Abstract
As in other organ systems, gene and drug delivery to ocular tissues such as the retina and cornea is hampered by inefficient penetration of therapeutic molecules across the plasma membrane. We describe the use of a novel peptide for ocular delivery (POD) with protein transduction properties, for delivery of small and large molecules across the plasma membrane. POD enters cells within 5 minutes in a temperature dependent manner. POD can compact and deliver plasmid DNA, achieving transgene expression in >50% of human embryonic retinoblasts. Delivery of small interfering RNA (siRNA) duplexes to cells using POD, allowed for silencing of transgene expression by >50%. POD could also be used to deliver quantum dots in vitro and in vivo. Upon ocular delivery, POD rapidly entered neural retina and localized to retinal pigment epithelium (RPE), photoreceptor, and ganglion cells. Additionally, POD was able to enter corneal epithelium, sclera, choroid, and the dura of the optic nerve via topical application. POD also functions as a bacteriostatic, a useful property for a carrier of molecules to post mitotic neural ocular tissues.
INTRODUCTION
According to the National Institute of Health, ocular diseases that lead to blindness are one of the most common causes of disability in the United States.1 Some common diseases of the retina include age-related macular degeneration (AMD), retinitis pigmentosa and glaucoma, which are associated with degeneration of the retinal pigment epithelium (RPE), photoreceptors, and retinal ganglion cells, respectively.2,3 Infections, dystrophies, or rejection of transplanted corneas are also amongst the common causes of vision loss.4 Improvements in healthcare that have increased human life expectancy ironically result in a significant increase in the frequency of diseases such as AMD or extend the burden associated with currently untreatable diseases such as retinitis pigmentosa.1 Therefore, the need to develop gene and drug delivery technologies for treatment of genetic and acquired ocular disorders is considerable.
As in other tissues, proteins, or drugs directed at intracellular ocular targets need to be sufficiently polar to be easily administered.5 However, for the majority of such molecules, the plasma membrane represents an impermeable barrier. Remarkably, a few select proteins such as the human immunodeficiency virus Tat,6 herpes simplex virus VP22,7 and the Drosophila melanogaster Antennapedia homeodomain8 possess the ability to traverse intact biological membranes. Interrogation of the structures of such proteins has led to the hypothesis that these proteins contain modules that confer the property of “protein transduction” and hence such sequences are generally referred to as protein transduction domains (PTDs) as reviewed in ref. 9. PTDs can be isolated and incorporated into heterologous proteins and peptides, conferring novel protein transduction properties to such recombinant molecules. Initial experiments by several groups on the use of PTDs generated conflicting data and substantial debate regarding their properties and mode of action.10,11 Some of the issues addressed were whether the phenomenon of protein transduction was in fact real or only an artifact of fixation and whether the transduction was receptor, energy, and/or temperature dependent.12 In agreement with many other investigators studying PTDs, we have previously demonstrated that the PTD of both human immunodeficiency virus Tat13 and herpes simplex virus VP2214 have valid transduction properties, at least in ocular cell lines and tissues.
The goal of the current study was to examine whether the novel peptide GGG(ARKKAAKA)4, MW = 3.5 kd that has not previously been shown to have PTD properties can efficiently deliver small molecules, including fluorescent probes and siRNA, and large molecules, including plasmid DNA and quantum dots, to cells in culture and to murine ocular tissues in vivo. Our results indicate that GGG(ARKKAAKA)4 is an efficient “peptide for ocular delivery (POD)” of small and large molecules across the plasma membrane, both in vitro and in vivo and hence may have potential for the delivery of genes and drugs to human ocular tissues.
RESULTS
POD is a cell penetrating, non-membrane-permeabilizing peptide
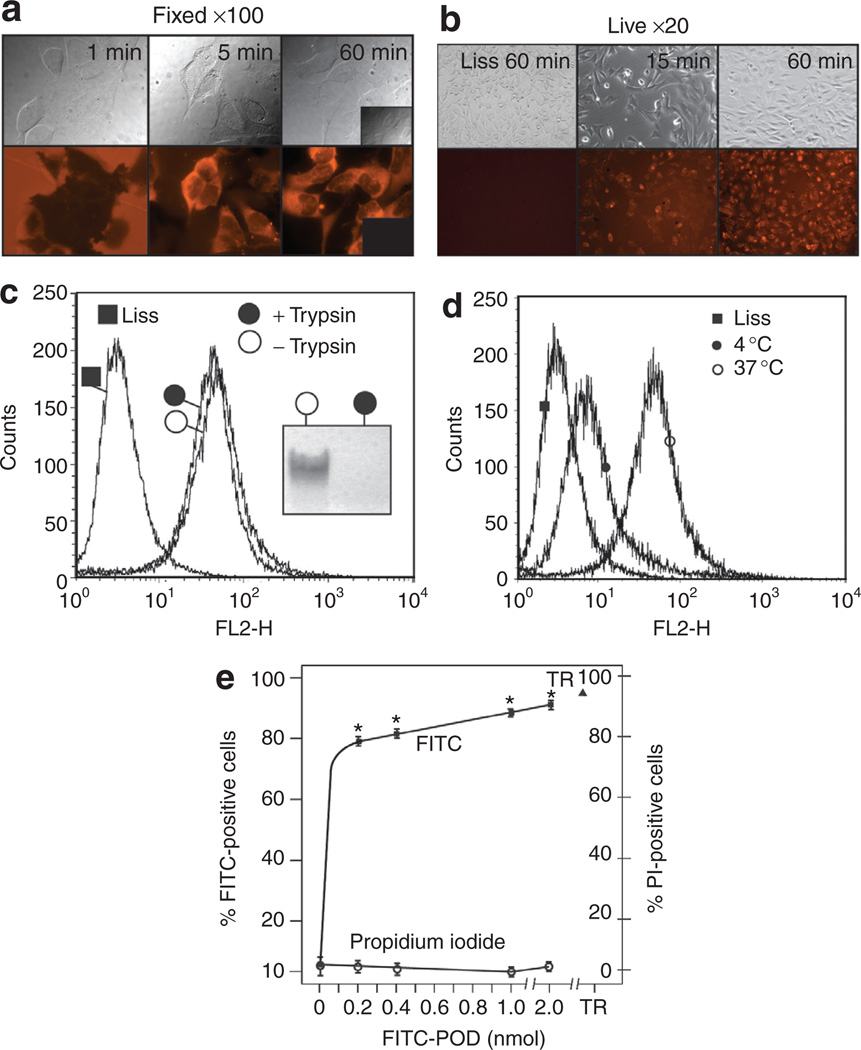
Human embryonic retinal (HER) 911 cells15 incubated with 2 nmol (8 µmol/l) lissamine-conjugated POD (L-POD), followed by formalin fixation, became opaque to 558 nm excitation (emission measured at 583 nm) within 1 minute but appeared normal in bright field (Figure 1a). Within 5 minutes, L-POD was seen within the cytoplasm with both a diffuse cytoplasmic and slightly punctate pattern (Figure 1a). The majority of cells showed cytoplasmic staining after 60 minutes. Furthermore, there was no evidence of uptake of lissamine only (inset, Figure 1a). Cells that were not fixed, i.e., live cells, showed a similar rate of L-POD uptake but with a pattern of localization that was almost exclusively punctate at both 15 minutes and 60 minutes. Again, there was no evidence of uptake of lissamine only (Figure 1b).
