Abstract
Clinical diagnosis of grade 1 acute graft-versus-host disease (GVHD) marks the beginning of a potentially progressive and fatal course of GVHD after hematopoietic stem cell transplantation (HSCT). However, interventional studies to treat early GVHD are lacking. We conducted a single-arm prospective phase II trial to test the hypothesis that treatment of newly-diagnosed grade 1 acute GVHD with etanercept and topical corticosteroids would reduce progression to grade 2–4 within 28 days. Study patients (n=34) had a median age of 51 years (range, 10–67 years) and had undergone unrelated (n=22) or related (n=12) donor HSCT. Study patients were treated with etancercept (0.4 mg/kg, maximum 25 mg/dose) twice weekly for 4–8 weeks. Ten of 34 patients (29%) progressed to grade 2–4 acute GVHD within 28 days. The cumulative incidence of grade 2–4 and grade 3–4 acute GVHD at 1-year were 41% and 3%, respectively. Non-relapse mortality was 19% and overall survival was 63% at 2-years. Among a contemporaneous control cohort of patients that were diagnosed with grade 1 acute GVHD and treated with topical corticosteroids but not etanercept during the study period, 12 of 28 patients (43%) progressed to grade 2–4 GVHD within 28 days, with 1-year incidence of grade 2–4 GVHD and grade 3–4 GVHD of 61% (41% vs 61%, p=0.08) and 18% (3% vs 18%, p=0.05), respectively. Patients treated with etanercept also experienced less increase in GVHD plasma biomarkers ST2 (p=0.06) and Reg3α (p=0.01) 28 days after grade 1 acute GVHD diagnosis compared to contemporaneous control patients. This study was terminated early due to poor accrual. Future prospective studies are needed to identify patients with grade 1 acute GVHD at risk of swift progression to more severe GVHD and to establish consensus for the treatment of grade 1 acute GVHD. This trial is registered with ClinicalTrials.gov, number NCT00726375.
Introduction
Allogeneic hematopoietic stem-cell transplantation (HSCT) is an important therapy for many malignant and non-malignant conditions [1]. A significant barrier to the more widespread application of HSCT is the potentially severe and fatal complication of acute graft-versus-host disease (GVHD) [2]. While prophylaxis strategies have lowered the risk of life-threatening GVHD, 40–70% of patients are still at risk of developing the complication [3–7]. Moreover, in these patients, treatment approaches have provided inconsistent outcomes [8]. High-dose systemic corticosteroids remain the standard initial therapy for grade 2–4 acute GVHD, yet carry significant risks [9], and complete response rates range between 25–40% [10–13]. Patients who do not have at least a partial response to therapy within the first 28 days are at high risk for non-relapse mortality (NRM) six months from the onset of therapy [14–17].
The standard treatment of grade 1 (skin stage 1 or 2 only) acute GVHD is topical corticosteroid therapy [9]. However, in clinical practice, it is likely that far more patients with grade 1 acute GVHD are treated with systemic corticosteroids than are reported. In a recent multicenter Blood and Marrow Transplant Clinical Trials Network phase II trial, up to 13% of study patients had a clinical diagnosis of grade 1 acute GVHD and were treated with systemic steroids in conjunction with a secondary agent [18]. Nonetheless, to our knowledge, interventional studies targeted at treatment of grade 1 acute GVHD have not been previously reported. We reasoned that a strategy allowing early, standardized treatment of grade 1 acute GVHD would reduce progression in the first 28 days of diagnosis.
TNF-alpha (TNFα) is an important component of the inflammatory cascade that evolves into acute GVHD [19–22]. Our group has previously shown that the magnitude of increase in TNF-receptor-1 (TNFR1), a surrogate for TNFα, 7 days after HSCT relative to pre-HSCT baseline levels, strongly correlates with increased GVHD incidence, NRM, and decreased overall survival in adults and children [19, 20]. Etanercept, a recombinant human soluble TNFα receptor fusion protein, competes for TNFα binding and renders it inactive [23]. Etanercept attenuated rising TNFR1 levels early after HSCT in patients that received non-TBI conditioning and correlated with good clinical outcomes when used in combination with standard immunosuppression for GVHD prophylaxis [24]. Based on pre-clinical and clinical studies implicating a role for TNF-α in the etiology of acute GVHD [19–22, 24], we hypothesized that TNF-α blockade with etanercept for treatment of grade 1 acute GVHD would reduce the progression to grade 2–4 within 28 days.
Subjects and Methods
Study cohort
A prospective, open-label, single-arm phase II trial of etanercept combined with topical corticosteroid therapy for grade 1 acute GVHD after allogeneic HSCT was conducted between May 2008 and April 2013. Patients with a clinical diagnosis of grade 1 acute GVHD (stage 1 or 2 skin rash covering < 50% body surface area) were eligible for inclusion in the study if sufficient rash was present to biopsy and the results were consistent with the clinical diagnosis of GVHD. Patients of any age who underwent HSCT with donor cells from any source following either a myeloablative or nonmyeloablative preparative regimen and with clinical grade 1 acute GVHD were eligible. Patients with grade 2–4 acute GVHD or with an active infection unresponsive to antibiotics were ineligible for this study. Patients who used systemic steroids at any previous time for treatment of GVHD and patients who received etanercept for any other purpose were also ineligible. The protocol and informed consents were approved by the Institutional Review Board at the University of Michigan. All patients and their legal guardians signed informed consents in accordance with the Declaration of Helsinki.
The GVHD prophylaxis regimens consisted of tacrolimus initiated on day −3 before HSCT (titrated and maintained at a level of 8–12 ng/mL) either with mini-methotrexate administered at a dose of 5 mg/m2 i.v. on days 1, 3, 6 and 11 following HSCT or with mycophenolate mofetil at 10mg/kg/dose every 8 hours on days 0 through 28. In the absence of GVHD, tacrolimus was tapered starting from day 56 post-HSCT and was discontinued by day 180.
Supportive care therapies were administered according to institutional clinical practice guidelines. Antimicrobial prophylaxis included levofloxacin 500 mg once daily for prevention of bacterial infections, voriconazole 200 mg twice daily, acyclovir 400 mg twice daily for viral prophylaxis, and sulfamethoxazole/trimethoprim or pentamidine for prevention of Pneumocystis carinii pneumonia. Pediatric patients received age/weight equivalent dosing of antibiotics. Cytomegalovirus (CMV) DNA was monitored weekly by quantitative PCR [25] and preemptive therapy with antiviral agents begun in the event of a positive assay. Intravenous immunoglobulin (Ig) replacement therapy (400 mg/kg) was given for IgG levels < 400 mg/dL.
Infection
Infections were enumerated for each patient for 180 days beginning on the first day of etanercept treatment. An infection was defined using the following criteria: one or more positive blood/fluid cultures, or the detection of DNA in the plasma by quantitative PCR. Proven, probable, and possible invasive fungal infections were classified according to international consensus criteria [26].
