 34 successes were observed. Secondary endpoints included AE profile, confirmed response rates (RR), PFS, and OS.
OS was defined as the time from registration to death due to any cause. PFS was defined as the time from registration to the first date of disease progression or death as a result of any cause. PFS was censored at the date of the last contact for patients alive and progression free at the time of this analysis. Exact binomial confidence intervals for the proportion of successes were constructed. The distribution of PFS and OS time was estimated using the Kaplan-Meier (KM) method.16
QOL scores were translated onto a 0–100 point scale with higher values indicating worse symptoms,17 and summarized descriptively at each time point. Changes from baseline in overall and individual item QOL scores were analyzed using a paired sample t-test.
Patient genotypes were correlated in an exploratory analysis with primary (PFS6) and secondary (AE, RR, PFS and OS) endpoints. Logistic regression models were used to compare the PFS6 status, AE patterns and ORR between the different tagged SNP subgroups. KM curves were used to visually compare the OS and PFS distributions between the different tagged SNP subgroups, and Cox regression models were used to assess the impact of the genotype subgroups on OS and PFS. No adjustments for multiple comparisons were performed in this exploratory exercise.
Each participant signed an IRB-approved, protocol-specific informed consent in accordance with federal and institutional guidelines. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center.
RESULTS
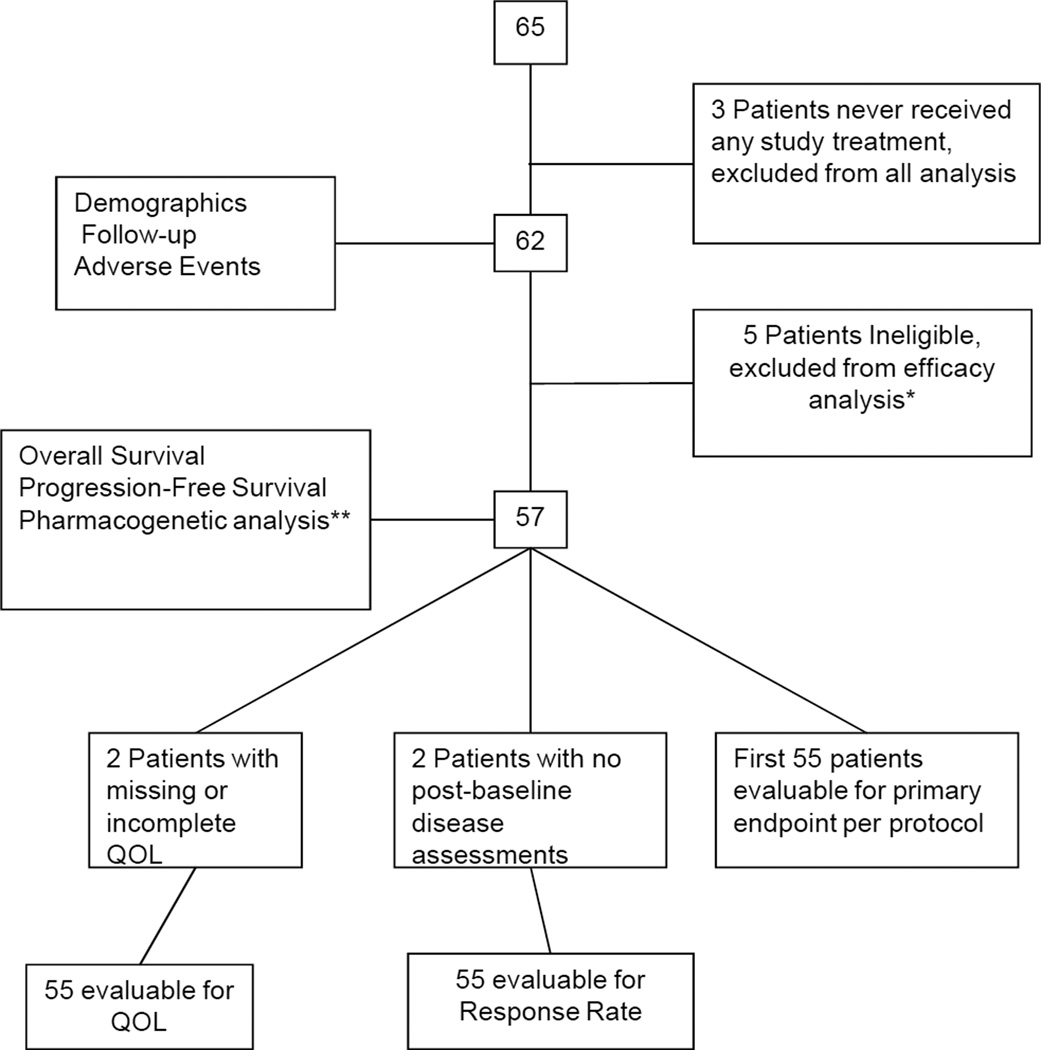
This protocol was activated on December 12, 2008 and permanently closed to patient accrual on October 1, 2010 after accruing 65 patients. Final analysis as of November 6th, 2012 is reported. Figure 1 shows the CONSORT diagram of the study enrollment. Three patients who never received any study treatment are excluded from all analyses. Table 1 summarizes the demographic and baseline characteristics of the 62 patients who received treatment on this study as well as follow-up information. One patient was still receiving active treatment at the time of data lock. Median number of cycles received by all 62 patients treated was 6 (range 1–43). Median number of cycles of the triplet combination received by all 62 patients treated was 6 (range 1–6). Twenty eight patients received a median of 4 cycles of pemetrexed and bevacizumab only (range 1–37). Twenty-four patients received at least one dose reduction, most commonly to manage a non-hematologic AE (63% of all dose modifications between the three agents). Median follow-up for the 15 living patients is 25.4 months (range: 1.1 to 37.4 months). All 62 patients who received treatment are included in the AE summary. Five patients who were deemed ineligible are excluded from all efficacy analysis. The patient cohorts evaluable for the different endpoints are described in the CONSORT diagram.
