Abstract
AIM: To evaluate the antioxidant enzymes and oxidative products in overweight and obese Saudi children before the onset of metabolic complications.
METHODS: The study was carried out on 231 Saudi children. They were classified into three groups: uncomplicated overweight, uncomplicated morbid obesity, and the matched age group as control. All subjects underwent anthropometric measurements and activities of superoxide dismutase, catalase, glutathione peroxidase (GSH-Px), glutathione reductase, the concentrations of reduced GSH, malondialdehyde (MDA) oxidized low-density lipoprotein (ox-LDL) and advanced oxidation protein products (AOPPs) were measured in the blood of these groups.
RESULTS: Overweight and obese children had a significantly higher body mass index, while obese children only had a significantly higher waist-to-hip ratio compared to that of the control group. The enzyme activities under study were significantly elevated in the overweight group, although they were significantly reduced among obese children. The concentration of GSH was reduced in both the overweight and obese groups. The mean values of ox-LDL, MDA and AOPP were non-significantly increased in overweight children, while they were significantly elevated in obese children compared to that of normal weight children. A significant disturbance of oxidant-antioxidant status was observed in severely morbid children.
CONCLUSION: The increase of oxidative stress in obese children is associated with the increase in AOPPs and MDA which reflects an imbalance between reactive oxygen species production and antioxidant defense.
Keywords: Saudi children, Body mass index, Malondialdehyde, Superoxide dismutase, Catalase, Glutathione peroxidase, Glutathione reductase, Reduced glutathione
Core tip: Childhood obesity is growing at an alarming rate and is concomitant with an increasing prevalence of oxidative stress. The association between obesity and oxidative stress is illustrated in the present study that showed that obese children with body mass index greater than 35 kg/m2 had higher oxidative products, e.g., malondialdehyde, advanced oxidation protein products and oxidized low-density lipoprotein concentrations with lower antioxidants, e.g., superoxide dismutase, catalase, glutathione peroxidase (GSH-Px) and glutathione reductase, and GSH. Therefore, the early recognition of these changes in oxidant status in children is important for preventing the long-term complications of obesity and targeting individual subjects who are particularly at risk. In addition, improving the oxidant status in overweight and obese children may reduce obesity-related comorbidities in adulthood.
INTRODUCTION
Obesity is one of the most common health problems among children and adolescents in developed and high resource countries[1]. In addition, it is a principal causative factor in the development of various diseases, such as dyslipidemia, atherosclerosis, cardiovascular and others, and increases the risk of premature illness and death later in life, raising public health concerns[2-4].
Oxidative stress results from an imbalance between the production of reactive oxygen species (ROS), such as superoxide (O2•-) and hydroxyl (.OH) radicals, with antioxidants defenses, which leads to oxidative damage of lipids, proteins and DNA and might be a major mechanism underlying obesity-related complications[5].
The human body has developed several mechanisms to protect biomolecules from the deleterious effects of ROS. These include the antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GSSG-R) and glutathione peroxidase (GSH-Px), as well as water and lipid-soluble antioxidants, such as GSH, ascorbate (vitamin C), α-tocopherol (vitamin E) and β-carotene[6]. They either detoxify ROS, convert superoxide radicals (O2•-) into H2O2, or metabolize peroxide organic molecules.
Glutathione protects the body organs against oxidative stress of ROS, either directly as an antioxidant or indirectly by maintaining other cellular antioxidants in a functional state[7].
The accumulation of oxidative modified macromolecules has been demonstrated in obese adults. The whole body oxidative stress is best reflected by systemic levels of lipid peroxidation, e.g., malondialdehyde (MDA) and oxidized low-density lipoprotein (ox-LDL), which are considered the most reliable oxidative biomarkers[8]. The levels of MDA were significantly increased in obese children compared with non-obese children. ox-LDL, another marker of oxidative stress, is associated with obesity[9].
Proteins are recognized as oxidants by ROS, which then may undergo structural and functional modifications, leading to endothelial dysfunction. Advanced oxidation protein products (AOPPs) are novel markers of increased oxidative stress, which has some advantages over other markers because of their relatively early formation, greater stability, ease of determination and reliability, and also their longer life span[10], and are considered reliable markers to estimate the degree of oxidant-mediated protein damage.
Although the prevalence of morbid obesity and obesity-related complications in children has greatly increased in the eastern region of Saudi Arabia, the antioxidant enzymes and the more important oxidatively modified macromolecules, e.g., MDA, ox-LDL and AOPP, have not been completely explored in overweight and obese children because only data on antioxidant vitamin levels are available[11-13]. The current study aims to evaluate the activities of SOD, CAT, GSH-Px, GSSG-R, levels of MDA, ox-LDL and AOPP in Saudi overweight, obese and normal weight children to identify obese children who are at risk of complications.
MATERIALS AND METHODS
Children and study protocol
The investigations were carried out in overweight and obese children referred to the Hospital Paediatric Clinic and attendees of the Polyclinic Center at King Faisal University in Al-Ahsa, Saudi Arabia between January 2011 and March 2012. The present study comprised a total of 213 children (6-12 years, mean age 9.5 ± 1.5 years; boys n = 143, 67% and girls n = 70, 33%). The children were free from endocrinological and liver disorders and genetic syndromes associated with obesity. Clinically they were stable without symptoms of any acute infections in order to avoid the possible influence of such conditions on the parameters examined. None of the children were smokers.