GVHD treatment
All study patients were treated with topical corticosteroids (0.1% triamcinolone crème applied to affected areas three times daily) at the time of grade 1 acute GVHD diagnosis according to institutional clinical practice guidelines. Etanercept (0.4 mg/kg, maximum 25 mg/dose) was administered subcutaneously twice weekly on non-consecutive days for 4 weeks, as previously reported at our center [20], for a total of 8 doses. In some patients, etanercept was continued for an additional 4 weeks, as described below. Doses were held and not replaced in patients with bacteremia, hemodynamic instability, fever, or persistent viral infection. If signs and symptoms of infection resolved (blood pressure stable, negative blood cultures for a minimum of 48 hours, 50% reduction in viral copy number) before needing to hold a third consecutive dose of trial drug, etanercept dosing was resumed and the patient was allowed to continue on the trial. In patients who progressed to grade 2–4 acute GVHD, standard high-dose systemic corticosteroid therapy was initiated and etanercept was permanently discontinued.
GVHD scoring and evaluation of response
GVHD was monitored weekly in all patients using the modified Glucksberg criteria [27]. Formal GVHD grading to evaluate response in all patients was performed at the start of etanercept treatment (day 0 of trial), week 4, and week 8. Overall GVHD grade was used to determine the response. A complete response (CR) was defined as the complete resolution of all manifestations of GVHD (all organs grade 0). Patients achieving a CR at 4 weeks stopped etanercept treatment (8 total doses). Patients with no change in overall grade of GVHD (stable disease, SD) at 4 weeks received 4 additional weeks of etanercept treatment (16 total doses). Treatment success was defined as having CR or SD at the 4-week assessment (i.e. no systemic corticosteroid therapy). Treatment failure was defined as progression to grade 2–4 acute GVHD within the first 4 weeks of etanercept treatment. All patients, including treatment failures, were re-evaluated for GVHD grade and response at 8 weeks. Chronic GVHD was diagnosed and staged according to published criteria [28] and treated according to institutional clinical practice guidelines.
Comparison with a contemporaneous control cohort
A contemporaneous control cohort was constructed from the BMT Program Clinical Database to provide a comparator for the study patients, given a lack of published data on clinical outcomes associated with grade 1 acute GVHD and its treatment. The contemporaneous control cohort is comprised of all patients who underwent HSCT according to study criteria and were diagnosed with grade 1 acute GVHD during the same time interval as the study patients (2008–2013) but who chose not to enroll in the study (n=28). All contemporaneous control patients were treated with topical corticosteroids (0.1% triamcinolone crème applied to affected areas three times daily) at the time of grade 1 acute GVHD diagnosis according to institutional clinical practice guidelines. GVHD was monitored weekly using the modified Glucksberg criteria [27] and standard high-dose systemic corticosteroid therapy was initiated in patients who progressed to grade 2–4 acute GVHD.
Correlative studies: Plasma Biomarkers
Peripheral blood samples were collected on the day of clinical grade 1 GVHD diagnosis and 14 and 28 days thereafter. Plasma was obtained after Ficoll (Amersham, Piscataway, NJ) gradient centrifugation on the day of collection. Samples were dispensed without additives into cryovials and frozen at −80°C for later analysis. ST2, Reg3α, TNFR1 and elafin concentrations in each plasma sample were determined using an enzyme-linked immunosorbant assay (ELISA) as previously described [20, 24, 29–31]. Day 28 biomarker levels were compared between study and contemporaneous control patients after normalizing to day 0 levels (i.e. ratio of day 28 over day 0 concentration) for each patient. The normalized value thus indicates the fold-change in biomarker concentration from day 0 to day 28. Samples and standards were run in duplicate. Absorbance was measured using a Synergy HT plate reader and results were calculated using Gen 5.1 software (BioTek Instruments, Winooski, VT). Assays were performed at the Immunologic Monitoring Core of the University of Michigan Cancer Center.
Statistical Analysis
This trial was designed to enroll 50 patients in order to have sufficient power (90%) to detect a decrease in the percentage of patients who progressed within 4 weeks of initiation of treatment for grade 1 acute GVHD, from 58% observed historically between 2001 and 2006 at the University of Michigan Medical Center to 38%, assuming a type 1 error rate of 5%. At the time of study design in 2007–2008, grade 1 acute GVHD was diagnosed in approximately 21 patients per year at the University of Michigan. Assuming 80% of patients would be eligible and consent to study participation, we expected accrual to take approximately three years. However, due to competing studies at our center and unforeseen difficulties obtaining approval for insurance coverage of the study drug, we accrued a total of 34 patients over a five-year period (2008–2013). Based soley on the slow accrual of the study, the decision was made by the BMT Team in concert with recommendations provided by the University of Michigan Comprehensive Cancer Center Data Safety Monitoring Board to terminate the study early. Herein, we report the findings in 34 subjects. All subjects who enrolled in the study and received at least one dose of etanercept were included in the analysis.
Overall survival (OS) was measured from the date of transplantation to the earlier of death from any cause or end of follow-up, and was estimated with the methods of Kaplan-Meier [32]. The cumulative incidence of relapse, NRM, grade 2 or higher acute GVHD, and chronic GVHD were estimated using the method of Fine and Gray [33]. Relapse and NRM were competing risks for each other and relapse and death were competing risks for acute and chronic GVHD. Baseline characteristics were compared between the study and contemporaneous control cohorts with the use of the Wilcoxon rank-sum test for continuous variables and chi-square or Fisher’s exact tests for categorical characteristics. Concentrations of biomarker proteins in plasma samples were compared using the Wilcoxin rank-sum test. These statistical analyses were performed in R (R Project for Statistical Computing at www.r-project.org) and GraphPad Prism 6.0 (GraphPad Software, Inc. La Jolla, CA). A two-sided p-value of less than 0.05 was considered to indicate statistical significance. This trial is registered with clinicaltrials.gov, number NCT00726375.
Results
Patient characteristics
A total of 34 patients were enrolled in this phase II trial and received treatment with etanercept for newly diagnosed grade 1 acute GVHD (May 2008–April 2013). Sixteen patients received a full-course of etanercept treatment (8 doses over 4 weeks). Seven patients received two courses of etanercept (15–16 doses over 8 weeks). Eleven patients received less than a full course of treatment due to early removal from the study as a result of GVHD progression (n=8), development of idiopathic pneumonia syndrome (IPS, n=1) or having missed ≥ 2 consecutive doses due to viral infection (n=2).