Figure 1. Peptide for ocular delivery (POD) is a cell penetrating, non-membrane-permeabilizing peptide.
(a) Formalin-fixed human embryonic retinoblast cells after incubation with lissamine (Liss)-conjugated POD (L-POD) for 1, 5, and 60 minutes indicates potential aggregation of L-POD on the cell surface within 1 minute and internalization within 5 minutes with a cytoplasmic pattern. Inset, lissamine only treated cells. (b) Live cells exposed to L-POD indicate a pattern of localization different from formalin-fixed cells, being mostly punctate, with no evidence of lissamine uptake. (c) Treatment of human embryonic retinal (HER) cells with trypsin following incubation with L-POD does not reduce the number of lissamine-positive cells, indicating that L-POD is internalized and not simply membrane associated. Treatment of C-POD with trypsin prior to loading on an acrylamide gel digests the peptide (inset). (d) Uptake of L-POD is temperature dependent, being substantially more efficient at 37 °C than at 4 °C. (e) Concentrations of fluorescein isothiocyanate (FITC)-conjugated POD ranging from 0 to 2 nmol result in an increase in the number of FITC-positive HER cells without an increase in propidium iodide (PI)-positive cells. Incubation with FITC-POD resulted in a significant increase in FITC fluorescence relative to control samples (*P < 0.0002). PI uptake was not statistically different between the control and FITC-POD treated cells (P > 0.05). TR, 1 % Triton-X1 00. POD, peptide for ocular delivery.
To determine whether L-POD was internalized and not simply associated with the plasma membrane, HER cells incubated with 2 nmol (8 µmol/l) L-POD for 15 minutes were treated with trypsin at 37 °C prior to counting of lissamine-positive cells by fluorescence-activated cell sorting (FACS). A total of 90.10 ± 1.89% or 92.23 ± 0.65% of cells were lissamine-positive with or without incubation with trypsin, respectively (Figure 1c), supporting the hypothesis that the majority of L-POD was internalized and not membrane associated. The sensitivity of POD peptide to trypsin-mediated digestion was confirmed by incubation of 2.5 nmol of cysteine-containing POD (C-POD) with trypsin prior to loading on an acrylamide gel (inset, Figure 1c). Whereas 92.23 ± 0.65% of HER cells were lissamine-positive when incubated with L-POD at 37 °C, only 48.74 ± 2.32% of cells were lissamine-positive when incubated with L-POD at 4 °C (Figure 1d). Hence, uptake of L-POD is temperature dependent.
In order to determine whether uptake of POD required plasma membrane disruption, we incubated HER cells with increasing concentrations (0.2–2.0 µmol/l) of fluorescein isothiocyanate–conjugated POD (F-POD) followed by a FACS measurement of the number of permeabilized cells by incubation with propidium iodide (PI). In this experiment F-POD rather than L-POD was utilized to reduce bleed of the lissamine-signal into that of PI. An average of 3.98 ± 0.70% of cells were PI-positive when pre-incubated with F-POD, similar to the number of PI-positive cells in the absence of any peptide—5.18 ± 3.00% (Figure 1e). In contrast, 91.44 ± 9.66% were PI-positive when incubated with the cell permeabilizing detergent, 1% Triton-X100 (Figure 1e). We conclude that F-POD enters HER cells without substantially disrupting the plasma membrane.
POD-mediated delivery of small and large molecules in cell culture
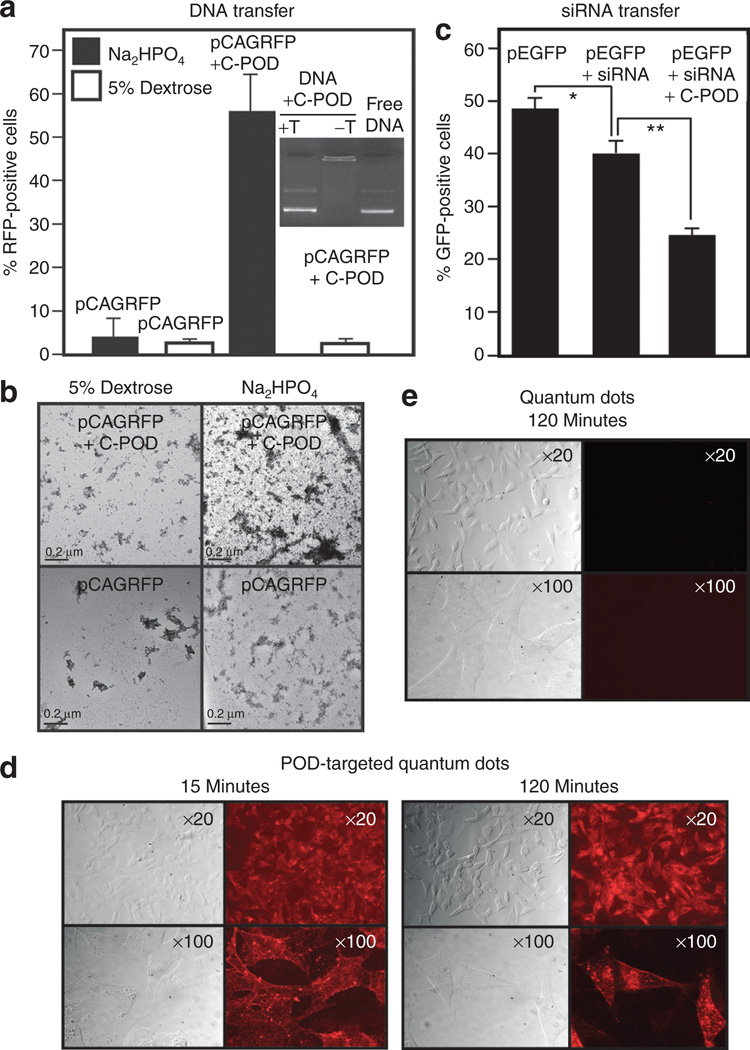
We examined the potential use of an N terminal–biotinylated POD (B-POD) peptide or an N terminal C-POD peptide in delivering small and relatively large molecules to cells in culture. To establish proof-of-principle, we decided to deliver siRNA, plasmid DNA and streptavidin-coated CdSe quantum dots. The use of biotin was to allow the future conjugation of a large variety of biologically relevant compounds to POD via a streptavidin bridge, as we show here for quantum dots, or direct chemical linkage to cysteine is possible through the free sulfhydryl bond. First, we electrostatically conjugated C-POD with a 5.5 kilobase plasmid containing a red fluorescent protein (RFP) expression cassette (pCAGRFP) and demonstrated that C-POD could compact DNA and prevent its migration in an agarose gel and that the DNA could be liberated by trypsin-digestion of the complex (inset, Figure 2a). Such complexes prepared in 5% dextrose were incubated with HER 911 cells and analyzed by FACS 48 hours later. pCAGRFP alone or pCAGRFP complexed with C-POD resulted in only 2.45 ± 0.31% and 2.20 ± 0.46% RFP-positive cells, respectively (Figure 2a). In contrast, when pCAGRFP was complexed with C-POD in Na2HPO4 buffer, 55.58 ± 8.23% of HER cells were RFP-positive. pCAGRFP without C-POD in Na2HPO4 buffer resulted in only 3.80 ± 2.86% RFP-positive cells (Figure 2a) indicating that C-POD and the specific buffer were important components of the gene delivery complex. Examination of C-POD/pCAGRFP complexes in the two differently performing buffers by electron microscopy indicated that complexes prepared in Na2HPO4 had an average area of 63.31 nm2, while those prepared in dextrose were 29.66 nm2 (Figure 2b). This difference may in part play a role in the different rates of gene transfer in the two buffers.