Children were classified into three groups: overweight, obese and healthy weight based on body mass index (BMI) (kg/m2) using the International Obesity Task Force criteria[14]. Group I included 66 uncomplicated overweight children with a BMI = 25-30 kg/m2 or 85th percentile < BMI < 95th percentile. Group II included 83 children with uncomplicated morbid obesity of BMI ≥ 95th percentile or BMI ≥ 30 kg/m2 or more. Group III included 64 children of the same age as normal control of BMI = 18.5-25 kg/m2 or < 85th percentile. BMI was calculated as weight in kilograms divided by height in meters squared[14]. BMI z-scores were calculated based on the United States Center for Disease Control and Prevention reference data[15].
Ethical approval
The study was approved by the Ethical Committee of the King Faisal University.
Data collection
Anthropometry: Anthropometric measures followed the protocols of the International Society for the Advancement of Kinanthropometry[16]. Height was measured with a wall-mounted stadiometer (SECA 770, Hamburg, Germany) in a relaxed position with arms hanging freely and without shoes to the nearest 0.3 cm. Weight was measured using electronic digital scales (TANITA ultimate scale 2000 scales, Tanita Corporation, Tokyo, Japan) to the nearest 0.1 kg, with children wearing only a hospital gown and underwear. Measurements were taken by a single technician to overcome inter-rater error.
Calculation of waist-to-hip ratio: To calculate waist-to-hip ratio (WHR), the waist circumference was measured at its smallest point between the iliac crest and the rib cage and the hip circumference at its largest width over the greater trochanters. Blood pressure was measured using a mercury gravity manometer with proper cuff size in standard conditions and ambulatory blood pressure monitoring was carried out[17].
Demographics/background information: Parents completed a questionnaire to collect information about household income, maternal education, child medications and any medical conditions and oral consent was given by all children.
Blood sampling: Blood samples were freshly withdrawn from the vein of various children groups after an overnight fasting on heparin as inpatients at the Hospital Pediatric Clinic and Polyclinic Center and were immediately transferred to our laboratory at the College of Medicine, King Faisal University in an icebox. Blood samples were immediately centrifuged at 4000 rpm at 4 °C and plasma was stored at -20 °C until analysis. The 50 μL of RBC were taken and lysed with 1.0 mL ice cold water and the clear lysate obtained after spinning down the cell debris at 8500 g for 10 min at 4 °C was used for the assays.
Biochemical analysis
Determination of hemoglobin (%): Determination of hemoglobin (Hb) was estimated spectrophotometrically (Boeco S-20 Spectrophotometer, Hamburg, Germany) by using a kit obtained from Biodiagnostic Cairo, Egypt according to the method of Ranganathanand and Gunasekaran[18]. The values were expressed as g/dL.
Estimation of blood glucose: Blood glucose concentration was estimated spectrophotometrically (Boeco S-20 Spectrophotometer, Hamburg, Germany) through application of the method described by Freund et al[19] using an enzymatic test kit (glucose oxidase) supplied by Biodiagnostic, Cairo, Egypt. The results were expressed as mg/dL.
Estimation of total serum protein: The total plasma proteins were estimated by using a spectrophotometric (Boeco S-20 Spectrophotometer, Hamburg, Germany) method of Buiret[20]. The results were expressed as g/dL.
Measurement of concentrations of oxidative products and activities of antioxidant enzymes: (1) Determination of MDA: MDA level, an end product of lipid peroxidation of erythrocytes, was assayed spectrophotometrically (Boeco S-20 Spectrophotometer, Hamburg, Germany) using a diagnostic kit supplied by Biodiagnostic, Cairo, Egypt using the method of Stocks et al[21]. The results were expressed as nmol/g Hb; (2) Determination of plasma ox-LDL: ox-LDL level was estimated by using an enzyme-linked immunosorbent assay (Merocdia, Inc., Winston-Salem, NC, United States) kit according to the method described by Hoogerbrugge et al[22]. The concentration of ox-LDL was expressed in mg/g protein; and (3) AOPP: determination of AOPP was based on spectrophotometric detection of chloramin T at 340 nm according to the method of Witko-Sarsat et al[23]. Concentration of AOPP was expressed in chloramine units (μmol/g protein).
SOD activity (EC 1.15.1.1): The Jaiswal et al[24] method was used to estimate the total SOD activity spectrophotometrically (Boeco S-20 Spectrophotometer, Hamburg, Germany) in RBC hemolysate by using a test kit obtained from SpinReact Biodiagnostic, Cairo, Egypt. The results were expressed as U/g Hb.
GSH-Px (EC 1.11.1.9): The activity of GSH-Px in erythrocytes was estimated spectrophotometrically (Boeco S-20 Spectrophotometer, Hamburg, Germany) using the method described by Paglia et al[25] using a diagnostic kit provided by Biodiagnostic, Cairo, Egypt. The results were expressed as mU/g Hb.