Patient demographics and clinical characteristics are summarized in Table 1. The median age was 51 years (range, 10–67 years). Sixty-two percent of patients had intermediate-to-high disease risk status, classified according to the American Society of Blood and Marrow Transplantation (ASBMT) 2013 disease classification index [34]. Twelve patients received HLA-matched related grafts and sixteen patients received matched unrelated grafts. Four patients received one-locus HLA-mismatched grafts from unrelated donors and two patients received unrelated double cord grafts mismatched at 2 loci. Twenty-four patients (71%) received myeloablative conditioning. These regimens were busulfan-based (12.8 mg/kg; n=20), TBI-based (total body irradiation, 1200 cGy; n=3) or BCNU-based (carmustine, 300 mg/m2; n=1). Ten patients received reduced-intensity conditioning regimens consisting of fludarabine and busulfan (fludarabine, 120 mg/m2; busulfan, 6.4mg/kg; n=6), fludarabine and melphalan (fludarabine 120 mg/m2; melphalan, 140 mg/m2; n=3) or cyclophosphamide and ATG (cyclophosphamide, 200 mg/kg; thymoglobulin, 7.5mg/kg; n=1). All patients received standard GVHD prophylaxis with a calcineurin inhibitor and mini-methotrexate (n=18) or mycophenolate mofetil (n=16) following transplant.
Table 1.
Baseline demographics and clinical characteristics of the study participants and controls.
| Characteristic | Study | Control | P value | |
|---|---|---|---|---|
| No. (%) or Median (IQR) | No. (%) or Median (IQR) | |||
| Age (years) | 51 (30) | 56 (12) | 0.10 | |
| Gender | Male | 26 (76) | 18 (64) | 0.44 |
| Female | 8 (24) | 10 (36) | ||
| Race/Ethnicity | White | 30 (88) | 24 (86) | 0.54 |
| Black or African American | – | 1 (4) | ||
| Other† | 4 (12) | 3 (11) | ||
| Diagnosis | Malignant | 32 (94) | 28 (100) | 0.56 |
| Acute myelogenous leukemia | 13 (38) | 8 (29) | ||
| Acute lymphoblastic leukemia | 6 (18) | 7 (25) | ||
| Multiple myeloma | 4 (12) | 2 (7) | ||
| Myelodysplastic syndrome | 4 (12) | 3 (11) | ||
| Non-Hodgkin’s lymphoma | 2 (6) | 4 (14) | ||
| Myelofibrosis | 2 (6) | 1 (4) | ||
| Chronic lymphocytic leukemia | 1 (3) | 2 (7) | ||
| Chronic myelogenous leukemia | – | 1 (4) | ||
| Non-malignant disease | 2 (6) | – | ||
| Disease Risk Status | Low | 13 (38) | 11 (39) | 0.74 |
| Intermediate | 5 (15) | 6 (21) | ||
| High | 16 (47) | 11 (39) | ||
| Donor | Matched related | 12 (35) | 10 (36) | 0.46 |
| Matched unrelated | 16 (47) | 12 (43) | ||
| Mismatched related | – | 2 (7) | ||
| Mismatched unrelated | 6 (18) | 4 (14) | ||
| CMV Status | Recipient (R) or Donor (D) positive | 24 (71) | 20 (71) | 0.80 |
| R+, D+ | 9 (26) | 10 (36) | ||
| R+, D– | 12 (35) | 9 (32) | ||
| R–, D+ | 3 (9) | 1 (4) | ||
| Recipient and Donor negative | 10 (29) | 8 (29) | ||
| Conditioning | Full | 24 (71) | 21 (76) | 0.92 |
| Busulfan-based‡ | 20 (59) | 18 (65) | ||
| TBI-based§,£ | 3 (9) | 3 (11) | ||
| BCNU-based¶ | 1 (3) | – | ||
| Reduced | 10 (30) | 7 (25) | ||
| Fludarabine, busulfan | 6 (18) | 6 (21) | ||
| Fludarabine, melphalan | 3 (9) | 1 (4) | ||
| Cyclophosphamide, ATG | 1 (3) | – | ||
| CD34+ Count (×106 cells/kg) | 6.3 (2.2) | 5.7 (1.2) | 0.36 | |
| GVHD Prophylaxis | Calcineurin Inhibitor, MTX | 18 (53) | 13 (46) | 0.80 |
| Calcineurin Inhibitor, MMF | 16 (47) | 15 (54) | ||
| Time HSCT to Grade 1 acute GVHD Diagnosis (days) | 28 (35) | 32 (19) | 0.84 | |
| Time to Progression, Grade 1 to Grade 2–4 GVHD (days) | 24 (50) | 7 (27) | 0.08 | |
Study: Asian (n=1), Hispanic or Latino (n=3); Control: American Indian/Alaskan Native (n=1), Hispanic or Latino (n=2)
Study:fludarabine and busulfan (n=14), clofarabine and busulfan (n=6); Control: fludarabine and busulfan (n=10), clofarabine and busulfan (n=8)
Study:cyclophosphamide and TBI (n=1), thiotepa, cyclophosphamide and TBI (n=1), campath, fludarabine, cyclophosphamide and TBI (n=1); Control: cyclophosphamide and TBI (n=3)
Study: rituximab, carmustine, etoposide, cytarabine and melphalan (n=1)
Abbreviations: TBI, total body irradiation; BCNU, carmustine; MTX, mini-methotrexate; MMF, mycophenolatemofetil
Safety and Infection
The safe use of etanercept in patients following HSCT has been demonstrated at our institution previously [11, 18, 24]. Treatment with etanercept in this study was well-tolerated. There were no reactions related to the subcutaneous injection of etanercept. Etanercept was discontinued early in two patients whose treatment was held according to study design, and whose viral infection (RSV, CMV) did not adequately resolve before the third consecutive dose was held.
Infections were monitored for 180 days, beginning at the onset of etanercept treatment. Viral reactivations were the most common type of infection observed in study patients. Twelve patients developed a total of 18 viral reactivations; 11 were CMV reactivations. There were 7 cases of Gram-positive bacteremia (5 single organism and 2 polymicrobial infections) and 3 cases of Gram-negative bacteremia in study patients. Bacterial pneumonia accounted for 1 death, which occurred 13 weeks after etanercept treatment. One patient developed fatal invasive fungal infection 12 weeks after etanercept. Both fatal infections occurred during treatment with systemic corticosteroids for progressive acute GVHD. A complete list of bacterial, viral and fungal infections, and time to their development, are listed in Table 2. The number and severity of infectious complications were consistent with the patient population, and were not different from the incidence of infection in the contemporaneous control cohort who did not receive etanercept.
Table 2.