Figure 2. Peptide for ocular delivery (POD)-mediated delivery of small and large molecules in cell culture.
(a) Cysteine-containing POD (C-POD) can compact plasmid DNA and reduce its migration into an agarose gel, an effect that can be alleviated by the addition of trypsin (inset). pCAGRFP compacted with C-POD in Na2HPO4 can transduce significantly (P < 0.0003) more 911 cells in culture relative to uncompacted pCAGRFP in the same buffer, whereas DNA only or the compaction performed in 5% dextrose does not transduce a significant (P > 0.05) number of cells relative to the compaction performed in Na2HPO4. (b) Electron micros-copy of C-POD complexed with plasmid DNA in either 5% dextrose or Na2HPO4 buffer, horizontal bar = 0.2 µm. (c) C-POD can deliver small interfering RNA (siRNA) to human embryonic retinal (HER) cells and silence green fluorescent protein (GFP) expression more efficiently than siRNA alone (*P < 0.007, **P < 0.0002). (d) Biotin-conjugated POD (B-POD) conjugated to quantum dots via a streptavidin bridge allows uptake of the quantum dots within 15 minutes. (e) No evidence of quantum dot uptake at 120 minutes in HER cells in the absence of B-POD. T, trypsin.
C-POD was subsequently used to deliver a plasmid containing an expression cassette for green fluorescent protein (pEGFP) to HER cells, resulting in 49.13 ± 2.23% GFP-positive cells as determined by FACS analysis (Figure 2c). Transfected cells were then incubated with either free siRNA duplex or siRNA duplex complexed with C-POD, resulting in 39.86 ± 2.20% and 24.79 ± 1.18% GFP-positive cells, respectively (Figure 2c). This indicates that delivery of siRNA by C-POD is enhanced as compared to that of siRNA alone and that this approach may be a useful method for delivery of siRNA to enhance the knock down of gene expression.
To examine the potential of delivering larger cargo with POD, we conjugated B-POD with streptavidin-coated quantum dots (QDPOD). HER cells incubated with QDPOD resulted in QDPOD-associated fluorescence within 15 minutes. QDPOD was also found in a punctate pattern within cells at 120 minutes (Figure 2d). In contrast, streptavidin-coated quantum dots without B-POD were not taken up by HER cells (Figure 2e).
Uptake of POD is inhibited by proteoglycans and POD has bacteriostatic activity
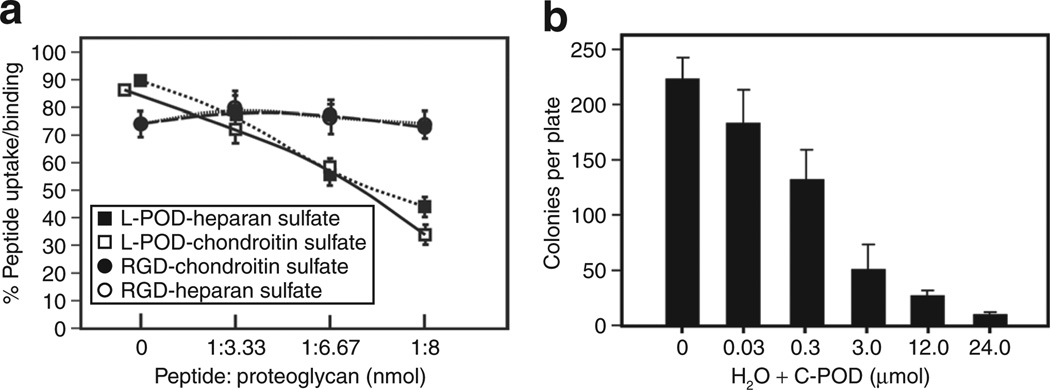
In order to determine whether L-POD uses cell-surface proteoglycans for binding and cell entry, we pre-incubated L-POD with varying ratios of either chondroitin sulfate or heparan sulfate prior to addition of the presumed complex to HER cells. Based on preliminary studies, we arbitrarily selected ratios of L-POD: proteoglycan of 1:3.33, 1:6.67, and 1:8. Without pre-incubation of L-POD with any proteoglycan, 90.07 ± 0.64 % of cells were lissamine-positive, whereas increasing ratios of chondroitin sulfate led to a decrease in uptake of L-POD to 71.24 ± 1.77%, 59.43 ± 1.55%, and 34.38 ± 3.13% at 1:3.33, 1:6.67, and 1:8 molar ratios, respectively (Figure 3a). Similarly, pre-incubation of L-POD with heparan sulfate reduced uptake to 78.60 ± 0.35%, 57.78 ± 2.83%, and 44.37 ± 2.17% respectively at the same ratios described above (Figure 3a). In contrast, the uptake of a tetramethylrhodamine isothiocyanate–conjugated peptide containing an integrin-binding RGD motif was not affected by pre-incubation with either proteoglycan (Figure 3a).
Figure 3. Peptide for ocular delivery (POD) uptake is influenced by cell-surface proteoglycans and POD has microbicidal activity.
(a) Preincubation of lissamine-conjugated POD (L-POD) with either heparan sulfate or chondroitin sulfate significantly (P < 0.0001) reduces uptake of L-POD with all concentrations of proteoglycan but these same proteoglycans have no significant (P > 0.05) influence on uptake of an integrin-binding RGD-containing tetramethylrhodamine isothiocyanate-labeled peptide. (b) Incubation of cysteine-containing POD (C-POD) with Escherichia coli prior to plating significantly (P < 0.05) reduces number of colonies per plate in a concentration dependent manner.
Since endophthalmitis, an infection within the eye, regularly acts as a complicating factor in drug delivery to ocular tissues,16 we examined the potential bacteriostatic activities of C-POD. Escherichia coli grown to mid-log phase were incubated with either H2O only or C-POD suspended in H2O at concentrations ranging from 0.03 to 60 µmol/l prior to plating on Luria-Bertani agar. A significant inhibition of bacterial growth was observed at C-POD concentrations of 0.30 µmol/l (Figure 3b), and almost complete inhibition at 24.0 µmol/l C-POD. Greater concentrations of C-POD completely eliminated bacterial growth (data not shown). Hence, we conclude that C-POD has bacteriostatic activity and that it is concentration dependent.