GSSG-R (1.6.4.2): Erythrocyte GSSG-R activity was determined spectrophotometrically (Boeco S-20 Spectrophotometer, Hamburg, Germany) using a diagnostic kit provided by Biodiagnostic, Cairo, Egypt, as described by Worthington et al[26]. The results were expressed as mU/g Hb.
CAT (EC 1.11.1.6): CAT activity was measured spectrophotometrically (Boeco S-20 Spectrophotometer, Hamburg, Germany) using a standard CAT assay kit Biodiagnostic, Cairo, Egypt, through following the decomposition rate of H2O2 at 240 nm, according to the method of Al-Essa et al[27]. The results were expressed as U/g Hb.
GSH: GSH was assayed using the method of Giustarini et al[28]. The results were expressed as mg/g Hb.
Statistical analysis
The data were reported as mean ± SD and analyzed with the SPSS 16.0.7 (SPSS, Chicago, IL, United States) for Microsoft Windows XP (Redmond, WA, United States) statistical software package. Differences between the groups were evaluated using the Student’s independent samples t test. Group comparison was performed by using a one-way analysis of variance. Differences were considered statistically significant at P < 0.05. The graphic was drawn by Graph Pad Prism-5.
RESULTS
Basic characteristics
Clinical characteristics of the normal weight, overweight and obese children groups and their control, glucose and Hb are demonstrated in Table 1. A total of 213 children (normal weight = 66, overweight = 83, obese = 64) were included, with age range from 6-12 years (mean age = 9.7 ± 1.5); boys, n = 143; 67.1% and girls, n = 70; 32.9%. There were no differences in age, gender distribution and levels of fasting glucose and Hb between the different groups. Most of the overweight and obese children resided in the urban region. Body weight and BMI were significantly of higher levels among overweight and obese children compared to the controls. WHR were significantly higher in obese children than in the normal controls, whereas no difference was observed in the WHR of overweight children (Table 1).
Table 1.
Characteristics, glucose and hemoglobin levels of obese and control children enrolled in the study n (%)
| Characteristic |
Subjects |
||
| Normal weight | Overweight | Obese | |
| (n = 66) | (n = 83) | (n = 64) | |
| Gender | |||
| Boys | 42 (63.6) | 60 (72.3) | 41 (64.1) |
| Girls | 24 (33.4) | 23 (27.7) | 23 (35.9) |
| Age, yr | |||
| Mean ± SE | 9.5 ± 1.2 | 9.7 ± 1.8 | 9.4 ± 1.5 |
| P value | 0.089 | 0.1431 | 0.1631 |
| Residence | |||
| Urban | 44 (66.7) | 55 (66.3) | 40 (62.5) |
| Rural | 22 (33.3) | 28 (33.7) | 24 (37.5) |
| Anthropometry | |||
| Height in cm | 141.9 ± 2.1 | 142.3 ± 2.4 | 141.1 ± 2.9 |
| Mean ± SE | |||
| P value | 0.7031 | 0.9941 | |
| Body weight in kilogram | 67.7 ± 5.4 | 139.7 ± 5.9 | |
| Mean ± SE | 39.2 ± 3.4 | 0.00012 | 0.00012 |
| P value | 22.4 ± 2.3 | 32 ± 3.8 | |
| BMI | 0.00012 | 0.00012 | |
| Mean ± SE | 16.4 ± 1.7 | ||
| P value | 0.89 ± 1.28 | 2.16 ± 2.64 | |
| BMI z-score | 0.00012 | 0.00012 | |
| Mean ± SE | |||
| P value | 0.03 ± 0.32 | ||
| Waist/hip ratio | |||
| Mean ± SE | 0.77 ± 0.15 | 0.79 ± 0.16 | 0.88 ± 0.16 |
| P value | 0.6021 | 0.00042 | |
| Age in years | |||
| Mean ± SE | 9.9 ± 1.6 | 9.9 ± 1.9 | 9.6 ± 2.1 |
| P value | 0.6161 | 0.7241 | |
| Glucose (mg/dL) | |||
| Mean ± SE | 80.1 ± 2.9 | 81.7 ± 2.3 | 83.5 ± 2.2 |
| P value | 0.7191 | 0.0751 | |
| Hb (g %) | |||
| Mean ± SE | 14.2 ± 1.1 | 13.9 ± 3.4 | 13.8 ± 3.9 |
| P value | 0.6741 | 0.7411 | |
| Systolic blood pressure (mmHg) | |||
| Mean ± SE | 115.6 ± 6.8 | 115.5 ± 7.4 | 116.2 ± 9.1 |
| P value | 0.8741 | 0.7941 | |
| Diastolic blood pressure (mmHg) | |||
| Mean ± SE | 68.8 ± 6.4 | 67.6 ± 5.1 | 68.8 ± 7.1 |
| P value | 0.7281 | 0.8351 | |
Values are presented in means ± SE. BMI: Weight in kg/height in m2.
non-significant values of overweight and obese groups vs control;
significant values of overweight and obese groups vs control. Comparison of the means was evaluated by ANOVA. BMI: Body mass index; Hb: Hemoglobin; ANOVA: Analysis of variance.