Figure 1.
CONSORT diagram
Table 1.
Patient demographics and Follow up
| TOTAL N=62 |
|
|---|---|
| Age (Years) | |
| Median | 74.5 |
| Range | (70–86) |
| Gender | |
| Female | 31 (50.0%) |
| ECOG Performance Status | |
| 0 | 30 (48.4%) |
| 1 | 32 (51.6%) |
| Race | |
| White | 58 (93.5%) |
| Black or African American | 2 (3.2%) |
| Unknown | 2 (3.2%) |
| Histologic Type | |
| Adenocarcinoma | 44 (71.0%) |
| *Other | 18 (29.0%) |
| NSCLC Stage (TNM 6th Edition) | |
| Stage IIIB | 9 (14.5%) |
| Stage IV | 53 (85.5%) |
| Follow-up Time (Months) in Alive Patients | |
| N | 15 |
| Median | 25.4 |
| Range | (1.1–37.4) |
| Cycles of Therapy Given | |
| N | 527 |
| Median | 6.0 |
| Range | (1–43) |
| Patients Off Study | |
| N | 61 (98.4%) |
| Reason Ended Treatment | |
| Refused Further Treatment | 10 (16.4%) |
| Adverse Event | 22 (36.1%) |
| Disease Progression | 27 (44.3%) |
| Other** | 2 (3.3%) |
This table includes the 3 patients who were eventually deemed ineligible due to incorrect histology upon central review (see CONSORT diagram).
1 Patient due to lack of insurance coverage; 1 MD discretion.
Primary Endpoint
Thirty three of the 55 patients evaluable for PFS were progression-free at 6 months, resulting in 60% PFS6 (95% CI: 45.9–73%). Two patients were lost to follow-up prior to 6 months, and were considered as failures for the PFS6 endpoint. Thus, the trial did not meet the primary endpoint of at least 70% PFS6. A Kaplan-Meier estimate (considering the two lost to follow-up patients as censored observations) of the PFS6 for the first 55 evaluable patients yields 64.2% (95% CI: 49.7–75.5%).
Secondary efficacy outcomes