Infections*
| Study Cohort | ||||
|---|---|---|---|---|
|
| ||||
| Category | Total | 0–59d† | 60–180d | Organisms |
| Gram positive bacteria | 5 | 3 | 2 | Coagulase negative Staphylococcus sp.‡, Bacillus sp., Mycobacterium gordonae, Enterococcus faecium |
| Gram negative bacteria | 3 | 1 | 2 | Escherichia coli, Klebsiella oxytoca, Enterobacter cloacae |
| Viral | 18 | 11 | 7 | Cytomegalovirus (CMV), Human herpesvirus 6 (HHV-6), Herpes simplex virus 1 (HSV-1), Human respiratory syncytial virus (RSV), Epstein-Barr virus (EBV), Human adenovirus |
| Fungal | 1 | 1§ | – | Rhizopus sp. |
| Polymicrobial | 2 | 2 | – | 1 patient: gram positive α Streptococcus sp., Rhodococcus gordonia and coagulase negative Staphylococcus sp.; 1 patient: HHV-6 and gram positive α Streptococcus sp. |
|
| ||||
| Total Events | 29 | 18 | 11 | |
|
| ||||
| Alive at interval start | N=34 | N=34 | N=33 | |
| Contemporaneous Cohort | ||||
|---|---|---|---|---|
|
| ||||
| Category | Total | 0–59d | 60–180d | Organisms |
| Gram positive bacteria | 9 | 2 | 7 | Coagulase negative Staphylococcus sp., Lactobacillus sp., Enterococcus faecium, aerobic gram positive rod NOS¶, Nocardia asteroides, Streptococcus pneumoniae |
| Gram negative bacteria | 1 | 1 | – | Klebsiella pneumoniae |
| Viral | 12 | 8 | 4 | CMV, HHV-6, RSV, EBV |
| Fungal | 3 | – | 3£ | Rhizopus sp., Aspergillus fumigatus |
| Polymicrobial | 2 | – | 2 | 1 patient: gram positive coagulase negative Staphylococcus sp., gram negative Sphingomonas paucimobilis and Stenotrophomonas maltophilia; 1 patient: CMV and HSV-1. |
|
| ||||
| Total Events | 27 | 11 | 16 | |
|
| ||||
| Alive at interval start | N=28 | N=28 | N=26 | |
Listed are the counts of new infection events during the 180 days from the start of Enbrel treatment (study cohort) or grade 1 GVHD diagnosis (contemporaneous control cohort) and the count by interval within these 180 days. Patients with polymicrobial infections (concurrent infection by >1 organism) received a single infection count unless otherwise specified. In addition to the count of events, the table shows the count of patients alive (N) at the start of each interval. Organisms not captured include enterococci from stool, rectum, skin; Clostridium difficile from stool; BK virus from urine; and oral thrush.
Enbrel treatment duration was 28 or 56 days.
sp.; abbreviation for species.
concomitant coagulase negative Staphylococcus sp. infection, thought to be a contaminant, counted as a separate gram positive event.
NOS, abbreviation for not otherwise specified.
concomitant Streptococcus pneumonia infection in 1 patient, counted as a separate gram positive event.
Acute GVHD
The median time to onset of grade 1 acute GVHD in study patients was 28 days (range, 7–235 days) after HSCT (Table 1). We hypothesized that treatment of grade 1 acute GVHD with etanercept would reduce the proportion of patients who progressed to grade 2–4 acute GVHD within 4 weeks of diagnosis from 58% historically observed at our institution to 38%. Etanercept limited the progression to grade 2–4 GVHD within 4 weeks of grade 1 GVHD diagnosis (i.e. study failure) to 10 of 34 patients (29%, Figure 1). Fourteen (41%) of the study patients achieved a CR at 4 weeks, and 10 patients (29%) had stable disease after etanercept and topical corticosteroid treatment. Of the 10 patients who failed etanercept treatment and started systemic corticosteroid therapy, 5 (50%) and 2 (20%) achieved CR and SD, respectively, by 4 weeks. In the remaining three patients who failed etanercept therapy, two (20%) developed progressive GVHD by 4 weeks but achieved CR by 8 weeks; one developed idiopathic pneumonia syndrome after two doses of etanercept and died.
Figure 1.
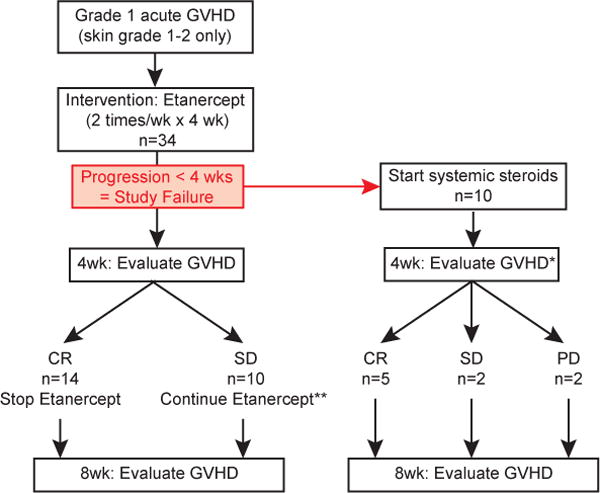
Study schema. Thirty-four patients diagnosed with skin grade 1–2 acute GVHD (overall grade 1) were treated with etanercept twice weekly for four weeks. Patients whose GVHD progressed to grade 2–4 within the first 28 days were considered study failures. In these patients, etanercept therapy was stopped and treatment with systemic steroids was initiated. GVHD was formally evaluated 4 and 8 weeks after diagnosis and onset of etanercept therapy. CR, complete resolution of GVHD; SD, stable disease; PD, progressive disease. *, 1 patient died of idiopathic pneumonia syndrome (IPS) before the 4 week GVHD evaluation; **, 7 of 10 patients with SD at the 4 week evaluation received an additional 4 week course of etanercept treatment.
The cumulative incidence of grade 2–4 acute GVHD at 1-year was 41% with a median time of onset of 24 days (range, 2–352 days) after grade 1 GVHD diagnosis (Figure 2A and Table 1). Only one study patient developed overall grade 3–4 acute GVHD, resulting in a cumulative incidence at 1-year of 3% (Figure 2A). This patient developed grade 3 GI GVHD (grade 3 overall GVHD) three days after grade 1 acute GVHD diagnosis and after a single dose of etanercept.
Figure 2.