Oxidant markers
Table 2 depicts the activities of antioxidant enzymes SOD, CAT, GSH-Px and GSSG-R in erythrocytes under study. The erythrocyte activities of SOD, CAT, GSH-Px and GSSG-R were significantly elevated in overweight children compared to the corresponding activities of the control group (P < 0.001). The mean activity values of these enzymes were decreased in the obese group compared to the corresponding activities of the normal weight and overweight groups (P < 0.001). The glutathione levels were decreased in both overweight and obese children in comparison to the corresponding level in the normal control group.
Table 2.
Erythrocyte activities of superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase, and reduced glutathione concentration
| Group | SOD | CAT | GSH-Px | GSSG-R | GSH |
| (U/g Hb) | (mg/g Hb) | (mg/g Hb) | (mg/g Hb) | (mg/g Hb) | |
| Normal weight | |||||
| n | 66 | 66 | 66 | 66 | 66 |
| Mean ± SE | 224.6 ± 5.3 | 85.4 ± 2.7 | 41.4 ± 2.6 | 42.4 ± 1.6 | 69.5 ± 1.9 |
| Over weight | |||||
| n | 83 | 83 | 83 | 83 | 83 |
| Mean ± SE | 252.7 ± 6.5 | 99.7 ± 3.7 | 56.8 ± 3.5 | 56.3 ± 2.5 | 66.7 ± 2.8 |
| P value | 0.0011 | 0.0011 | 0.0011 | 0.0011 | 0.011 |
| Obese | |||||
| n | 64 | 64 | 64 | 64 | 64 |
| Mean ± SE | 181.7 ± 7.2 | 68.3 ± 5.1 | 37.3 ± 5.9 | 32.9 ± 4.6 | 33.3 ± 2.1 |
| P value | 0.00112 | 0.00112 | 0.00112 | 0.00112 | 0.00112 |
Values are presented in means ± SE.
Significant values of overweight and obese vs control;
Significant values of obese vs overweight group. Comparison of the means was evaluated by ANOVA. SOD: Superoxide dismutase; CAT: Catalase; GSH-Px: Glutathione peroxidase; GSSG-R: Glutathione reductase; GSH: Reduced glutathione concentration.
The concentrations of MDA, OxLDL and AOPP are shown in Figure 1 respectively. MDA, OxLDL and AOPP were non-significantly increased in the overweight children, although highly increased in obese children compared to the normal weight children (P < 0.001).
Figure 1.
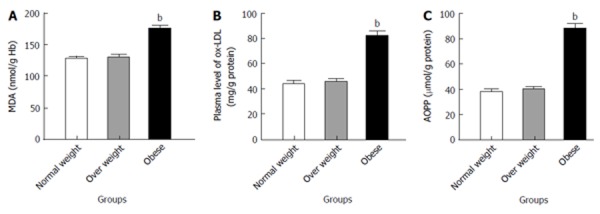
Erythrocyte levels of malondialdehyde (mg/g Hb, A), plasma levels of oxidized low-density lipoproteins (B) and plasma levels of advanced oxidation protein products (C) in normal weight, overweight and obese children. bP < 0.001 vs control and overweight groups. Results are expressed as mean ± SE. MDA: Malondialdehyde; ox-LDL: Oxidized low-density lipoprotein; AOPP: Advanced oxidation protein products.
DISCUSSION
The present study estimated the glucose level to exclude the children with hyperglycemia because this condition may affect the levels of the present parameters. In addition, the present study avoided inherited obesity to exclude its effect on the same parameters and thus investigated the effect of the acquired fatness on the antioxidant status in children. The prevalence of overweight and obese children in the urban region may be attributed to habits of eating fatty foods and lack of physical activity due to the very hot weather.
Growing evidence indicates that the mitochondria of white adipose tissue (WAT), particularly of obese persons, are the main site of ROS generation, e.g., superoxide radicals (O2•-) and H2O2, accompanied by augmented expression of NADPH oxidase and decreased expression of antioxidative enzymes[29]. This finding is confirmed by Mahadev et al[30] who reported that mRNA expression of NADPH oxidase increased in WAT of obese mice.
The antioxidant defenses against ROS include non-enzymatic, e.g., thiol-containing compounds, and antioxidant enzymes[7]. The major antioxidant enzymes include SOD, GSH-Px, GSSG-R and CAT. In the present study, the overweight children showed an elevation in the activities of SOD, GSH-Px, GSSG-R and CAT, but their activities are depleted in obese children (Table 2). The activation of these antioxidant enzymes in overweight children may be to counteract the effect of oxidative stress generated by ROS. The present findings are concordant with prior reports on obese humans and animal models which found that in the early stages of obesity development there may be an initial elevation in antioxidant enzymes to counteract oxidative stress[31]. The depletion of the antioxidant activities in obese children may be attributed to the high production of ROS which may destroy these antioxidant enzymes[32-34]. In addition, the decrease of erythrocyte GSH level (Table 2), which is an essential cofactor for GSH-Px, may lead to reduction of GSH-Px activity in obese children[35,36]. Furthermore, the reduced activities of these antioxidant enzymes in obese children may be attributed to the decreased expression of their mRNA. This finding is confirmed by the studies of Li et al[37] and Furukawa et al[38] which showed that the mRNA expression levels of antioxidant enzymes, such as SOD, CAT and GSH-Px, decreased in WAT of obese mice. The excess production of MDA (Figure 1A) has additional toxic effects for antioxidant enzymes. MDA may modify the amino acid side chains and oxidation of thiol groups of these enzymes, resulting in a partial or complete loss of their activities and functions[39]. Fang et al[40] found that the Ox-LDL correlated negatively with SOD expression and they reported that the decreased activity of SOD may be attributed to excess production of ox-LDL which inhibits the expression of SOD.