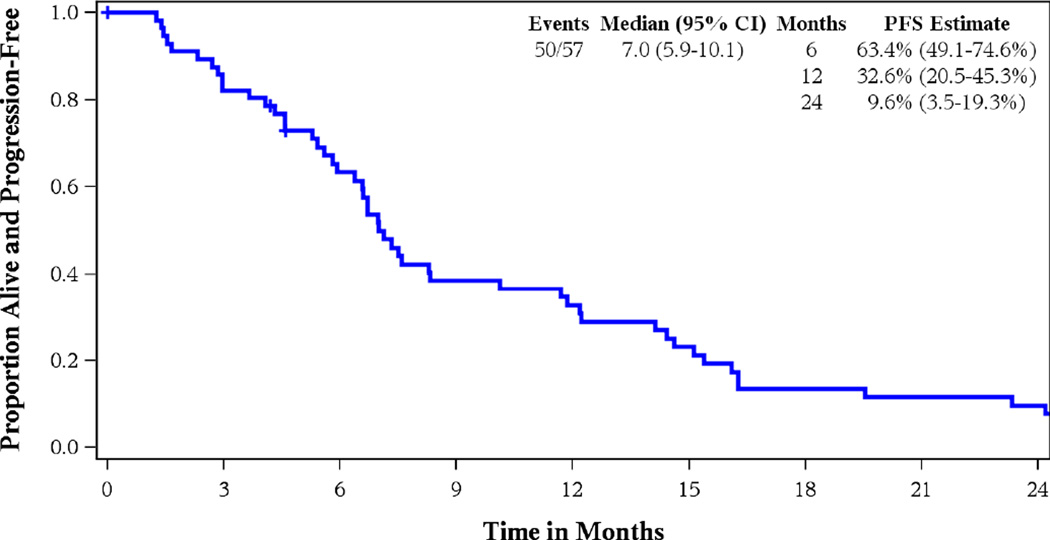
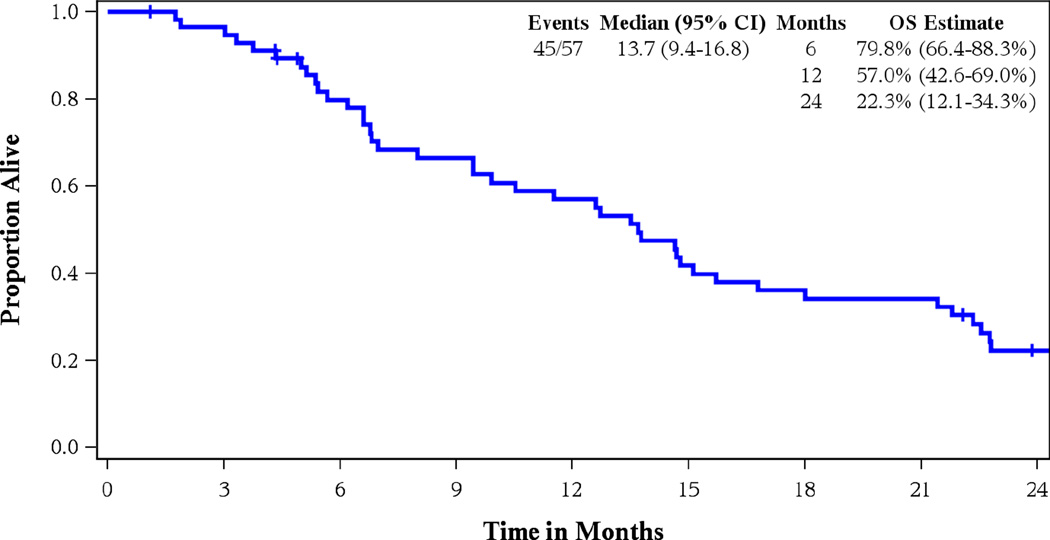
Twenty eight out of 55 patients evaluable for tumor response had a partial or complete response for an ORR of 50.9% (95% CI 37.1–64.7%). Confirmed RR was seen in 22 patients (40%, 95% CI 27.0–54.1%), one of whom had a complete response. Median PFS was 7 months (95% CI: 5.9–10.7), median OS was 13.7 months (95% CI: 9.4–16.8) for a 1-year survival estimate of 57% (42.6–69.0%). Figures 2 and 3 show the Kaplan-Meier curves for PFS and OS, respectively for all 57 eligible patients.
Figure 2.
Kaplan Meier Curve for Progression-Free Survival
Figure 3.
Kaplan Meier Curve for Overall Survival
AE profile
All 62 patients are evaluable for AEs. Table 2 summarizes the observed toxicities. Grade 3 or higher AE were reported in 54 (87%) patients. There were no treatment-related deaths. The grade 3 non-hematologic AEs occurring in at least 10% of patients were fatigue (26%) and hypertension (11%). The grade 3 hematologic AEs occurring in at least 10% of patients were neutropenia (29%) and thrombocytopenia (18%). Grade 3 or higher anemia occurred in 4.8%. Grade 3 or higher hemorrhages occurred in 5%. One patient had neutropenic fever and developed typhlitis during his hospitalization. Twenty two (36%) patients ended study treatment due to AEs (however, additional data on specific AEs were not collected). Eight of the 22 patients discontinued treatment during the maintenance phase whereas the remaining 14 patients ended study within the first six cycles of treatment. 29 patients had at least one dose delay implemented during their course of therapy.
Table 2.