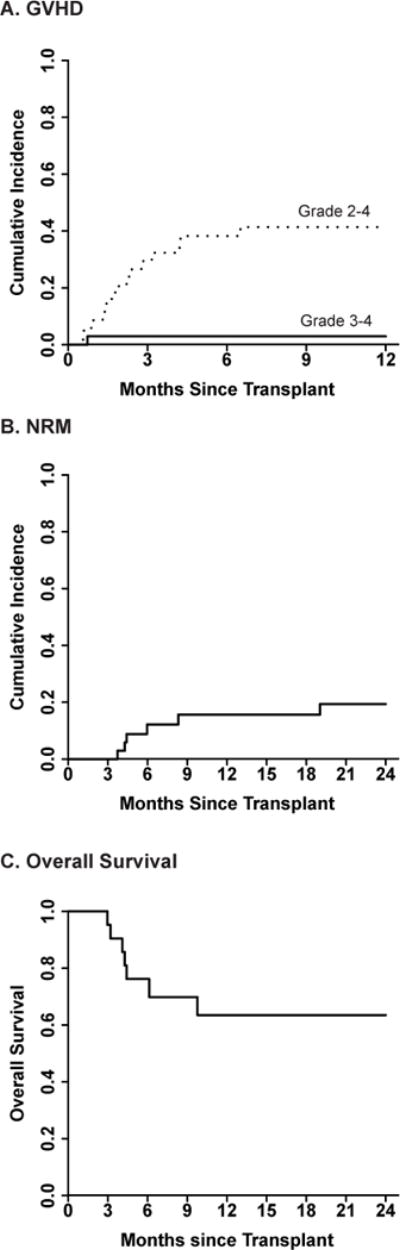
Incidence of acute graft-versus-host disease, NRM and OS in study patients (n=34). (A) 1-year cumulative incidence of acute GVHD. Dashed line, grade 2–4 acute GVHD; Solid line, grade 3–4 acute GVHD. (B) 2-year NRM. (C) 2-year OS.
Relapse and survival
The 2-year cumulative incidence of relapse was 32%, with a median time from HSCT to relapse of 251 days (range, 73–854). All-cause 2-year NRM was 19% (Figure 2B) and acute GVHD-related mortality was 13%. The causes of NRM were GVHD-related complications (n=3), idiopathic pneumonia syndrome (n=1) and a cardiac event unrelated to GVHD (n=1). With a median follow-up of 20 months (range, 3.6–63.4 months), overall 2-year survival was 63% (Figure 2C).
Comparison with contemporaneous controls
Patients diagnosed with grade 1 acute GVHD in the contemporaneous control cohort were treated with topical corticosteroids. Baseline clinical characteristics of the control cohort are reported in Table 1. There were no significant differences in baseline patient characteristics, including age, gender, race, diagnosis at transplant, disease risk status [34], degree of donor HLA-match, CMV serostatus, conditioning regimen, donor CD34+ cells infused, or GVHD prophylaxis between the control cohort and study patients. The incidence of infection events in the first 180 days after grade 1 GVHD diagnosis also was not different between the two groups (Table 2).
As expected, the median time from HSCT to the onset of grade 1 acute GVHD did not differ between the control cohort and study patients (32 versus 28 days, respectively; Table 1). However, control patients progressed to grade 2–4 acute GVHD sooner than the study patients, with a median time of 7 days from grade 1 diagnosis to progression, compared to 24 days in study patients (p=0.08; Table 1). Forty-three percent of control patients (n=12) progressed to grade 2–4 acute GVHD and required systemic corticosteroid therapy within 28 days of grade 1 GVHD diagnosis, compared to 29% of study patients. The cumulative incidence of grade 2–4 GVHD was higher in the control cohort compared with study patients (61% versus 41%, p=0.08; Figure 3A) at 1-year. Control patients also experienced a significantly higher incidence of severe grade 3–4 acute GVHD at 1-year (18% vs 3%, p=0.05; Figure 3B). The cumulative incidence of chronic GVHD (cGVHD) did not significantly differ between the two groups (p=0.4; Figure 3C).
Figure 3.
Incidence of graft-verus-host disease in study (n=34) and contemporaneous control (n=26) patients. (A) Cumulative incidence of grade 2–4 acute GVHD. Study versus control, p=0.08. (B) Cumulative incidence of grade 3–4 acute GVHD. Study versus control, p=0.05. (C) Cumulative incidence of moderate-to-severe chronic GVHD. Study versus control, p=0.4. Dashed line, contemporaneous control patients; Solid line, study patients treated with etanercept.
The cumulative incidence of relapse at 2-years in the control cohort was 41%, with a median time from HSCT to relapse of 95 days (range, 28–833 days). NRM was 15% at 2-years and was caused by GVHD-related complications (n=5) and an acute hemorrhagic event (n=1). The overall survival of the control cohort was 56% at 2-years. These were not different from the study patients who had incidences of relapse, NRM and survival of 32%, 19% and 63%, respectively.
Plasma biomarkers
Recently, biomarkers with relevance for GVHD have been identified using proteomics discovery and validation strategies [29–31, 35–37] and may provide opportunity for early intervention and improved survival after HSCT [36]. These include elafin and Reg3α as skin-specific [31] and GI-specific [30, 35] biomarkers of acute GVHD, respectively, and ST2 as a predictor for therapy resistant GVHD and death without relapse [29]. Although the small sample size in this study precluded the ability to predict later outcomes, such as non-relapse mortality and overall survival, we measured the concentrations of ST2, Reg3α and elafin in the plasma of study patients at the time of grade 1 acute GVHD diagnosis (day 0, prior to etanercept onset), as well as 14 and 28 days later. Findings were compared between study patients and contemporaneous control patients that did not get etanercept. ST2 and Reg3α were detectable in the plasma of study patients on day 0 and remained unchanged on day 28 (Figure 4A and B, left panels). In contrast, ST2 levels in the control cohort progressively increased between diagnosis and day 28 (medians, 19.2 to 46.6 ng/mL; p=0.004; Figure 4A). Reg3α also increased more than two-fold in the controls, from a median concentration of 8.6 ng/mL at diagnosis to 19.6 ng/mL on day 28 (p=0.005; Figure 4B). We compared the magnitude of change in ST2 and Reg3α concentrations in the first 28 days between study patients and contemporaneous controls by normalizing day 28 levels to day 0 levels (i.e. ratio of day 28 over day 0 concentration) for each patient. Study patients experienced less increase in ST2 (medians, 1.3-fold versus 2.0-fold; p=0.06) and Reg3α levels (medians, 0.7-fold versus 1.5-fold; p=0.01) than the control cohort 28 days after grade 1 GVHD diagnosis (Figure 4C). The levels of elafin also decreased during etanercept treatment from a median concentration of 17.8 ng/mL at diagnosis to 12.2 ng/mL on day 28 (p<0.0001; Figure 4D) but did not change in contemporaneous control patients that were not treated with etanercept (data not shown).
Figure 4.