POD-mediated delivery of small and large molecules to retina in vivo
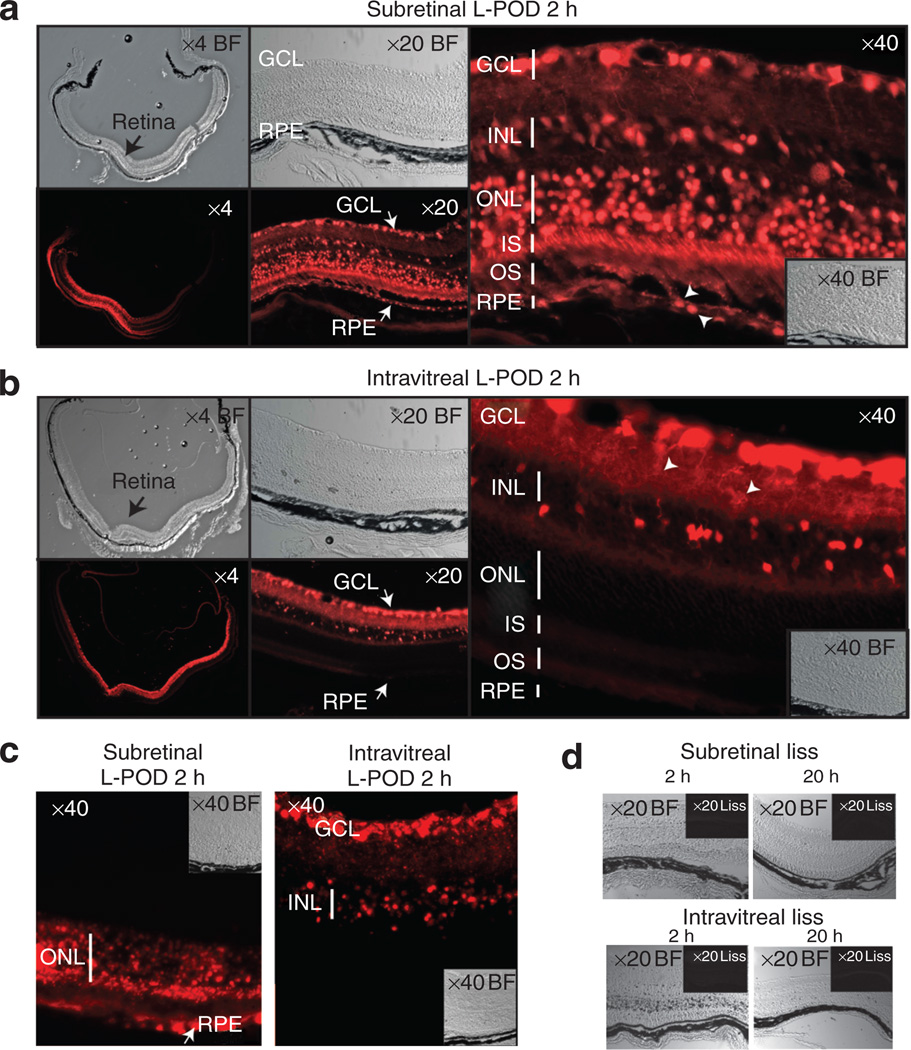
Delivery of L-POD into the subretinal space (a space created between the photoreceptors and the RPE following delivery of fluids) of adult C57BL/6J mice followed by harvesting of tissue 2 hours later resulted in ~40% transduction of neural retina (Figure 4a, ×4). Within this region, there was substantial transduction of several layers of the retina including the RPE (Figure 4a, ×20). Closer examination reveals significant transduction of photoreceptor cell bodies, photoreceptor inner segments, cells in the inner nuclear layer and ganglion cells (Figure 4a, ×40). Although nuclei were not strongly positive in the cell culture experiments described above, in vivo transduction of retina indicated that L-POD localized to nuclei in the case of RPE transduction (arrowheads, Figure 4a, ×40). As the nuclei of photoreceptor cells are large relative to the cell body, nuclear versus cytoplasmic localization could not be differentiated for those cells.
Figure 4. Peptide for ocular delivery (POD) penetrates and carries cargo to retinal tissues in vivo.
(a) Subretinal injection of lissamine-conjugated POD (L-POD) into adult mice transduces ~40% of neural retina after 2 hours (x4) and can be seen in several layers of the retina (×20). L-POD penetrates retinal pigment epithelium (RPE), inner and outer segments (IS, OS), cell bodies in the outer nuclear layer (ONL), cells in the inner nuclear layer (INL) and ganglion cells (GC)—×40. (b) Intravitreal injection of L-POD transduces approximately 85% of the neural retina within 2 hours (×4). Transduction is primarily of the GCL, INL (×20) and dendrites, with no significant transduction of RPE or ONL (×40). (c) Accumulation of L-POD almost exclusively in the outer and inner retina 20 hours after subretinal or intravitreal administration respectively. (d) Lissamine (liss) only does not penetrate retinal cells at 2 or 20 hours by subretinal or intravitreal injection.
Delivery of L-POD into the intravitreal space (the region between the lens and retina) transduced ~85% of the neural retina (Figure 4b, ×4) within 2 hours. Strong staining was seen in the ganglion cell layer, inner plexiform layer and inner nuclear layer (Figure 4b, ×20). Closer examination revealed significant transduction of the ganglion cells and dendrites associated with the inner plexiform layer (arrowheads, Figure 4b, ×40). There was no significant transduction of the outer layers of the retina by intravitreal injection at the 2 hour time point. Whether transduction of inner layers of the retina in addition to the outer layers following subretinal delivery was due to leakage of L-POD from the subretinal space into the vitreous, or to direct penetration of the retina by L-POD within 2 hours could not be determined. After 20 hours, L-POD accumulated almost exclusively in the outer nuclear layer and RPE after subretinal administration, or the inner layers of the retina including ganglion cells after intravitreal injection (Figure 4c). Lissamine only injected into either the subretinal or intravitreal space did not penetrate the retina at either 2 or 20 hours (Figure 4d).
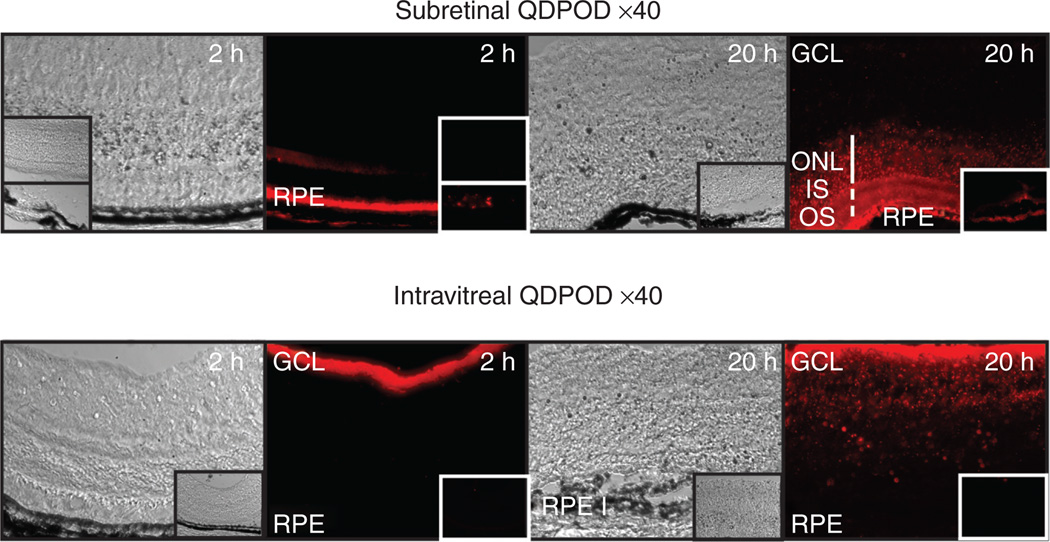
In contrast to L-POD, delivery of the significantly larger QDPOD complexes into the subretinal or intravitreal space appeared to permit cell binding but not uptake per se in the 2 hour time period following injection (Figure 5). However, a longer incubation period of 20 hours revealed uptake of QDPOD into the outer nuclear layer following subretinal injection, or into the inner layers of the retina upon intravitreal injection (Figure 5). In contrast, the control quantum dots that were coated with strepta-vidin only, showed only weak uptake by the RPE in 2 hours and slightly greater but still minimal uptake in 20 hours by the RPE (insets, Figure 5).