Adverse Events
| Adverse Event (Max Grade per Event) |
No. of Patients (Total=62) |
% |
|---|---|---|
| Patients with at least one of the following: | ||
| Grade 3/4/5 AE | 54 | 87.1 |
| Grade 4/5 AE | 21 | 33.9 |
| Grade 3/4 hematologic AE | 33 | 53.2 |
| Grade 4 hematologic AE | 13 | 21.0 |
| Grade 3/4/5 nonhematologic AE | 45 | 72.6 |
| Grade 4/5 nonhematologic AE | 10 | 16.1 |
| All Grade 4 hematologic AEs | ||
| Anemia | 1 | 1.6 |
| Lymphocytopenia | 1 | 1.6 |
| Neutropenia | 10 | 16.1 |
| Thrombocytopenia | 4 | 6.5 |
| Leukopenia | 1 | 1.6 |
| All Grade 4 nonhematologic AEs | ||
| Abdominal pain | 1 | 1.6 |
| Dyspnea | 1 | 1.6 |
| Fracture | 1 | 1.6 |
| Hypertension | 1 | 1.6 |
| Myocardial ischemia | 1 | 1.6 |
| Thrombosis | 1 | 1.6 |
| Ventricular tachycardia | 1 | 1.6 |
| Lower gastrointestinal hemorrhage | 1 | 1.6 |
| Sepsis | 1 | 1.6 |
| All Grade 5 nonhematologic AEs | ||
| Disease progression | 2 | 3.2 |
| Grade 3 or higher AEs occurring in ≥10% of Patients | ||
| Fatigue | 16 | 25.8 |
| Hypertension | 7 | 11.3 |
| Neutropenia | 18 | 29.0 |
| Thrombocytopenia | 11 | 17.7 |
| Grade 3 or higher Anemia/Hemorrhage | ||
| Anemia | 3 | 4.8 |
| Pulmonary hemorrhage | 1 | 1.6 |
| Lower gastrointestinal hemorrhage | 1 | 1.6 |
| Jejunal hemorrhage | 1 | 1.6 |
QOL analysis
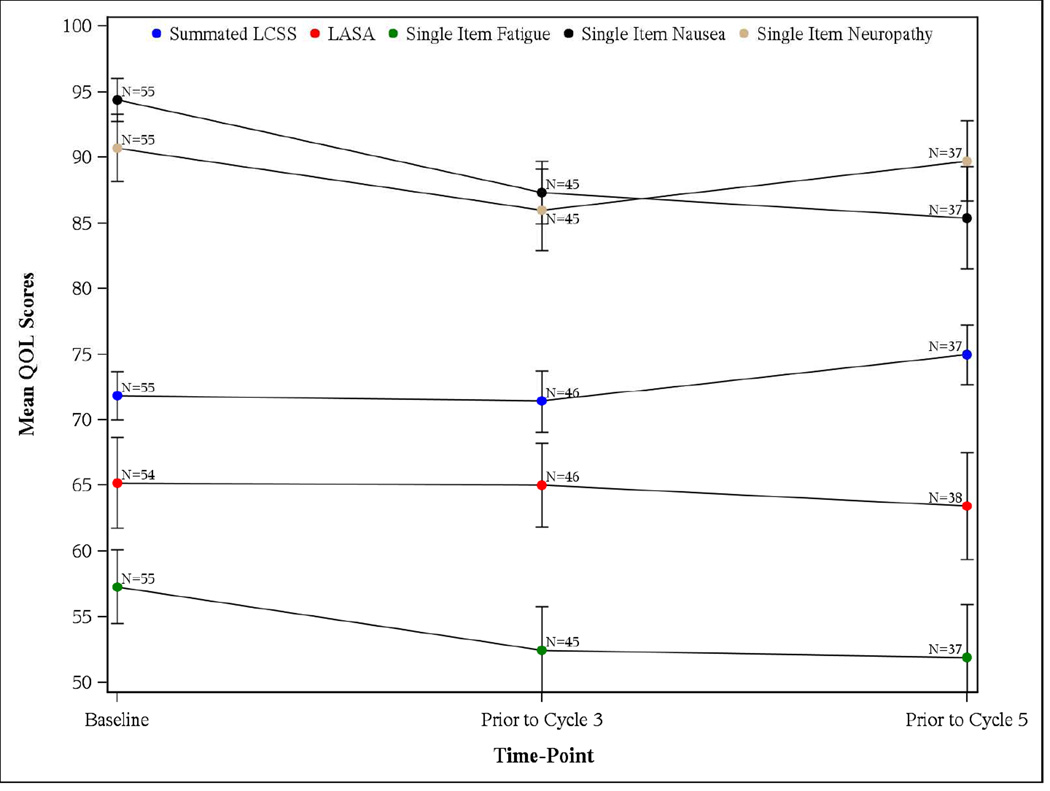
At baseline, the median scores for all individual questions were greater than 50, with a summated LCSS score of 72.2 (inter-quartile range (IQR): 58.9–84.4) and overall LASA score of 70 (IQR: 50–90). While there was a statistically significant worsening in the pain QOL domain of LCSS, the overall QOL as evaluated by the LASA or the LCSS did not show any significant change over time. The median (IQR) baseline neuropathy, nausea and fatigue were respectively 100.0 (IQR: 90–100), 100.0 (IQR: 90–100) and 60.0 (IQR: 40–70), where higher values indicate worse symptoms. While there were no significant changes in the fatigue or neuropathy as assessed by the single item questions, a statistically significant improvement from baseline was observed for nausea at cycle 3 (mean score: 94.36 versus 87.33; p=0.0142) and cycle 5 assessment (mean score: 94.36 versus 85.41; p=0.0191). Figure 4 shows the temporal changes for various QOL measures.
Figure 4.
