Abstract
Background
We have previously shown that a novel synthetic peptide for ocular delivery (POD) can efficiently compact DNA and deliver it to cells in vitro. This observation prompted us to develop use of POD as a nonviral vector in vivo.
Methods
POD peptide was modified using poly(ethylene) glycol (PEG-POD) and used to compact DNA into nanoparticles that were then analysed using electron microscopy, dynamic light scattering, and fluorescent labeling. Transfection efficiency and localization were determined 48 h post-injection into the subretinal space of the mouse eye using luciferase and LacZ, respectively. Efficiency of ocular transfection was compared to two other PEGylated peptides: PEG-TAT and PEG-CK30.
Results
PEG-POD can compact DNA and form discrete nanoparticles of approximately 136 nm that can penetrate and transduce the retinal pigment epithelium (RPE) in vivo. PEG-POD significantly increased expression of plasmid DNA by 215-fold, PEG-TAT by 56.52-fold, and PEG-CK30 by 24.73-fold relative to DNA injected alone. In all cases β-galactosidase was observed primarily in the RPE layer after subretinal injection. Electrophysiological analyses of PEG-POD transduced retina indicates an absence of PEG-POD-mediated toxicity. PEG-POD can protect plasmid DNA from DNaseI digestion, resulting in significant transfection of the lung after intravenous injection in mice.
Conclusions
PEG-POD was found to significantly increase gene delivery relative to both DNA alone and other pegylated peptides. These findings highlight the use of pegylated peptides, and specifically PEG-POD, as novel gene delivery vectors.
Keywords: cell penetrating peptide, gene delivery, nonviral, POD
Introduction
The development of nonviral gene transfer technologies for the treatment of genetic diseases has seen significant progress over the last 10 years. However, efficient gene transfer to post-mitotic cells or tissues in vivo, such as the retina or brain, remains a significant barrier for the widespread use of nonviral vectors in the clinic. Retinitis pigmentosa (RP) and age-related macular degeneration (AMD) collectively describe a common group of disorders that lead to blindness. In RP and AMD, the retinal photoreceptor cells or the retinal pigment epithelium (RPE) are among the first group of cells to degenerate. Hence, the protection of these cells is a target application of nonviral gene delivery.
Recently, we described a novel cell penetrating peptide for ocular delivery (POD) that can bind the plasma membrane of human embryonic retinoblasts in culture and enter the cytoplasm [1]. The design of POD [CGGG(ARKKAAKA)4] was based on the glycosaminoglycan-binding domain of acidic and basic fibroblast growth factor. When covalently linked to small dye molecules such as lissamine (0.6 kDa), POD retained the ability to bind and traverse the plasma membrane. POD was demonstrated to enter retinal cells in vivo, including RPE, photoreceptors and ganglion cells. Although this property of POD may potentially be useful for the delivery of small molecule drugs to the retina, the ability to deliver substantially larger molecules such as DNA (approximately 4.3 MDa) to post-mitotic cell nuclei in vivo remains a challenge. To achieve transgene expression in post-mitotic cells in vivo, POD/DNA complexes would need to overcome the barrier of the plasma membrane, escape endosomes, avoid degradation in the cytoplasm and cross the intact nuclear membrane pore [2]. Although viruses or recombinant viral gene transfer vectors are very efficient at overcoming each of these barriers, nonviral vectors have thus far enjoyed limited success, preventing their widespread use in gene transfer to post-mitotic tissues in vivo.
Because POD is a positively-charged peptide, we wished to examine whether POD may compact and protect DNA from endonucleases at the same time as retaining its cell-penetrating properties. The addition of poly(ethylene) glycol (PEG) has been previously demonstrated to stabilize protein–nucleic acid complexes [3]. Therefore, we postulated whether DNA compacted with pegylated POD may form discrete nanoparticles suitable as a formulation for gene delivery to post-mitotic tissue in vivo.