Figure 5. Delivery of peptide for ocular delivery (POD)-conjugated quantum dots (QDPOD) to retina in vivo.
Administration of QDPOD to the subretinal space after 2 hours permits binding to rod outer segments/ RPE but no significant uptake by the outer nuclear layer (ONL). Longer incubation times of 20 hours indicate significant uptake by retinal pigment epithelium (RPE) and ONL. Insets are control quantum dots without POD at similar time points. Intravitreal injection of QDPOD indicates binding to inner limiting membrane and ganglion cell layer (GCL) after 2 hours and uptake within 20 hours. Insets are control quantum dots without POD at similar time points.
POD-mediated delivery of cargo to ocular tissues by topical application in vivo
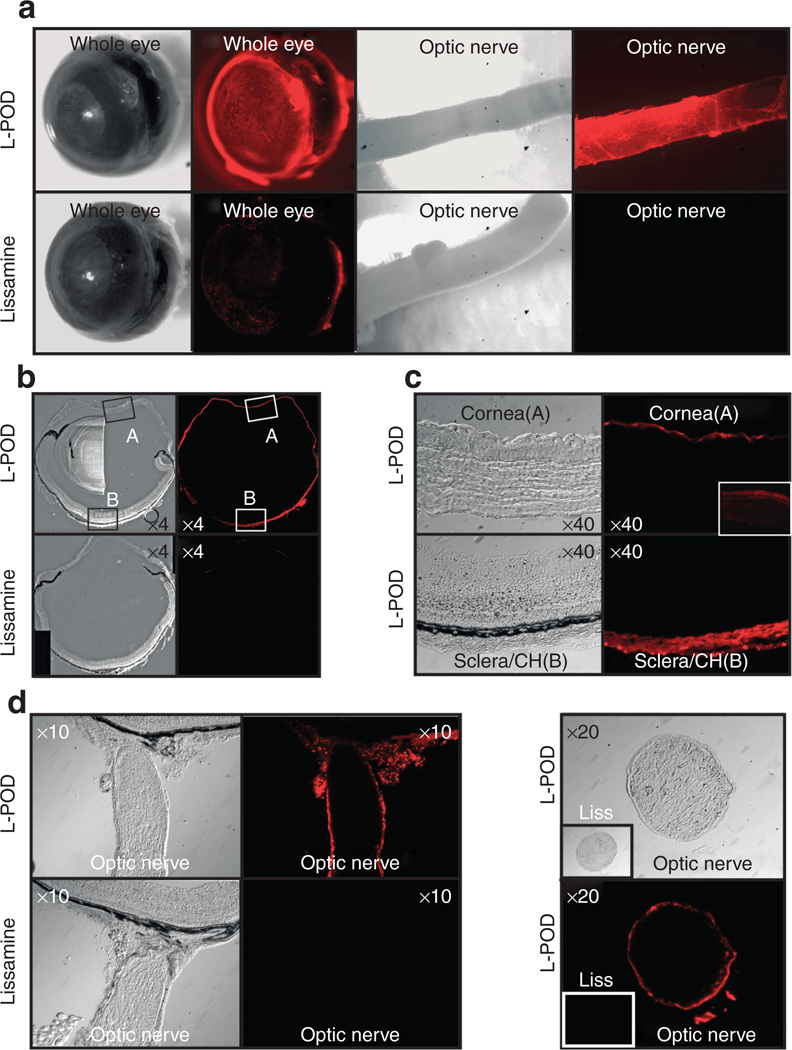
Delivery of drugs to ocular tissues via topical administration to the cornea is common practice, with drainage of large amounts of the drug occurring via the lacrimal ducts. In order to assess penetration of the cornea and sclera with L-POD, we topically applied 10 nmol L-POD to the mouse eye in vivo for 45 minutes followed by harvesting of ocular tissues. We found that within 45 minutes, L-POD bound strongly to the cornea, sclera and unexpectedly, also the dura of the optic nerve in vivo (Figure 6a). In contrast, lissamine only weakly stained the cornea and sclera and did not stain the dura of the optic nerve (Figure 6a). Frozen cross-sections of these eyes revealed that the entire outer ocular surface was positive for L-POD in contrast to lissamine, which only weakly stained these tissues (Figure 6b). Closer examination of cornea and sclera indicated that there was strong staining of the corneal epithelium and sclera/choroid within this 45 minute time period (Figure 6c). In ~50% of animals, the L-POD associated signal was persistent at 24 hours (inset, Figure 6c). However the signal at this later time point was weaker and seemed to occur deeper in the cornea. Longitudinal and cross-sections of the optic nerve indicated that the outer layers of the optic nerve were transduced with L-POD (Figure 6d) at the 45 minute time point. There was no staining observed with lissamine only (Figure 6d) in similar experiments. The L-POD associated signal in these tissues did not persist substantially at 24 hours (data not shown).
Figure 6. Peptide for ocular delivery (POD) penetrates ocular tissues by topical application in vivo.
(a) Topical application of lissamine-conjugated POD (L-POD) to mouse eye in vivo for 45 minutes transduces most of the external ocular tissues including cornea, sclera, and dura of the optic nerve. Lissamine only weakly stains cornea and sclera with no staining of dura of the optic nerve. (b) Cross-sections of the same eye in a indicate that all of the outer surfaces of the eye are transduced by L-POD. (c) Close examination of region A and B from Figure 4b indicates that L-POD is taken up by corneal epithelium and sclera/choroid. (d) Longitudinal and cross-sections of the optic nerve indicate that the outer layers of the optic nerve are transduced by L-POD but not lissamine at the 45 minute time point. Insets are lissamine only controls, except in (c) where the inset represents cornea at the 24 hour time point, a stage at which the signal was observed faintly but in deeper layers of the cornea in some (50%) of eyes. CH, choroid.
DISCUSSION
One goal of this study was to identify a peptide that would act efficiently as a PTD in ocular tissues such as the retina in vivo. We postulated that such a peptide should resemble the glycos-aminoglycan binding regions of proteins abundantly present in the retina. Two such proteins are the acidic and basic fibroblast growth factors.17 The glycosaminoglycan chondroitan sulphate is known to be abundantly present in adult retina. Heparan sulfate is abundantly expressed during development but its expression is substantially reduced in adult retina. Molecular modeling of protein–glycosaminoglycan interactions of the acidic fibroblast growth factor and basic fibroblast growth factor indicates that they contain basic heparin-binding regions of the form XBBBXXBX, where X and B are hydropathic and basic residues, respectively18 Following these observations, Verrecchio and colleagues19 have shown that peptides of the form (XBBBXXBX)n and (XBBXBX)n where n = 1–6, bind heparan. In addition, circu-lar dichroism indicated that heparan-binding peptides converted from a charged coil to an alpha-helix upon heparan binding. We hypothesized that such peptides may have protein transduction properties and tested a variety of sequences for protein transduction (data not shown) prior to the detailed examination of POD presented in this report.