Median and Standard errors of the QOL scores by Time-Point
Pharmacogenetic analysis
Sixty-four tagSNPs generated from the 8 genes with minor allele frequency (MAF) ≥5% were successfully genotyped. Most of the SNPs genotyped were in Hardy-Weinberg equilibrium (HWE), supplementary Table 1. Genotypes observed in <5 patients were regrouped and if the regrouped frequency was ≤ 10%, the SNP was excluded from the analyses with the clinical outcomes. SNPs that were not in HWE were also excluded from analysis with clinical outcomes. Several of the SNPs genotyped were associated significantly with the clinical outcomes at p≤0.05 (supplementary Tables 2–4). For example, in KDR (VEGFR2) rs7671745 polymorphism, the AG+AA genotypes, compared to the homozygous GG genotype, were associated with superior confirmed response rate (OR=4, 95% CI 1.11 -14.47, p=0.029) and PFS6 (OR=4.062, 95% CI=1.29–12.78 p=0.014). For the VEGFA rs3025018 polymorphism, CG+GG genotypes compared to the homozygous CC genotype, were associated with inferior PFS (HR=6.9, 95% CI= 2.21–21.3, p=0.0001) and OS (HR=6.8, 95% CI= 2.14–21.53, p=0.0002). The CT+TT genotypes in VEGFA rs3025035 polymorphism, compared to the homozygous CC genotype, were associated with reduced toxicity specifically grade 3+ AEs (OR=0.152, 95% CI= 0.026–0.880, p=0.0209) and grade 3+ non-hematologic AEs (OR=0.158, 95% CI=0.032–0.776, p=0.0137). In addition, there was a significant association with reduced toxicity for TYMS rs2847153 G>A (OR=0.244, 95% CI=0.071–0.847, p=0.0218) and GGH rs3780130 A>T (OR=0.181, 95% CI=0.035–0.923, p=0.0277) polymorphisms, while the AA genotype for SLC19A1 rs2838958 was associated with inferior confirmed response compared to the GG genotype (p=0.0312).
DISCUSSION
Bearing in mind the usual caveats about cross-trial comparisons, the toxicities and OS endpoint seen in this study were overall comparable to those reported by Spigel et al in a randomized phase II study of a similar elderly patient population treated in the carboplatin, pemetrexed and bevacizumab arm.18 Of note, there are some differences in the regimen utilized in the Spigel study. The carboplatin dose was slightly lower (AUC 5 in contrast to AUC of 6 utilized in our study) and its maintenance phase utilized bevacizumab alone (pemetrexed was combined with bevacizumab during maintenance in our study). In comparison with another phase II study of a similar elderly patient population treated with six cycles of carboplatin AUC 5 with pemetrexed 500 mg/’m2 followed by observation (no maintenance phase), the outcomes with median ORR of 29%, PFS of 5.5 months, OS of 10.4 months and 1-year OS rate of 42%, appeared to be somewhat inferior to results seen in our study.19 In the phase II Patel study wherein the median age of patients treated was 63.5 years (range 34–80.5),6 the median PFS of 7.8 months, OS 14.1 months, PFS6 rate of 59% and 1-year OS rate of 61% were comparable as well to what was observed in our cohort of geriatric patients with a median age of 74.5 years(7 months, 13.7 months, 60% and 57%, respectively). However, in contrast to the toxicity rates described in the phase II Patel study,6 our patient population generally encountered higher rates of grade ≥ 3 AEs (e.g. neutropenia, thrombocytopenia, hemorrhage and fatigue). This is not unexpected, in retrospect, as a subsequent dose-escalation study of carboplatin and pemetrexed (with a subsequent maintenance pemetrexed phase) in seventeen Japanese patients ≥ 75 years observed dose-limiting myelosuppression (grade 4 thrombocytopenia and grade 3 febrile neutropenia) in three of seven patients treated at carboplatin AUC 6, leading to the recommended dose of carboplatin AUC 5 in combination with pemetrexed 500 mg/m2 in the elderly.20
Our efficacy data were also consistent with the results seen among patients randomized to this treatment regimen in the recently reported phase III POINTBREAK study (34.1% and 12.6 months) where it was compared to carboplatin, paclitaxel and bevacizumab.21 The safety results from this phase III study showed that the side effect profile may favor the pemetrexed combination over the paclitaxel combination in terms of lower rates of grade 3/4 neutropenia (25.8% versus 40.6%), febrile neutropenia (1.4% versus 4.1%) and sensory neuropathy (0% versus 4.1%). However, fatigue (5% versus 10.9%) and thrombocytopenia (5.6% versus 23.3%) rates were higher with the pemetrexed combination, such that study discontinuations due to AEs or serious AEs and drug-related deaths were similar between the two treatment arms.21 Subgroup efficacy analyses in patients > 70 years show OS and PFS results consistent with the intention-to-treat population. In a pooled exploratory analysis based on age in the phase III E4599 and POINTBREAK studies, patients ≥75 years who received bevacizumab in combination with carboplatin and paclitaxel had a higher incidence of grade 3 AEs relative to carboplatin and paclitaxel alone with no statistically significant survival benefit.22 Despite the grade 3+ fatigue documented in more than a quarter of our patients, the fatigue domain of LCSS and single-item LASA in fact indicated less fatigue reported by patients who remained on study at the time of cycle 3, though this change was not statistically significant. Our QOL data suggest that there is no worsening of patient QOL scores over time when this regimen is used as first-line treatment for a geriatric population.