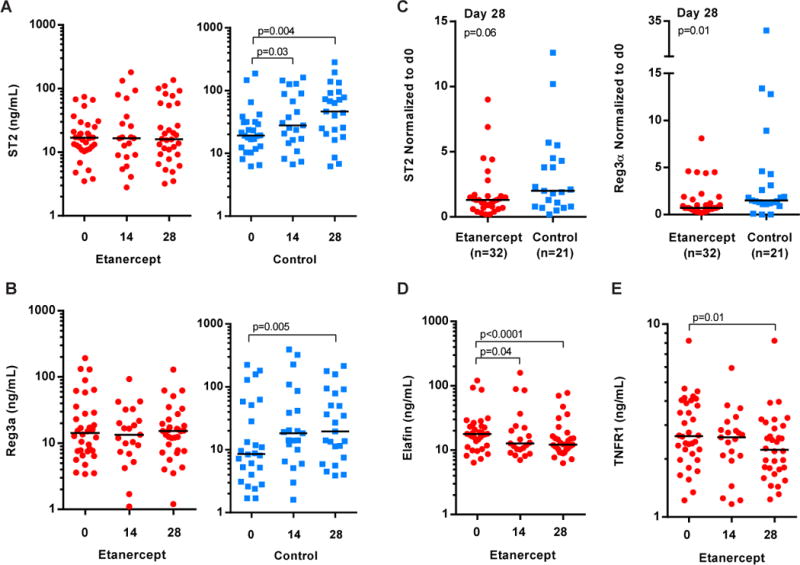
Biomarkers of acute GVHD in the plasma of study and contemporaneous control patients. Red circles denote patients in the study who received etanercept treatment. Blue squares are contemporaneous control patients. Horizontal black lines show the median values on each plot. (A and B) Plasma ST2 and Reg3α concentrations at the time of grade 1 diagnosis and treatment onset (day 0), day 14 and day 28. (A) Plasma ST2. (B) Plasma Reg3α. (C) Day 28 ST2 (left) and Reg3α (right) concentrations normalized to the concentration at GVHD diagnosis (ratio of day 28 over day 0). The number (n) of patients for whom samples were available to calculate the normalized value is indicated on each plot. Values plotted using linear-scale y-axis. (D) Plasma elafin. (E) Plasma TNFR1. (A, B, D, E) Study patient samples available for analysis: day 0, n=34; day 14, n=21; day 28, n=32. Control samples: day 0, n=26; day 14, n=21; day 28, n=23. Biomarker concentrations were plotted using a logarithmic-scale (Log10) y-axis to allow visualization of the full range of data at each time point.
In addition to the utility of TNFR1 as a biomarker for acute GVHD, the protein is also targeted by etanercept treatment. We therefore measured the concentration of TNFR1 in the plasma of patients to determine whether etanercept reduced plasma TNFR1 levels over the course of 28 days. We found that plasma TNFR1 levels in study patients did not differ from levels in the control cohort at the time of diagnosis (p=0.4; data not shown), but that levels were significantly reduced in study patients by day 28 (p=0.01; Figure 4E).
We also compared biomarker concentrations at each time point between patients whose grade 1 GVHD progressed to grade 2–4 at any time after HSCT and patients whose GVHD remained grade 1 or resolved with treatment. Plasma ST2 concentrations were significantly higher in both study and contemporaneous control patients whose GVHD progressed to grade 2–4 compared to patients whose GVHD did not progress, despite the small sample size of this study (Figure 5). This difference was evident in the control patients at the time of grade 1 GVHD diagnosis (day 0, p=0.02) and reached significance in study patients on day 14 (p=0.002). There were no significant differences in median ST2 concentrations between study and control patients at diagnosis (day 0) or at later time points, when the levels were analyzed by progression status (all p>0.2). Reg3α, elafin and TNFR1 plasma concentrations were not significantly different in patients whose GVHD progressed to grade 2–4 compared to patients whose GVHD remained grade 1 (data not shown).
Figure 5.
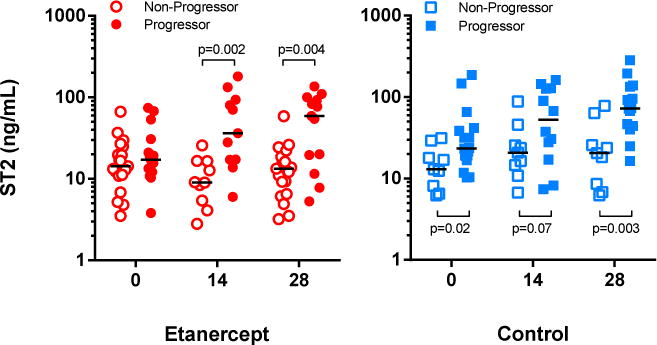
Patients whose acute GVHD progressed to grade 2–4 had higher plasma ST2 levels than patients whose GVHD did not progress. Plasma ST2 concentrations at the time of grade 1 diagnosis and treatment onset (day 0), day 14 and day 28 in patients whose GVHD remained grade 1 or resolved with treatment (Non-Progressor) and patients whose GVHD progressed to grade 2–4 (Progressor) at any time after diagnosis. (left) Study patients. Non-Progressors with samples available for analysis: day 0, n=19; day 14, n=10; day 28, n=19. Progressors: day 0, n=15; day 14, n=11; day 28, n=13. (right) Contemporaneous control patients. Non-Progressors with samples available for analysis: day 0, n=9; day 14, n=9; day 28, n=9. Progressors: day 0, n=17; day 14, n=12; day 28, n=14. Each plotted point represents a single patient. Horizontal black lines represent median ST2 concentrations plotted on a logarithmic-scale (Log10) y-axis.
Discussion
This study reports the clinical outcomes of patients with newly-diagnosed grade 1 (skin stage 1 or 2 only) acute GVHD who were treated with etanercept in addition to topical corticosteroids as first-line therapy.
The single-arm design and lack of published data on systemic treatment of grade 1 acute GVHD and its progression are limitations of this study. We constructed a contemporaneous control population consisting of patients who were diagnosed with grade 1 acute GVHD and were treated with topical corticosteroids but not etanercept at our institution during the study period, to provide a comparator for our study population while mitigating potential differences in clinical practice that might be encountered using historical controls. Control patients fulfilled all study eligibility criteria and were not selected on the basis of clinical characteristics. Although no significant differences between the study and control group were noted (Table 1), bias cannot be completely excluded, especially in the decision to treat grade 1 acute GVHD with topical corticosteroids as opposed to systemic corticosteroids at diagnosis. It is possible that a decision to treat with topical steroids at the time of diagnosis was indicative of a clinical opinion that the patient was lower-risk or that progression of acute GVHD was not an imminent risk. The small sample size of both the study and control cohorts also diminishes the ability of statistical methods to identify small differences in patient demographics. This study was powered based on enrollment of 50 patients over three years, but the decision was made to close the study after enrolling 34 patients over a five-year period (2008–2013), solely due to slow accrual.
Forty-three percent of control patients progressed to grade 2–4 acute GVHD within 28 days of grade 1 diagnosis with a median time to progression of 7 days. The 1-year cumulative incidence of grade 2–4 and grade 3–4 acute GVHD in these patients were 61% and 18%, respectively. It is encouraging that during the same time period, 29% of patients treated with etanercept on our study progressed to grade 2–4 acute GVHD within 28 days of grade 1 diagnosis with a median time to progression from grade 1 to grade 2–4 acute GVHD of 24 days. Study patients experienced 1-year cumulative incidence of grade 2–4 and grade 3–4 acute GVHD of 41% and 3%, respectively. Importantly, the decrease in grade 3–4 GVHD in study patients was not a consequence of fewer mismatched unrelated HSCT, which carry higher risk for more severe GVHD, and did not result in increased incidence of relapse in study patients. Etanercept treatment also did not change the incidence of chronic GVHD in the study patients compared to patients that did not receive etanercept. Nonetheless, only a prospective randomized trial can adequately compare outcomes after etanercept treatment with outcomes in the absence of etanercept.