Due to initial controversies surrounding the phenomenon of protein transduction, we have shown that POD enters cells without the need for fixation, although fixation does lead to an artifact in terms of cellular localization. While we observed primarily cytoplasmic localization in formalin-fixed cells within 5 minutes, POD appeared punctate and localized perhaps to endocytic vesicles in live cells even after 60 minutes. Interestingly, at 1 minute post-incubation with POD, cells became opaque to 558 nm excitation—potentially due to aggregation or capping of cell-surface proteoglycans upon POD binding.20 Evidence for uptake and not simply plasma membrane binding was demonstrated by treatment of live cells with trypsin, which failed to reduce the fluorescence associated with POD-transduced cells. Uptake was also shown to be temperature dependent. One concern in using a peptide such as POD for ocular gene or drug delivery would be potential toxicity during the process of traversing the cell membrane. To address this issue, we demonstrated that there is no increase in the uptake of PI by cells, following incubation with POD. Although additional work needs to be done to demonstrate the lack of toxicity, these initial results are encouraging. While our data support a temperature-dependent mechanism, we found no reduction in peptide uptake in the presence of inhibitors of endocytosis (data not shown). Chlorpromazine reportedly blocks clathrin coated pit-dependent endocytosis and genistein and filipin are known inhibitors of caveolae. However, we found no significant reduction in the uptake of L-POD with these inhibitors (data not shown).
Our observation that POD can deliver small molecules like siRNA efficiently across the plasma membrane may have implications for the treatment of ocular disease in humans. Currently there are several ongoing clinical trials that target the degradation of messenger RNA associated with either the vascular endothelial growth factor or its receptor for the treatment of AMD.21 Indeed, the use of siRNA for treatment of ocular disease in humans is at a particularly advanced stage of development as compared to treatment of other diseases.22 Since the target of siRNAs is intracellular, efficient delivery across the plasma membrane in a non-toxic manner is highly desirable. POD may potentially enhance siRNA delivery to the retina, allowing for the use of reduced concentrations of siRNA. This can also lead to reduced side effects such as those associated with non-specific siRNA mediated silencing. Enhanced delivery of RNA molecules such as the aptamer macugen that is currently used to target vascular endothelial growth factor in the treatment of AMD23 may also be potentially achieved. POD may also be used to deliver siRNA and DNA in cell culture to study a variety of cellular processes.
Delivery of POD to the subretinal and intravitreal space allowed for penetration of a variety of cell types in the retina. Transduction of photoreceptors can lead to applications in the treatment of diseases such as retinitis pigmentosa whereas transduction of RPE cells can be applied in the treatment of AMD. Conversely, intravitreal injection allowed for the targeting of ganglion cells, the degeneration of which is associated with glaucoma. POD-mediated delivery of growth factors or antiapoptotic factors to the ganglion cells may slow the rate of degeneration of such cells. While quantum dots took substantially longer to penetrate the retina than did L-POD, perhaps due to their larger size, that it was possible to deliver such large molecules is further support for the potential use of POD in the delivery of biologicals to the retina. These biologicals may include antibodies or antibody fragments such as avastin and lucentis respectively, that are currently also used in the treatment of AMD.24 One may envision fusion proteins with POD for rapid and enhanced penetration of retinal tissues.
Interestingly, L-POD administered topically reached the dura of the optic nerve within 45 minutes. This may have relevance for the treatment of diseases such as optic nerve sheath meningioma or possibly Leber’s hereditary optic neuropathy or ischemic optic neuropathy if POD were to accumulate in the optic nerve itself over time. Penetration of the entire sclera and potentially the choroid may allow for non-invasive delivery of drugs or genes to choroidal blood vessels and endothelial cells, proliferation of which is involved in a variety of ocular diseases including AMD. Topical administration of POD-conjugated drugs to the cornea will also allow enhanced delivery of drugs to the corneal epithelium and into the anterior chamber. This may be particularly useful given that currently very little drug penetrates into ocular tissue following topical administration.25 Finally, since levels of heparan sulfate are increased in diseased retina,26 the peptide described in this report may be applied in the treatment of a variety of retinal degenerations.
Several reports have suggested that cell-penetrating peptides may have microbicidal action due to their structural and chemical similarities with naturally occurring antimicrobial peptides.27–29 Human immunodeficiency virus TAT concentrations of 3–24 µmol/l have been found to be antifungal, resulting from the peptide binding nucleic acids and capable of disrupting the cell cycle.27 Similarly, Pep-1-K, an analogue of Pep-1 designed specifically to target microbes, was strongly antibacterial in cultures of Gram-positive and Gram-negative bacteria with a minimal inhibitory concentration of 1–2 µmol/l. The authors of this study suggested Pep-1-K killed the microorganism through the formation of small channels that allow transit of ions or protons.28 POD appears to have a bacteriostatic activity similar to that of Pep-1-K as it successfully and significantly reduced the growth of Gram-negative bacteria in culture. However, it is important to note that significant reductions in colony number were seen with much lower concentrations of POD than with the other peptides. A concentration as low as 0.3 µmol/l was sufficient to reduce E. coli growth, a valuable finding as lower doses of POD are likely to be more clinically applicable. Injections of POD-conjugated to therapeutic agents could possibly provide additional prophylaxis against infections resulting from invasive clinical procedures.30 Furthermore, the discovery of POD’s bacteriostatic activities suggests that the peptide may act as a therapeutic agent on its own, at least for E coli associated endophthalmitis that appears commonly in some parts of the world31 or randomly in some surgical procedures.30 Whether POD functions as a bacteriostatic against additional bacterial species or also as a microbicide is currently under investigation.
In summary, in this report we introduce a novel cell-penetrating peptide—POD, and demonstrate its ability to penetrate and deliver fluorophores, siRNA, DNA and quantum dots to cells in culture and retinal and ocular tissues in vivo. POD also acts as a microbicide, potentially providing a safer and more efficacious method of delivering molecules to ocular tissues in vivo. Although this has not been examined as yet, it is likely that POD can penetrate cells in other organ systems and hence may also be applied outside the field of ophthalmology.
MATERIALS AND METHODS
Materials and reagents
Heparan sulfate, chondroitin sulfate, and PI were purchased from Sigma. GFP Duplex I was purchased from Dharmacon (Lafayette, CO) and pd2-EGFPN1 was from Clontech (Mountain View, CA). N terminal–conjugated peptides (lissamine, biotin, cysteine) with GGG(ARKKAAKA)4 were custom synthesized and high performance liquid chromatography purified by Sigma Genosys (Woodlands, TX). N terminal fluorescein isothiocyanate–conjugated GGG(ARKKAAKA)4 was synthesized by the University of Utah peptide facility. All other commercially available materials and reagents, including the Qdot655 Streptavidin conjugate, were purchased from Invitrogen (Carlsbad, CA) unless otherwise noted.