The benefit of combining bevacizumab with carboplatin and pemetrexed compared to the platinum/pemetrexed combination as first-line therapy can only be speculated as there are no phase III data addressing this question. In three phase II and one phase III predominantly non-Asian studies of carboplatin pemetrexed in younger populations and including some squamous histologies, grade 3/4 neutropenia ranged between 10.3% to 33%, objective response rates ranged between 22% to 34%, median TTP/PFS were 5.4 to 6 months, median OS was 10.3 to 14.9 months, and 1-year OS ranged from 43.9% to 56%.23–26 Whether the benefit:risk ratio favors incorporating bevacizumab into the carboplatin/pemetrexed regimen for well-selected fit elderly patients is yet to be demonstrated. Although maintenance pemetrexed is thought to be non-standard in elderly patients, our study demonstrates the feasibility of this approach as nearly half of our patients received maintenance treatment, with a median for four cycles administered. Pemetrexed should thus be offered to eligible patients with advanced nonsquamous NSCLC and good PS, regardless of age, based on the known survival benefit of this approach compared with placebo.27
Although prognostic and predictive associations of VEGFA and VEGFR polymorphic variants have been reported in NSCLC and other tumor types,8–11, 28–34 some of these reports have conflicted in part due to the heterogeneity of patient populations and treatment regimens across studies. In this study, the variant alleles of bevacizumab-associated genes such as VEGFA SNP rs3025018 was significantly associated with both inferior PFS and OS and rs3025035 with reduced toxicity while KDR (VEGFR2) SNP rs1870377 was associated with inferior PFS6. Furthermore, the pemetrexed related gene SLC19A1 rs2838958 SNP, which showed reduced association with confirmed response rate is linked to SLC19A1 rs1051298 SNP (r2=0.8) which correlated previously with inferior survival.7 Finally, TYMS, the target for pemetrexed and 5FU, contained SNP rs2847153 which correlated with toxicity in this study and which had been reported previously to be associated with 5FU cytotoxicity.35
In conclusion, bevacizumab in combination with carboplatin-pemetrexed as first-line therapy in nonsquamous NSCLC patients ≥ 70 years was well tolerated and was associated with an encouraging PFS rate, but did not meet the specified efficacy endpoint of this phase 2 trial. Several candidate SNPs may have prognostic and predictive implications for treatment outcomes using this regimen and may be explored in future studies. This study however was limited by lack of a control group and lack of available information regarding molecular subtypes of tumors among patients enrolled. Future clinical trials should incorporate this data into study design and analytical tools refined to account for such variations or missing information.
Supplementary Material
ACKNOWLEDGEMENTS
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group, Mayo Clinic and the Alliance and was supported in part by Public Health Service grants CA-25224, CA-37404, CA-63849, CA-35113, CA-35267, CA-35269, CA-35103, CA-35415, CA-35090, and CA-63848 from the National Cancer Institute, Department of Health and Human Services. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Cancer Institute or the National Institute of Health.
Additional participating institutions include: Mayo Clinic Arizona, Scottsdale, AZ 85259 (Michele Y. Halyard, M.D..); Montana Cancer Consortium, Billings, MT 59101 (Benjamin T. Marchello, M.D.); Essentia Duluth CCOP, Duluth, MN 55805 (Daniel A. Nikcevich, M.D.); Toledo Community Hospital Oncology Program CCOP, Toledo, OH 43623 (Rex B. Mowat, M.D.); Michigan Cancer Research Consortium, Ann Arbor, MI 48106 (Philip J. Stella, M.D.); Columbus CCOP, Columbus, OH 53215 (J. Philip Kuebler, M.D., Ph.D.); Hematology & Oncology of Dayton, Inc., Dayton, OH 45415 (Howard M. Gross, M.D.); Colorado Cancer Research Program, Denver, CO 80224 (Keren Sturtz, M.D.); St. Vincent Regional Cancer Center CCOP, Green Bay, WI 54303 (Anthony J. Jaslowski, M.D.); Northern Indiana Cancer Research Consortium, South Bend, IN 46601 (Robin T. Zon, M.D.); Siouxland Hematology-Oncology Associates, Sioux City, IA 51105 (Donald B. Wender, M.D.). We are also grateful to the patients and their families who participated and supported this study.
Footnotes
The results of this study had been presented in a poster session in the 12th Annual meeting of the American Society of Clinical Oncology in June 2012.