GSH plays multiple roles in the cell, including being a free radical scavenger as a primary antioxidant defense[7]. The significant decrease of erythrocyte GSH content in overweight and obese children may be due to its increased turnover into its oxidized form (GSSG) through its detoxification of ROS and other peroxides to challenge the prevailing oxidative stress generated by ROS[41]. This is consistent with GSH function to scavenge oxidants by binding with them covalently[42]. Furthermore, the reduction in erythrocyte GSH content may be attributed to its use in the recycling of vitamin E and semihydroascorbic radicals and reduced oxidized molecules, such as lipid hydroperoxides[43]. In addition, a decrease in the GSH level in the red cells may result from depressed GSSG-R activity (Table 2)[44].
The common approach in the measurement of oxidative stress is the determination of MDA, a product of lipid peroxidation. Thus, the excess production of ROS with insufficient antioxidant enzymes in obese children may have a serious adverse effect on RBC membranes, resulting in lipid peroxidation enhancing production of MDA concentrations similar to our cases (Figure 1A)[38]. This finding is in agreement with the study of Ustundag et al[45] which showed the elevation of plasma MDA in smaller groups of obese children when compared with healthy controls.
The current study showed the increase level of ox-LDL (Figure 1B), the second approach in the measurement of oxidative stress in obese children. Increased levels of ox-LDL may be related to excess oxidative stress with lowered antioxidant defense[46]. This finding was demonstrated in 1992 by Parthasarathy et al[47] who reported that obese children and adolescents have higher levels of ox-LDL due to generation of ROS compared to a normal weight group.
AOPPs are considered reliable markers to estimate the degree of oxidant-mediated protein damage. The observed increases in AOPP levels in the present study (Figure 1C) suggest that proteins might be an important oxidative target of accumulation of oxidative stress in severe childhood obesity[48]. This argument has been confirmed by the study of Atabek et al[49] which found that AOPP levels were increased in obese children and adolescents.
ACKNOWLEDGMENTS
The author thanks the technicians who carried out the blood analysis and Dr. Mosaad Seif, Professor of Biochemistry and Medical Genetics, who aided in the interpretation of the data.
The antioxidant enzymes and the more important oxidatively modified macromolecules, e.g., MDA, ox-LDL and AOPP, have not been completely explored in overweight and obese children because only data on antioxidant vitamin levels are available[11-13]. The current study aimed to evaluate the activities of SOD, CAT, GSH-Px, GSSG-R, levels of MDA, ox-LDL and AOPP in Saudi overweight, obese and normal weight children to identify obese children who are at risk of complications.
COMMENTS
Background
Alarmingly, obesity and its complications have especially increased in the last three decades among pediatric populations, reaching epidemic proportions in developed and developing countries. Excess body fat expressed as body mass index (BMI) is highly correlated with systemic oxidative stress, which is considered an imbalance between the concentrations of reactive oxygen species (ROS) and the oxidative defense mechanisms. The oxidant-antioxidant status has not been completely studied in overweight and obese children. Therefore, the present study investigates oxidatively modified macromolecules, e.g., malondialdehyde (MDA), oxidized low density lipoproteins (ox-LDL) and advanced oxidation protein products (AOPP), and antioxidants, e.g., superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), glutathione reductase (GSSG-R) and reduced glutathione, in overweight and obese children.
Research frontiers
Studies on obese adults show that oxidative stress is related to BMI. Therefore, the research hotspot is to investigate this relationship in overweight and obese children by estimating oxidative damage products, e.g., MDA, ox-LDL and AOPP, and antioxidant defenses, e.g., SOD, CAT, GSH-Px, GSSG-R and GSH, which are studied as marks of oxidative stresses in obese children.
Innovations and breakthroughs
Due to the lack of availability of information regarding oxidative stress markers in obese children, the novelty of this work is the investigation of oxidative stress within this age group. The children were classified according to their BMI, the precise evaluation of which may be done by a combination of biomarkers that represent a different aspect of oxidative damage products. Examples of such are MDA, AOPP and ox-LDL, and antioxidant defenses, such as SOD, CAT, GSH-Px, GSSG-R and GSH.
Applications
In the future, these findings will be translated into clinical evaluation prior to the overt manifestation of diseases or to assess the benefits of treatment of obese children. In addition, the measurement of oxidant-antioxidant parameters may be useful in understanding the pathophysiology of obesity-related health effects.
Terminology
The authors’ findings indicate that severe childhood obesity, represented by BMI, is associated with significantly increased AOPP and ox-LDL concentrations. In addition, the recognition of these changes early in childhood is important for preventing the long-term complications of obesity and targeting individual subjects who are particularly at risk. The authors’ results may be helpful in increasing research to expand both prevention and therapeutic strategies for obesity to minimize oxidative stress in children.