Cell-penetrating properties of POD
Unless otherwise stated, all experiments were performed at least twice in triplicate. HER cells were seeded on Lab Tek-II chamber slides and grown to ~70% confluence. Cells were washed twice with phosphate-buffered saline (PBS) and incubated with 1 nmol L-POD for 0, 5, 15, 30, 45, and 60 minutes or 1 nmol lissamine only for 60 minutes. Cells were fixed for 15 minutes in formalin at room temperature. Cells for live imaging were grown on 24-well plates and incubated with 2 nmol of L-POD. Following incubation, cells were washed three times with PBS, incubated in phenol red-free Dulbecco’s modified Eagle’s medium supplemented with 2% fetal bovine serum. Cells were visualized by light and fluorescent microscopy using either an Olympus IX51 (live cells) or an Olympus BX51 (fixed cells) with differential interference contrast, RFP, and GFP filters. Images were obtained using a Retiga 2000R FAST camera and QCapture Pro 5.0 (QImaging, British Columbia, Canada). To measure L-POD uptake in the presence of trypsin, 0.2 × 106 HER cells were incubated with 2.0 nmol L-POD in media for 15 minutes at 37 °C followed by incubation in 2.5 mg/ml trypsin for 12 minutes at 37 °C. For analysis of uptake at 4 °C, cells were cooled to 4 °C for 45 minutes prior to the addition of peptide and cold (4 °C) reagents were used for peptide administration. Following incubation with peptide, cells were washed twice with PBS and spun and resuspended in PBS for FACS analysis. For measurement of membrane permeabilization, HER cells were incubated with 2.0 nmol fluorescein isothiocyanate-heparin-binding protein for 30 minutes at 37 °C, washed twice, isolated, and suspended in PBS with 1 µmol/l PI. The number of PI-positive cells was analyzed by FACS. FACS analysis was performed using a FACSCalibur (Becton Dickinson, Franklin Lakes, NJ). Experiments were performed in triplicate and 10,000 events per sample were counted. Results were analyzed using CellQuest Pro software (Becton Dickinson).
POD-mediated delivery of siRNA, DNA and quantum dots in vitro
pCAGRFP was compacted with C-POD at a ratio of 400 peptide/plasmid molecules. Briefly, 2 µg of DNA and the appropriate concentration of peptide were each suspended in 15 µl of either 100 mmol/l sodium phosphate or 5% dextrose buffer. The peptide and DNA solutions were mixed and incubated for 30 minutes at room temperature prior to addition to HER cells. After 48 hours, cells were resuspended in PBS for FACS analysis. For electron microscopy, peptide/DNA complexes were prepared as above. Particles were bound to glow-discharged copper grids, stained with urinyl acetate, and visualized on a CM10 Transmission Electron Microscope (FEI, Hillsboro, OR) using Digital Micrograph software (Gatan, Pleasanton, CA). C-POD was used to compact both pd2-EGFP-N1 and GFP Duplex I at concentrations of 450 particles/DNA molecule and 25 particles/duplex molecule, respectively, in 100 mmol/l sodium phosphate buffer using the same procedures described above. 2 µg of compacted pd2-EGFP-N1 DNA and 70 pmol of GFP Duplex I diluted in Dulbecco’s modified Eagle’s medium + 2% fetal bovine serum were added simultaneously to HER cells for 4 hours. Growing medium was then added to cells and the incubation continued for 44 additional hours. Cells were isolated and resuspended in PBS for FACS analysis as described above. For delivery of quantum dots, 50 pmol of a Qdot655 streptavidin conjugate (Invitrogen, Carlsbad, CA) were added to 5 nmol of Biotin-conjugated POD (B-POD) in PBS and mixed for 1 hour by gentle rocking at room temperature. The Qdot–peptide complex (QDPOD) was dialyzed in PBS using a 50 K Ultrafree (Millipore, Billerica, MA) column and added to 0.2 × 106 HER cells for either 15 minutes or 2 hours. The amount of fluorescence resulting from the Qdot–peptide complex was determined using a fluorometer and an equivalent amount of unbound Strep–Qdot was added separately as control. Cells were washed three times with PBS, fixed as described above, and stained with 42,6-diamidino-2-phenylindole. Uptake of Qdots was determined by fluorescent microscopy as described above.
Inhibition of POD uptake by proteoglycans and POD-associated microbicidal activity
L-POD and either heparan sulfate or chondroitan sulfate were incubated together at molar ratios of 1:3.33, 1:6.67, and 1:8 in water for 30 minutes at room temperature. The complex was diluted in Dulbecco’s modified Eagle’s medium + 2% fetal bovine serum and added to 0.2 × 106 HER cells for 5 minutes at 37 °C. Following incubation, cells were trypsinized and suspended in PBS for FACS analysis as described above. To measure microbicidal activity of C-POD, XL-1 Blue cells (Stratagene, La Jolla, CA) were grown to mid logarithmic phase (OD600 ≈ 0.600) and washed twice in a solution of 10 mmol/l Tris, pH 7.4 and 5 mmol/l glucose. The cells were resuspended in 10 ml of the same solution and 5 µl of the suspension was incubated with C-POD in a final volume of 50 µl. The peptide/bacteria cell solution was rocked for 2 hours at 37 °C. The peptide-treated bacteria were diluted 1:4,500 with Luria–Bertani Broth, plated on agar, and grown overnight at 37 °C. The following day, the bacterial colonies on each plate were counted to quantify peptide-related reduction in growth.
POD-mediated delivery of molecules to ocular tissues in vivo
The use of animals in this work was in accordance with the Statement for the Use of Animals in Ophthalmic and Vision Research, set out by the Association for Research in Vision and Ophthalmology. C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME), bred and maintained in a 12-hour light–dark cycle and cared for in accordance with federal, state and local regulations. For each experiment at least 4 mice (8 eyes) were investigated, yielding roughly similar results. Mice were anesthetized by intraperitoneal injection of xylazine (10 mg/ml)/ketamine (1 mg/ml). Subretinal or intravitreal injections were performed with a 32G needle attached to a 5 µl glass syringe (Hamilton, Reno, NV) by a trans-scleral trans-choroidal approach to deliver 0.25 nmol of L-POD per eye. For topical delivery to cornea, male C57BL6/6J mice at 6 weeks of age were anesthetized by intraperitoneal injection of ketamine/xylazine and 10 nmol of either L-POD or lissamine only were dropped on the cornea and incubated for 45 minutes or 24 hours. Upon completion of the treatment, the animal was sacrificed by CO2 inhalation followed by cervical dislocation and the eyes harvested and washed three times in PBS. Fluorescent and bright field images were taken of each eye using a Nikon C-FMC microscope prior to fixation. Eyes were fixed overnight at 4 °C in 4% paraformaldehyde and embedded in Optimal Cutting Temperature Compound and 14 µm sections were collected using a Microm 550 cryostat.
ACKNOWLEDGMENTS
This study was supported by grants to R.K.-S. from the National Institutes of Health/National Eye Institute (EY014991 and EY013887), The Foundation Fighting Blindness, The Ellison Foundation and grants to the department of ophthalmology at Tufts University from the Lions Eye Foundation and Research to Prevent Blindness.