Results first reported as poster presentation in the 2012 Annual Meeting of the American Society of Clinical Oncology (abstr 7555)
REFERENCES
- 1.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 2.Ramalingam SS, Dahlberg SE, Langer CJ, et al. Outcomes for elderly, advancedstage non small-cell lung cancer patients treated with bevacizumab in combination with carboplatin and paclitaxel: analysis of Eastern Cooperative Oncology Group Trial 4599. J Clin Oncol. 2008;26:60–65. doi: 10.1200/JCO.2007.13.1144. [DOI] [PubMed] [Google Scholar]
- 3.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 4.Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–1440. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 5.Pujol JL, Paul S, Chouaki N, et al. Survival without common toxicity criteria grade 3/4 toxicity for pemetrexed compared with docetaxel in previously treated patients with advanced non-small cell lung cancer (NSCLC): a risk-benefit analysis. J Thorac Oncol. 2007;2:397–401. doi: 10.1097/01.JTO.0000268672.57002.69. [DOI] [PubMed] [Google Scholar]
- 6.Patel JD, Hensing TA, Rademaker A, et al. Phase II study of pemetrexed and carboplatin plus bevacizumab with maintenance pemetrexed and bevacizumab as first18 line therapy for nonsquamous non-small-cell lung cancer. J Clin Oncol. 2009;27:3284–3289. doi: 10.1200/JCO.2008.20.8181. [DOI] [PubMed] [Google Scholar]
- 7.Adjei AA, Mandrekar SJ, Dy GK, et al. Phase II trial of pemetrexed plus bevacizumab for second-line therapy of patients with advanced non-small-cell lung cancer: NCCTG and SWOG study N0426. J Clin Oncol. 2010;28:614–619. doi: 10.1200/JCO.2009.23.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lambrechts D, Claes B, Delmar P, et al. VEGF pathway genetic variants as biomarkers of treatment outcome with bevacizumab: an analysis of data from the AViTA and AVOREN randomised trials. Lancet Oncol. 2012;13:724–733. doi: 10.1016/S1470-2045(12)70231-0. [DOI] [PubMed] [Google Scholar]
- 9.Schneider BP, Wang M, Radovich M, et al. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol. 2008;26:4672–4678. doi: 10.1200/JCO.2008.16.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin M, Liao Z, Yuan X, et al. Polymorphisms of the vascular endothelial growth factor gene and severe radiation pneumonitis in non-small cell lung cancer patients treated with definitive radiotherapy. Cancer Sci. 2012;103:945–950. doi: 10.1111/j.1349-7006.2012.02229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang W, Dahlberg SE, Yang D, et al. Genetic variants in angiogenesis pathway associated with clinical outcome in NSCLC patients (pts) treated with bevacizumab in combination with carboplatin and paclitaxel: Subset pharmacogenetic analysis of ECOG 4599. J Clin Oncol. 2009;>27 abstr8032. [Google Scholar]
- 12.Hu Q, Li X, Su C, et al. Correlation between thymidylate synthase gene polymorphisms and efficacy of pemetrexed in advanced non-small cell lung cancer. Exp Ther Med. 2012;4:1010–1016. doi: 10.3892/etm.2012.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tiseo M, Giovannetti E, Tibaldi C, et al. Pharmacogenetic study of patients with advanced non-small cell lung cancer (NSCLC) treated with second-line pemetrexed or pemetrexed-carboplatin. Lung Cancer. 2012;78:92–99. doi: 10.1016/j.lungcan.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Smit E, Burgers S, Biesma B, et al. Randomized phase II and pharmacogenetic study of pemetrexed compared with pemetrexed plus carboplatin in pretreated patients with advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:2038–2045. doi: 10.1200/JCO.2008.19.1650. [DOI] [PubMed] [Google Scholar]
- 15.Adjei AA, Salavaggione OE, Mandrekar SJ, et al. Correlation between polymorphisms of the reduced folate carrier gene (SLC19A1) and survival after pemetrexed-based therapy in non-small cell lung cancer: a North Central Cancer Treatment Group-based exploratory study. J Thorac Oncol. 2010;5:1346–1353. doi: 10.1097/JTO.0b013e3181ec18c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan E, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 17.Sloan J, Dueck A. Issues for Statisticians in Conducting Analyses and Translating Results for Quality of Life End Points in Clinical Trials. J Biopharm Stat. 2004;14:73–96. doi: 10.1081/BIP-120028507. [DOI] [PubMed] [Google Scholar]
- 18.Spigel DR, Hainsworth JD, Shipley DL, et al. A randomized phase II trial of pemetrexed/gemcitabine/bevacizumab or pemetrexed/carboplatin/bevacizumab in the first-line treatment of elderly patients with advanced non-small cell lung cancer. J Thorac Oncol. 2012;7:196–202. doi: 10.1097/JTO.0b013e3182307efe. [DOI] [PubMed] [Google Scholar]