We analyzed biomarkers of acute GVHD in plasma samples at grade 1 onset as well as 14 and 28 days after initiation of treatment. These analyses were exploratory and were not analyzed for association with clinical outcomes such as NRM and survival due to the small sample size in this study. Patients treated with etanercept demonstrated reduced biomarker levels compared with contemporaneous controls who did not receive etanercept. These findings trend with the clinical findings that study patients had fewer severe stage 3–4 skin rashes (8 vs. 12, respectively) and fewer manifestations of severe stage 3–4 GI GVHD (1 vs. 6, respectively) compared to contemporaneous control patients. The small sample size of this study, and the even lower frequency of severe GVHD manifestations in target organs, precluded statistical correlation of biomarker levels with GVHD at specific sites (i.e. skin and GI). However, when biomarker concentrations were analyzed according to GVHD progression status, ST2 levels were significantly higher in study and control patients whose GVHD progressed to grade 2–4 at any time after grade 1 diagnosis compared to patients who did not progress, in accord with the recent report that ST2 is an independent predictor for therapy resistant GVHD [29]. That Reg3α, elafin and TNFR1 levels did not significantly differ in progressors compared to non-progressors in this study may simply be a result of our small sample size coupled with a narrow dynamic range of change in these markers, and because these biomarkers are typically assessed as components of a multi-biomarker panel, not as stand-alone indicators [36]. It is important to continue monitoring these biomarkers in large, randomized studies of patients to better understand their clinical significance and utility in earlier diagnosis of GVHD, as well as in predicting which patients will respond to treatment or are at risk for swift progression to more severe GVHD [29–31, 35–39]. Current reliance on clinical symptomology alone for GVHD diagnosis can result in a very short window of opportunity to treat mild GVHD before it becomes a more severe manifestation, a problem that is compounded by the fact that many patients, prior to their grade 1 GVHD diagnosis, are outpatients and are thus only seen in clinic on a weekly basis. These factors represent significant challenges in the study of grade 1 acute GVHD. Earlier identification of the first hint of GVHD may allow more efficacious treatment and delay or spare altogether the need for systemic steroid therapy by reducing the progression of acute GVHD [29].
Our findings suggest that grade 1 acute GVHD is not a benign process. Diagnosis of grade 1 acute GVHD among contemporaneous control patients resulted in a 1-year cumulative incidence of grade 2–4 acute GVHD of 61% despite initiating treatment with topical corticosteroids at grade 1 diagnosis. However, more effective treatment of grade 1 GVHD is hindered by a paucity of trials that address its clinical care. Many groups preemptively treat grade 1 acute GVHD with systemic corticosteroids based on risk assessment (i.e. mismatched, unrelated HSCT) or clinician best guess of potential for worsening acute GVHD. However, despite common use, systemic steroid therapy has not proven to unilaterally benefit this patient population and carries significant risk of infection and relapse secondary to immunosuppression [9]. Potential long-term complications associated with prolonged steroid use, such as hypertension, hyperglycemia, and avascular necrosis may also impact quality of life and influence the economic burden after HSCT. This trial uniquely studied the first-line treatment and evaluation of grade 1 acute GVHD. Treatment of newly diagnosed grade 1 acute GVHD with etanercept reduced the incidence of progression to grade 2–4 acute GVHD within 28 days compared with contemporaneous patients who were treated with topical corticosteroids but did not receive etanercept. Future prospective studies are needed to better identify patients with grade 1 acute GVHD who may warrant therapy to prevent progression to more severe GVHD as well as to provide published clinical outcomes associated with grade 1 acute GVHD.
Table 3.
Causes of transplant-related mortality
| Cause | Deaths (No.) |
|---|---|
| GVHD-related complication | 3 |
| Idiopathic pneumonia syndrome | 1 |
| Cardiac | 1 |
Acknowledgments
This protocol was developed at the American Society for Blood and Marrow Transplantation Clinical Research Training Course (Park City, UT). We thank the patients, their families, and the clinical personnel who participated in this study; the BMT Program Team at the Clinical Trials Office at the University of Michigan for data collection and management; the BMT Program research nurses and Joel Whitfield of the Immunologic Monitoring Core of the University of Michigan Cancer Center for research support, without which this study would not have been possible. This study was supported by grants to S.W.C. from St. Baldrick’s Foundation, the Michigan Institute for Clinical and Health Research, and the National Institutes of Health (1K23AI091623) and to S.P. from the National Institutes of Health (R01CA174667). S.W.C. is an A. Alfred Taubman Institute/Edith Briskin Emerging Scholar.
Support: This study was supported by grants to S.W.C. from St. Baldrick’s Foundation, the Michigan Institute for Clinical and Health Research, and the National Institutes of Health (1K23AI091623) and to S.P. from the National Institutes of Health (R01CA174667). S.W.C. is an A. Alfred Taubman Institute/Edith Briskin Emerging Scholar.
Footnotes
Financial Disclosure Statement: The authors have no financial relationships to disclose.