REFERENCES
- 1.National Eye Institute. A Report of the National Eye Council. Bethesda: National Institutes of Health; 1999–2003. [Google Scholar]
- 2.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 3.Rattner A, Nathans J. Macular degeneration: recent advances and therapeutic opportunities. Nat Rev Neurosci. 2006;7:860–872. doi: 10.1038/nrn2007. [DOI] [PubMed] [Google Scholar]
- 4.Moffatt SL, Cartwright VA, Stumpf TH. Centennial review of corneal transplantation. Clin Experiment Ophthalmol. 2005;33:642–657. doi: 10.1111/j.1442-9071.2005.01134.x. [DOI] [PubMed] [Google Scholar]
- 5.Kelder J, Grootenhuis PD, Bayada DM, Delbressine LP, Ploemen JP. Polar molecular surface as a dominating determinant for oral absorption and brain penetration of drugs. Pharm Res. 1999;16:1514–1519. doi: 10.1023/a:1015040217741. [DOI] [PubMed] [Google Scholar]
- 6.Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 7.Phelan A, Elliott G, O’Hare P. Intercellular delivery of functional p53 by the herpesvirus protein VP22 [see comments] Nat Biotechnol. 1998;16:440–443. doi: 10.1038/nbt0598-440. [DOI] [PubMed] [Google Scholar]
- 8.Derossi D, Joliot AH, Chassaing G, Prochiantz A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J Biol Chem. 1994;269:10444–10450. [PubMed] [Google Scholar]
- 9.Dietz GP, Bahr M. Delivery of bioactive molecules into the cell: the Trojan horse approach. Mol Cell Neurosci. 2004;27:85–131. doi: 10.1016/j.mcn.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Lundberg M, Johansson M. Is VP22 nuclear homing an artifact? Nat Biotechnol. 2001;19:713–714. doi: 10.1038/90741. [DOI] [PubMed] [Google Scholar]
- 11.Falnes PO, Wesche J, Olsnes S. Ability of the Tat basic domain and VP22 to mediate cell binding, but not membrane translocation of the diphtheria toxin A-fragment. Biochemistry. 2001;40:4349–4358. doi: 10.1021/bi002443l. [DOI] [PubMed] [Google Scholar]
- 12.Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, et al. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J Biol Chem. 2003;278:585–590. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- 13.Cashman SM, Morris DJ, Kumar-Singh R. Evidence of protein transduction but not intercellular transport by proteins fused to HIV tat in retinal cell culture and in vivo . Mol Ther. 2003;8:130–142. doi: 10.1016/s1525-0016(03)00131-x. [DOI] [PubMed] [Google Scholar]
- 14.Cashman SM, Sadowski SL, Morris DJ, Frederick J, Kumar-Singh R. ntercellular trafficking of adenovirus-delivered HSV VP22 from the retinal pigment epithelium to the photoreceptors-implications for gene therapy. Mol Ther. 2002;6:813–823. doi: 10.1006/mthe.2002.0806. [DOI] [PubMed] [Google Scholar]
- 15.Fallaux FJ, Kranenburg O, Cramer SJ, Houweling A, Van Ormondt H, Hoeben RC, et al. Characterization of 911: a new helper cell line for the titration and propagation of early region 1-deleted adenoviral vectors. Hum Gene Ther. 1996;7:215–222. doi: 10.1089/hum.1996.7.2-215. [DOI] [PubMed] [Google Scholar]
- 16.Ho J, Loewenstein JI. Endophthalmitis associated with intravitreal injections. Int Ophthalmol Clin. 2007;47:199–208. doi: 10.1097/IIO.0b013e318037795f. [DOI] [PubMed] [Google Scholar]
- 17.Bugra K, Hicks D. Acidic and basic fibroblast growth factor messenger RNA and protein show increased expression in adult compared to developing normal and dystrophic rat retina. J Mol Neurosci. 1997;9:13–25. doi: 10.1007/BF02789391. [DOI] [PubMed] [Google Scholar]
- 18.Cardin AD, Weintraub HJ. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1989;9:21–32. doi: 10.1161/01.atv.9.1.21. [DOI] [PubMed] [Google Scholar]
- 19.Verrecchio A, Germann MW, Schick BP, Kung B, Twardowski T, San Antonio JD. Design of peptides with high affinities for heparin and endothelial cell proteoglycans. J Biol Chem. 2000;275:7701–7707. doi: 10.1074/jbc.275.11.7701. [DOI] [PubMed] [Google Scholar]
- 20.Martinho RG, Castel S, Urena J, Fernandez-Borja M, Makiya R, Olivecrona G, et al. Ligand binding to heparan sulfate proteoglycans induces their aggregation and distribution along actin cytoskeleton. Mol Biol Cell. 1996;7:1771–1788. doi: 10.1091/mbc.7.11.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michels S, Schmidt-Erfurth U, Rosenfeld PJ. Promising new treatments for neovascular age-related macular degeneration. Expert Opin Investig Drugs. 2006;15:779–793. doi: 10.1517/13543784.15.7.779. [DOI] [PubMed] [Google Scholar]
- 22.Check E. Firm sets sights on gene silencing to protect vision. Nature. 2004;430:819. doi: 10.1038/430819b. [DOI] [PubMed] [Google Scholar]
- 23.Ng EW, Shima DT, Calias P, Cunningham ET, Jr., Guyer DR, Adamis AP. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov. 2006;5:123–132. doi: 10.1038/nrd1955. [DOI] [PubMed] [Google Scholar]
- 24.Pieramici DJ, Avery RL. Ranibizumab: treatment in patients with neovascular age-related macular degeneration. Expert Opin Biol Ther. 2006;6:1237–1245. doi: 10.1517/14712598.6.11.1237. [DOI] [PubMed] [Google Scholar]
- 25.Shell JW. Ophthalmic drug delivery systems. Surv Ophthalmol. 1984;29:117–128. doi: 10.1016/0039-6257(84)90168-1. [DOI] [PubMed] [Google Scholar]
- 26.Landers RA, Rayborn ME, Myers KM, Hollyfield JG. Increased retinal synthesis of heparan sulfate proteoglycan and HNK-1 glycoproteins following photoreceptor degeneration. J Neurochem. 1994;63:737–750. doi: 10.1046/j.1471-4159.1994.63020737.x. [DOI] [PubMed] [Google Scholar]
- 27.Jung HJ, Park Y, Hahm KS, Lee DG. Biological activity of Tat (47–58) peptide on human pathogenic fungi. Biochem Biophys Res Commun. 2006;345:222–228. doi: 10.1016/j.bbrc.2006.04.059. [DOI] [PubMed] [Google Scholar]
- 28.Zhu WL, Lan H, Park IS, Kim JI, Jin HZ, Hahm KS, et al. Design and mechanism of action of a novel bacteria-selective antimicrobial peptide from the cell-penetrating peptide Pep-1. Biochem Biophys Res Commun. 2006;349:769–774. doi: 10.1016/j.bbrc.2006.08.094. [DOI] [PubMed] [Google Scholar]
- 29.Palm C, Netzereab S, Hallbrink M. Quantitatively determined uptake of cell-penetrating peptides in non-mammalian cells with an evaluation of degradation and antimicrobial effects. Peptides. 2006;27:1710–1716. doi: 10.1016/j.peptides.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Balestrazzi A, Blasi MA, Primitivo S, Balestrazzi E. Escherichia coli endophthalmitis after trans-scleral resection of uveal melanoma. Eur J Ophthalmol. 2002;12:437–439. doi: 10.1177/112067210201200517. [DOI] [PubMed] [Google Scholar]
- 31.Jackson TL, Eykyn SJ, Graham EM, Stanford MR. Endogenous bacterial endophthalmitis: a 17-year prospective series and review of 267 reported cases. Surv Ophthalmol. 2003;48:403–423. doi: 10.1016/s0039-6257(03)00054-7. [DOI] [PubMed] [Google Scholar]