Peer review
This is a good descriptive study in which the author did many experiments which illustrated the relationship between BMI and oxidative stress in obese children. The manuscript was interesting to read and the results are interesting. The data and the results in this paper are also very clear and will help the relationship between obese and oxidant-antioxidant status to be understood.
Footnotes
P- Reviewers: Akgül S, Ahmed MN, Fujiwara N, Guerrero-Romero F S- Editor: Wen LL L- Editor: Roemmele A E- Editor: Liu SQ
References
- 1.Schmidt Morgen C, Rokholm B, Sjöberg Brixval C, Schou Andersen C, Geisler Andersen L, Rasmussen M, Nybo Andersen AM, Due P, Sørensen TI. Trends in prevalence of overweight and obesity in danish infants, children and adolescents--are we still on a plateau. PLoS One. 2013;8:e69860. doi: 10.1371/journal.pone.0069860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olusi SO. Obesity is an independent risk factor for plasma lipid peroxidation and depletion of erythrocyte cytoprotectic enzymes in humans. Int J Obes Relat Metab Disord. 2002;26:1159–1164. doi: 10.1038/sj.ijo.0802066. [DOI] [PubMed] [Google Scholar]
- 3.Freedman DS, Khan LK, Dietz WH, Srinivasan SR, Berenson GS. Relationship of childhood obesity to coronary heart disease risk factors in adulthood: the Bogalusa Heart Study. Pediatrics. 2001;108:712–718. doi: 10.1542/peds.108.3.712. [DOI] [PubMed] [Google Scholar]
- 4.Santoro N. Childhood obesity and type 2 diabetes: the frightening epidemic. World J Pediatr. 2013;9:101–102. doi: 10.1007/s12519-013-0410-8. [DOI] [PubMed] [Google Scholar]
- 5.Martín-Gallán P, Carrascosa A, Gussinyé M, Domínguez C. Changes in oxidant-antioxidant status in young diabetic patients from clinical onset onwards. J Cell Mol Med. 2007;11:1352–1366. doi: 10.1111/j.1582-4934.2007.00068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutiérrez-Salinas J, García-Ortíz L, Morales González JA, Hernández-Rodríguez S, Ramírez-García S, Núñez-Ramos NR, Madrigal-Santillán E. In vitro effect of sodium fluoride on malondialdehyde concentration and on superoxide dismutase, catalase, and glutathione peroxidase in human erythrocytes. ScientificWorldJournal. 2013;2013:864718. doi: 10.1155/2013/864718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Dong H, Thompson DC, Shertzer HG, Nebert DW, Vasiliou V. Glutathione defense mechanism in liver injury: insights from animal models. Food Chem Toxicol. 2013;60:38–44. doi: 10.1016/j.fct.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faienza MF, Francavilla R, Goffredo R, Ventura A, Marzano F, Panzarino G, Marinelli G, Cavallo L, Di Bitonto G. Oxidative stress in obesity and metabolic syndrome in children and adolescents. Horm Res Paediatr. 2012;78:158–164. doi: 10.1159/000342642. [DOI] [PubMed] [Google Scholar]
- 9.Lima SC, Arrais RF, Almeida MG, Souza ZM, Pedrosa LF. [Plasma lipid profile and lipid peroxidation in overweight or obese children and adolescents] J Pediatr (Rio J) 2007;80:23–28. [PubMed] [Google Scholar]
- 10.Liu SX, Hou FF, Guo ZJ, Nagai R, Zhang WR, Liu ZQ, Zhou ZM, Zhou M, Xie D, Wang GB, et al. Advanced oxidation protein products accelerate atherosclerosis through promoting oxidative stress and inflammation. Arterioscler Thromb Vasc Biol. 2006;26:1156–1162. doi: 10.1161/01.ATV.0000214960.85469.68. [DOI] [PubMed] [Google Scholar]
- 11.Decsi T, Molnár D, Koletzko B. Reduced plasma concentrations of alpha-tocopherol and beta-carotene in obese boys. J Pediatr. 1997;130:653–655. doi: 10.1016/s0022-3476(97)70253-1. [DOI] [PubMed] [Google Scholar]
- 12.Kuno T, Hozumi M, Morinobu T, Murata T, Mingci Z, Tamai H. Antioxidant vitamin levels in plasma and low density lipoprotein of obese girls. Free Radic Res. 1998;28:81–86. doi: 10.3109/10715769809097878. [DOI] [PubMed] [Google Scholar]
- 13.Strauss RS. Comparison of serum concentrations of alpha-tocopherol and beta-carotene in a cross-sectional sample of obese and nonobese children (NHANES III). National Health and Nutrition Examination Survey. J Pediatr. 1999;134:160–165. doi: 10.1016/s0022-3476(99)70409-9. [DOI] [PubMed] [Google Scholar]
- 14.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogge MM, Merrill E. Obesity education for nurse practitioners: perspectives from nurse practitioner faculty. J Am Assoc Nurse Pract. 2013;25:320–328. doi: 10.1111/j.1745-7599.2012.00785.x. [DOI] [PubMed] [Google Scholar]