- 19.Gervais R, Robinet G, Clement-Duchene C, et al. Pemetrexed and carboplatin, an active option in first-line treatment of elderly patients with advanced non-small cell lung cancer (NSCLC): A phase II trial. Lung Cancer. 2013;80:185–190. doi: 10.1016/j.lungcan.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Tamiya A, Tamiya M, Shiroyama T, et al. Dose escalation study of carboplatinpemetrexed followed by maintenance pemetrexed for elderly patients with advanced nonsquamous nonsmall-cell lung cancer. Ann Oncol. 2013;24:980–985. doi: 10.1093/annonc/mds544. [DOI] [PubMed] [Google Scholar]
- 21.Patel JD, Socinski MA, Garon EB, et al. PointBreak: A randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31:4349–4357. doi: 10.1200/JCO.2012.47.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langer CJ, Socinski MA, Patel JD, et al. Efficacy and safety of paclitaxel and carboplatin with bevacizumab for the first-line treatment of patients with nonsquamous non-small cell lung cancer (NSCLC): Analyses based on age in the phase III PointBreak and E4599 trials. J Clin Oncol. 2013;31 abstr 8073. [PubMed] [Google Scholar]
- 23.Zinner RG, Fossella FV, Gladish GW, et al. Phase II study of pemetrexed in combination with carboplatin in the first-line treatment of advanced nonsmall cell lung cancer. Cancer. 2005;104:2449–2456. doi: 10.1002/cncr.21480. [DOI] [PubMed] [Google Scholar]
- 24.Scagliotti GV, Kortsik C, Dark GG, et al. Pemetrexed combined with oxaliplatin or carboplatin as first-line treatment in advanced non-small cell lung cancer: a multicenter, randomized, phase II trial. Clin Cancer Res. 2005;11:690–696. [PubMed] [Google Scholar]
- 25.Socinski MA, Raju RN, Stinchcombe T, et al. Randomized, phase II trial of pemetrexed and carboplatin with or without enzastaurin versus docetaxel and carboplatin as first-line treatment of patients with stage IIIB/IV non-small cell lung cancer. J Thorac Oncol. 2010;5:1963–1969. doi: 10.1097/JTO.0b013e3181fd42eb. [DOI] [PubMed] [Google Scholar]
- 26.Rodrigues-Pereira J, Kim JH, Magallanes M, et al. A randomized phase 3 trial comparing pemetrexed/carboplatin and docetaxel/carboplatin as first-line treatment for advanced, nonsquamous non-small cell lung cancer. J Thorac Oncol. 2011;6:1907–1914. doi: 10.1097/JTO.0b013e318226b5fa. [DOI] [PubMed] [Google Scholar]
- 27.Paz-Ares LG, De Marinis F, Dediu M, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small cell lung cancer. J Clin Oncol. 2013;31:2895–2902. doi: 10.1200/JCO.2012.47.1102. [DOI] [PubMed] [Google Scholar]
- 28.Heist RS, Zhai R, Liu G, et al. VEGF polymorphisms and survival in early-stage nonsmall- cell lung cancer. J Clin Oncol. 2008;26:856–862. doi: 10.1200/JCO.2007.13.5947. [DOI] [PubMed] [Google Scholar]
- 29.Loupakis F, Ruzzo A, Salvatore L, et al. Retrospective exploratory analysis of VEGF polymorphisms in the prediction of benefit from first-line FOLFIRI plus bevacizumab in metastatic colorectal cancer. BMC Cancer. 2011;11:247. doi: 10.1186/1471-2407-11-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masago K, Fujita S, Kim YH, et al. Effect of vascular endothelial growth factor polymorphisms on survival in advanced-stage non-small-cell lung cancer. Cancer Sci. 2009;100:1917–1922. doi: 10.1111/j.1349-7006.2009.01253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim MK, Suh C, Chi HS, et al. VEGFA and VEGFR2 genetic polymorphisms and survival in patients with diffuse large B cell lymphoma. Cancer Sci. 2012;103:497–503. doi: 10.1111/j.1349-7006.2011.02168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim DH, Xu W, Karmel-Reid S, et al. Clinical relevance of vascular endothelial growth factor (VEGFA) and VEGF receptor (VEGFR2) gene polymorphism on the treatment outcome following imatinib therapy. Ann Oncol. 2010;21:1179–1188. doi: 10.1093/annonc/mdp452. [DOI] [PubMed] [Google Scholar]
- 33.Jain L, Sissung TM, Danesi R, et al. Hypertension and hand-foot skin reactions related to VEGFR2 genotype and improved clincal outcome following bevacizumab and sorafenib. J Exp Clin Cancer Res. 2010;29:95. doi: 10.1186/1756-9966-29-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia-Donas J, Esteban E, Leandro-Garcia LJ, et al. Single nucleotide polymorphism associations with response and toxic effects in patients with advanced renal-cell carcinoma treated with first-line sunitinib: a multicentre, observational, prospective study. Lancet Oncol. 2011;12(12):1143–1150. doi: 10.1016/S1470-2045(11)70266-2. [DOI] [PubMed] [Google Scholar]
- 35.Peters EJ, Kraja AT, Lin SJ, et al. Association of thymidylate synthase variants with 5-fluorouracil cytotoxicity. Pharmacogenet Genomics. 2009;19:399–401. doi: 10.1097/FPC.0b013e328329fdec. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.