References
- 1.Copelan EA. Hematopoietic stem-cell transplantation. The New England journal of medicine. 2006;354:1813–26. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–61. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. The New England journal of medicine. 2010;363:2091–101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jagasia M, Arora M, Flowers ME, Chao NJ, McCarthy PL, Cutler CS, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119:296–307. doi: 10.1182/blood-2011-06-364265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–8. [PubMed] [Google Scholar]
- 6.Storb R, Gyurkocza B, Storer BE, Maloney DG, Sorror ML, Mielcarek M, et al. Allogeneic hematopoietic cell transplantation following minimal intensity conditioning: predicting acute graft-versus-host disease and graft-versus-tumor effects. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2013;19:792–8. doi: 10.1016/j.bbmt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Storb R, Gyurkocza B, Storer BE, Sorror ML, Blume K, Niederwieser D, et al. Graft-versus-host disease and graft-versus-tumor effects after allogeneic hematopoietic cell transplantation. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31:1530–8. doi: 10.1200/JCO.2012.45.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alousi AM, Bolanos-Meade J, Lee SJ. Graft-versus-host disease: state of the science. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2013;19:S102–8. doi: 10.1016/j.bbmt.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deeg HJ. How I treat refractory acute GVHD. Blood. 2007;109:4119–26. doi: 10.1182/blood-2006-12-041889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hings IM, Severson R, Filipovich AH, Blazar BR, Kersey JH, Ramsay NK, et al. Treatment of moderate and severe acute GVHD after allogeneic bone marrow transplantation. Transplantation. 1994;58:437–42. doi: 10.1097/00007890-199408270-00008. [DOI] [PubMed] [Google Scholar]
- 11.Levine JE, Paczesny S, Mineishi S, Braun T, Choi SW, Hutchinson RJ, et al. Etanercept plus methylprednisolone as initial therapy for acute graft-versus-host disease. Blood. 2008;111:2470–5. doi: 10.1182/blood-2007-09-112987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacMillan ML, Weisdorf DJ, Wagner JE, DeFor TE, Burns LJ, Ramsay NK, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2002;8:387–94. doi: 10.1053/bbmt.2002.v8.pm12171485. [DOI] [PubMed] [Google Scholar]
- 13.Weisdorf DJ, Snover DC, Haake R, Miller WJ, McGlave PB, Blazar B, et al. Acute upper gastrointestinal graft-versus-host disease: clinical significance and response to immunosuppressive therapy. Blood. 1990;76:624–9. [PubMed] [Google Scholar]
- 14.Levine JE, Logan B, Wu J, Alousi AM, Ho V, Bolanos-Meade J, et al. Graft-versus-host disease treatment: predictors of survival. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16:1693–9. doi: 10.1016/j.bbmt.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacMillan ML, DeFor TE, Weisdorf DJ. The best endpoint for acute GVHD treatment trials. Blood. 2010;115:5412–7. doi: 10.1182/blood-2009-12-258442. [DOI] [PubMed] [Google Scholar]
- 16.Saliba RM, Couriel DR, Giralt S, Rondon G, Okoroji GJ, Rashid A, et al. Prognostic value of response after upfront therapy for acute GVHD. Bone marrow transplantation. 2012;47:125–31. doi: 10.1038/bmt.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westin JR, Saliba RM, De Lima M, Alousi A, Hosing C, Qazilbash MH, et al. Steroid-Refractory Acute GVHD: Predictors and Outcomes. Advances in hematology. 2011;2011:601953. doi: 10.1155/2011/601953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alousi AM, Weisdorf DJ, Logan BR, Bolanos-Meade J, Carter S, Difronzo N, et al. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood. 2009;114:511–7. doi: 10.1182/blood-2009-03-212290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitko CL, Paczesny S, Yanik G, Braun T, Jones D, Whitfield J, et al. Plasma elevations of tumor necrosis factor-receptor-1 at day 7 postallogeneic transplant correlate with graft-versus-host disease severity and overall survival in pediatric patients. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14:759–65. doi: 10.1016/j.bbmt.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi SW, Kitko CL, Braun T, Paczesny S, Yanik G, Mineishi S, et al. Change in plasma tumor necrosis factor receptor 1 levels in the first week after myeloablative allogeneic transplantation correlates with severity and incidence of GVHD and survival. Blood. 2008;112:1539–42. doi: 10.1182/blood-2008-02-138867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill GR, Teshima T, Rebel VI, Krijanovski OI, Cooke KR, Brinson YS, et al. The p55 TNF-alpha receptor plays a critical role in T cell alloreactivity. Journal of immunology. 2000;164:656–63. doi: 10.4049/jimmunol.164.2.656. [DOI] [PubMed] [Google Scholar]
- 22.Korngold R, Marini JC, de Baca ME, Murphy GF, Giles-Komar J. Role of tumor necrosis factor-alpha in graft-versus-host disease and graft-versus-leukemia responses. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2003;9:292–303. doi: 10.1016/s1083-8791(03)00087-9. [DOI] [PubMed] [Google Scholar]
- 23.Korth-Bradley JM, Rubin AS, Hanna RK, Simcoe DK, Lebsack ME. The pharmacokinetics of etanercept in healthy volunteers. The Annals of pharmacotherapy. 2000;34:161–4. doi: 10.1345/aph.19126. [DOI] [PubMed] [Google Scholar]
- 24.Choi SW, Stiff P, Cooke K, Ferrara JL, Braun T, Kitko C, et al. TNF-inhibition with etanercept for graft-versus-host disease prevention in high-risk HCT: lower TNFR1 levels correlate with better outcomes. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18:1525–32. doi: 10.1016/j.bbmt.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boeckh M, Huang M, Ferrenberg J, Stevens-Ayers T, Stensland L, Nichols WG, et al. Optimization of quantitative detection of cytomegalovirus DNA in plasma by real-time PCR. Journal of clinical microbiology. 2004;42:1142–8. doi: 10.1128/JCM.42.3.1142-1148.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2002;34:7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 27.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–8. [PubMed] [Google Scholar]
- 28.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2005;11:945–56. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Vander Lugt MT, Braun TM, Hanash S, Ritz J, Ho VT, Antin JH, et al. ST2 as a marker for risk of therapy-resistant graft-versus-host disease and death. N Engl J Med. 2013;369:529–39. doi: 10.1056/NEJMoa1213299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrara JL, Harris AC, Greenson JK, Braun TM, Holler E, Teshima T, et al. Regenerating islet-derived 3-alpha is a biomarker of gastrointestinal graft-versus-host disease. Blood. 2011;118:6702–8. doi: 10.1182/blood-2011-08-375006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paczesny S, Braun TM, Levine JE, Hogan J, Crawford J, Coffing B, et al. Elafin is a biomarker of graft-versus-host disease of the skin. Science translational medicine. 2010;2:13ra2. doi: 10.1126/scitranslmed.3000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 33.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 34.ASBMT. RFI 2013 – Disease Classifications Corresponding to CIBMTR Classifications. 2013 [Google Scholar]
- 35.Harris AC, Ferrara JL, Braun TM, Holler E, Teshima T, Levine JE, et al. Plasma biomarkers of lower gastrointestinal and liver acute GVHD. Blood. 2012;119:2960–3. doi: 10.1182/blood-2011-10-387357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levine JE, Logan BR, Wu J, Alousi AM, Bolanos-Meade J, Ferrara JL, et al. Acute graft-versus-host disease biomarkers measured during therapy can predict treatment outcomes: a Blood and Marrow Transplant Clinical Trials Network study. Blood. 2012;119:3854–60. doi: 10.1182/blood-2012-01-403063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paczesny S, Krijanovski OI, Braun TM, Choi SW, Clouthier SG, Kuick R, et al. A biomarker panel for acute graft-versus-host disease. Blood. 2009;113:273–8. doi: 10.1182/blood-2008-07-167098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levine JE, Paczesny S, Sarantopoulos S. Clinical applications for biomarkers of acute and chronic graft-versus-host disease. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18:S116–24. doi: 10.1016/j.bbmt.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paczesny S. Discovery and validation of graft-versus-host disease biomarkers. Blood. 2013;121:585–94. doi: 10.1182/blood-2012-08-355990. [DOI] [PMC free article] [PubMed] [Google Scholar]