- 16.Meyer NL, Sundgot-Borgen J, Lohman TG, Ackland TR, Stewart AD, Maughan RJ, Smith S, Müller W. Body composition for health and performance: a survey of body composition assessment practice carried out by the Ad Hoc Research Working Group on Body Composition, Health and Performance under the auspices of the IOC Medical Commission. Br J Sports Med. 2013;47:1044–1053. doi: 10.1136/bjsports-2013-092561. [DOI] [PubMed] [Google Scholar]
- 17.Soergel M, Kirschstein M, Busch C, Danne T, Gellermann J, Holl R, Krull F, Reichert H, Reusz GS, Rascher W. Oscillometric twenty-four-hour ambulatory blood pressure values in healthy children and adolescents: a multicenter trial including 1141 subjects. J Pediatr. 1997;130:178–184. doi: 10.1016/s0022-3476(97)70340-8. [DOI] [PubMed] [Google Scholar]
- 18.Ranganathan H, Gunasekaran N. Simple method for estimation of hemoglobin in human blood using color analysis. IEEE Trans Inf Technol Biomed. 2006;10:657–662. doi: 10.1109/titb.2006.874195. [DOI] [PubMed] [Google Scholar]
- 19.Freund A, Johnson SB, Rosenbloom A, Alexander B, Hansen CA. Subjective symptoms, blood glucose estimation, and blood glucose concentrations in adolescents with diabetes. Diabetes Care. 1986;9:236–243. doi: 10.2337/diacare.9.3.236. [DOI] [PubMed] [Google Scholar]
- 20.Flack CP, Woollen JW. Prevention of interference by dextran with biuret-type assay of serum proteins. Clin Chem. 1984;30:559–561. [PubMed] [Google Scholar]
- 21.Stocks J, Offerman EL, Modell CB, Dormandy TL. The susceptibility to autoxidation of human red cell lipids in health and disease. Br J Haematol. 1972;23:713–724. doi: 10.1111/j.1365-2141.1972.tb03486.x. [DOI] [PubMed] [Google Scholar]
- 22.Hoogerbrugge N, Zillikens MC, Jansen H, Meeter K, Deckers JW, Birkenhäger JC. Estrogen replacement decreases the level of antibodies against oxidized low-density lipoprotein in postmenopausal women with coronary heart disease. Metabolism. 1998;47:675–680. doi: 10.1016/s0026-0495(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 23.Witko-Sarsat V, Friedlander M, Nguyen Khoa T, Capeillère-Blandin C, Nguyen AT, Canteloup S, Dayer JM, Jungers P, Drüeke T, Descamps-Latscha B. Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J Immunol. 1998;161:2524–2532. [PubMed] [Google Scholar]
- 24.Jaiswal D, Rai PK, Mehta S, Chatterji S, Shukla S, Rai DK, Sharma G, Sharma B, Khair S, Watal G. Role of Moringaoleifera in regulation of diabetes-induced oxidative stress. Asian Pac J Trop Med. 2013;6:426–432. doi: 10.1016/S1995-7645(13)60068-1. [DOI] [PubMed] [Google Scholar]
- 25.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 26.Worthington DJ, Rosemeyer MA. Glutathione reductase from human erythrocytes. Molecular weight, subunit composition and aggregation properties. Eur J Biochem. 1975;60:459–466. doi: 10.1111/j.1432-1033.1975.tb21024.x. [DOI] [PubMed] [Google Scholar]
- 27.Al-Essa M, Dhaunsi GS, Al-Qabandi W, Khan I. Impaired NADPH oxidase activity in peripheral blood lymphocytes of galactosemia patients. Exp Biol Med (Maywood) 2013;238:779–786. doi: 10.1177/1535370213480692. [DOI] [PubMed] [Google Scholar]
- 28.Giustarini D, Dalle-Donne I, Milzani A, Fanti P, Rossi R. Analysis of GSH and GSSG after derivatization with N-ethylmaleimide. Nat Protoc. 2013;8:1660–1669. doi: 10.1038/nprot.2013.095. [DOI] [PubMed] [Google Scholar]
- 29.Roberts CK, Barnard RJ, Sindhu RK, Jurczak M, Ehdaie A, Vaziri ND. Oxidative stress and dysregulation of NAD(P)H oxidase and antioxidant enzymes in diet-induced metabolic syndrome. Metabolism. 2006;55:928–934. doi: 10.1016/j.metabol.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 30.Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, Goldstein BJ. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woo J, Shin KO, Yoo JH, Park S, Kang S. The effects of detraining on blood adipokines and antioxidant enzyme in Korean overweight children. Eur J Pediatr. 2012;171:235–243. doi: 10.1007/s00431-011-1518-2. [DOI] [PubMed] [Google Scholar]
- 32.Erdeve O, Siklar Z, Kocaturk PA, Dallar Y, Kavas GO. Antioxidant superoxide dismutase activity in obese children. Biol Trace Elem Res. 2004;98:219–228. doi: 10.1385/BTER:98:3:219. [DOI] [PubMed] [Google Scholar]
- 33.Lee YS, Kim AY, Choi JW, Kim M, Yasue S, Son HJ, Masuzaki H, Park KS, Kim JB. Dysregulation of adipose glutathione peroxidase 3 in obesity contributes to local and systemic oxidative stress. Mol Endocrinol. 2008;22:2176–2189. doi: 10.1210/me.2008-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Codoñer-Franch P, Valls-Bellés V, Arilla-Codoñer A, Alonso-Iglesias E. Oxidant mechanisms in childhood obesity: the link between inflammation and oxidative stress. Transl Res. 2011;158:369–384. doi: 10.1016/j.trsl.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Ozata M, Mergen M, Oktenli C, Aydin A, Sanisoglu SY, Bolu E, Yilmaz MI, Sayal A, Isimer A, Ozdemir IC. Increased oxidative stress and hypozincemia in male obesity. Clin Biochem. 2002;35:627–631. doi: 10.1016/s0009-9120(02)00363-6. [DOI] [PubMed] [Google Scholar]
- 36.Dincer Y, Akcay T, Alademir Z, Ilkova H. Effect of oxidative stress on glutathione pathway in red blood cells from patients with insulin-dependent diabetes mellitus. Metabolism. 2002;51:1360–1362. doi: 10.1053/meta.2002.35192. [DOI] [PubMed] [Google Scholar]
- 37.Li SL, Valente AJ, Zhao SJ, Clark RA. PU.1 is essential for p47(phox) promoter activity in myeloid cells. J Biol Chem. 1997;272:17802–17809. doi: 10.1074/jbc.272.28.17802. [DOI] [PubMed] [Google Scholar]
- 38.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doğruer ZN, Unal M, Eskandari G, Pata YS, Akbaş Y, Cevik T, Cimen MY. Malondialdehyde and antioxidant enzymes in children with obstructive adenotonsillar hypertrophy. Clin Biochem. 2004;37:718–721. doi: 10.1016/j.clinbiochem.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Fang X, Weintraub NL, Rios CD, Chappell DA, Zwacka RM, Engelhardt JF, Oberley LW, Yan T, Heistad DD, Spector AA. Overexpression of human superoxide dismutase inhibits oxidation of low-density lipoprotein by endothelial cells. Circ Res. 1998;82:1289–1297. doi: 10.1161/01.res.82.12.1289. [DOI] [PubMed] [Google Scholar]
- 41.Armstrong JS, Steinauer KK, Hornung B, Irish JM, Lecane P, Birrell GW, Peehl DM, Knox SJ. Role of glutathione depletion and reactive oxygen species generation in apoptotic signaling in a human B lymphoma cell line. Cell Death Differ. 2002;9:252–263. doi: 10.1038/sj.cdd.4400959. [DOI] [PubMed] [Google Scholar]
- 42.Yoshida K, Hirokawa J, Tagami S, Kawakami Y, Urata Y, Kondo T. Weakened cellular scavenging activity against oxidative stress in diabetes mellitus: regulation of glutathione synthesis and efflux. Diabetologia. 1995;38:201–210. doi: 10.1007/BF00400095. [DOI] [PubMed] [Google Scholar]
- 43.Pastore A, Ciampalini P, Tozzi G, Pecorelli L, Passarelli C, Bertini E, Piemonte F. All glutathione forms are depleted in blood of obese and type 1 diabetic children. Pediatr Diabetes. 2012;13:272–277. doi: 10.1111/j.1399-5448.2011.00806.x. [DOI] [PubMed] [Google Scholar]
- 44.Varma RN, Mankad VN, Phelps DD, Jenkins LD, Suskind RM. Depressed erythrocyte glutathione reductase activity in sickle cell disease. Am J Clin Nutr. 1983;38:884–887. doi: 10.1093/ajcn/38.6.884. [DOI] [PubMed] [Google Scholar]
- 45.Ustundag B, Gungor S, Aygün AD, Turgut M, Yilmaz E. Oxidative status and serum leptin levels in obese prepubertal children. Cell Biochem Funct. 2007;25:479–483. doi: 10.1002/cbf.1334. [DOI] [PubMed] [Google Scholar]
- 46.Kelly AS, Jacobs DR, Sinaiko AR, Moran A, Steffen LM, Steinberger J. Relation of circulating oxidized LDL to obesity and insulin resistance in children. Pediatr Diabetes. 2010;11:552–555. doi: 10.1111/j.1399-5448.2009.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parthasarathy S, Steinberg D, Witztum JL. The role of oxidized low-density lipoproteins in the pathogenesis of atherosclerosis. Annu Rev Med. 1992;43:219–225. doi: 10.1146/annurev.me.43.020192.001251. [DOI] [PubMed] [Google Scholar]
- 48.Krzystek-Korpacka M, Patryn E, Boehm D, Berdowska I, Zielinski B, Noczynska A. Advanced oxidation protein products (AOPPs) in juvenile overweight and obesity prior to and following weight reduction. Clin Biochem. 2008;41:943–949. doi: 10.1016/j.clinbiochem.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 49.Atabek ME, Keskin M, Yazici C, Kendirci M, Hatipoglu N, Koklu E, Kurtoglu S. Protein oxidation in obesity and insulin resistance. Eur J Pediatr. 2006;165:753–756. doi: 10.1007/s00431-006-0165-5. [DOI] [PubMed] [Google Scholar]