Materials and methods
Plasmids, peptide and cell lines
pCAGLuc was cloned by first placing the Luciferase cDNA from pGL3-Control (Promega, Madison, WI, USA) into pBluescript II KS (Stratagene, La Jolla, CA, USA) using HindIII and XbaI. An XhoI/NotI fragment of this plasmid was then inserted into pCAGEN (kindly provided by C. Cepko, Harvard Medical School, Boston, MA). A SmaI/NotI fragment of pCMVb (Clontech, Mountain View, CA, USA) was cloned into EcoRV/NotI-digested pCAGEN to generate pCAGLacZ. c-POD, CGGG(ARKKAAKA)4, was generated at Tufts University Peptide Synthesis Core Facility (Boston, MA, USA) and purified by high-performance liquid chromatography (HPLC). TAT (CGGGGYGRKKRRQRRR) was synthesized and purified by HPLC by Sigma Genosys (Woodlands, TX, USA). CK30 (CK30) was synthesized and purified by HPLC by PolyPeptide Laboratories (Torrance, CA, USA). Human embryonic retinoblasts (HER) 911 cells [4] were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA).
in vivo delivery of POD/DNA complexes
All experiments involving animals were in accordance with the Statement for the Use of Animals in Ophthalmic and Vision Research, set out by the Association for Research in Vision and Ophthalmology. C57BL/6J and BALB/cJ mice were purchased from Jackson Laboratories and maintained under 12:12 h dark/light cycles in accordance with federal, state, and local regulations. Mice were anesthetized by intraperitoneal injection of Xylazine (Xyla-Ject, Phoenix Pharmaceutical, Inc., St Joseph, MO, USA; 10 mg/ml)/Ketamine HCl (Ketaset, Fort Dodge Animal Health, Fort Dodge, IA, USA; 1 mg/ml). Subretinal injections were performed with a 32-G needle (Becton Dickinson, Franklin Lakes, NJ, USA) and a 5 µl glass syringe (Hamilton, Reno, NV, USA) by a trans-scleral trans-choroidal approach. Animals were sacrificed by CO2 inhalation followed by cervical dislocation.
c-POD/DNA compaction: in vitro and in vivo delivery
pCAGLuc was compacted using c-POD as previously described [1]. Briefly, 1.8 nmol c-POD and 2 µg (0.466 pmol) of DNA were each suspended in 15 µl of 100 mm sodium phosphate buffer. The DNA and c-POD solutions were mixed and incubated for 30 min prior to addition to the media of HER cells. Under serum starvation conditions, the cells were incubated with serum free DMEM for 24 h prior to transfection. Under nonserum starved conditions, the cells were maintained in 10% FBS until just prior to transfection, at which time the media was changed to 2% FBS. After 48 h, cells were prepared for luciferase assay according to the manufacturer’s protocol (Promega). Live cells were visualized with light and fluorescent microscopy using an Olympus IX51 with differential interference contrast and appropriate fluorescent filters (Olympus, Tokyo, Japan). Images were captured using a Retiga 2000R FAST camera and QCapture Pro 5.0 (QImaging, British Columbia, Canada). For use in explants and in vivo injection, c-POD/DNA complexes were prepared as above using 1.8 nmol c-POD to compact either 0.233 or 0.466 pmol DNA. Explants were prepared from 6–8-week-old C57BL/6J wild-type mice by enucleation and removal of the anterior chamber. The retina and RPE/sclera were separated, flattened and placed in 500 µl of 5% CO2 equilibrated DMEM with 10% FBS and 100 U/ml Pen-Strep. Compacted pCAGLuc (2 µg) or the plasmid alone was added to explants and incubated for 48 h. For injections in vivo, 0.2 µg of compacted DNA or naked DNA was injected into the subretinal space as described above. The eyes were harvested 48 h later and the anterior chamber removed. In both explants and in vivo injections, tissue was homogenized using a VWR PowerMax AHS 200 homogenizer (VWR, West Chester, PA, USA) in homogenization buffer (50 mm Tris HCl, pH 8.0, 150 mm NaCl) and assayed for luciferase expression according to the manufacturer’s protocol (Promega) using a Glomax 20/20 luminometer over a 10-s integration (Promega). Protein concentration was measured using a Quick Start Bradford Protein Assay (Bio-Rad, Hercules, CA, USA) and comparing to bovine serum albumin (Bio-Rad) standards in corresponding buffer.
Preparation and characterization of pegylated c-POD, TAT and CK30 nanoparticles
Peptides were resuspended in 0.1m sodium phosphate (pH 7.2), 5 mm ethylenediaminetetraacetic acid to form a 20 mg/ml solution. An equimolar amount of methoxy-PEG-maleimide − 10 kDa (Nektar Transforming Therapeutics, San Carlos, CA, USA) was resuspended in the same volume of dimethylsulfoxide. PEG was added dropwise to the peptide over approximately 10 min, vortexing between drops. The solution was shaken overnight at room temperature, and dialysed using a Bio-Gel P6 column (Bio-Rad) into 0.1% trifluoroacetic acid for POD and TAT and 50 mm ammonium acetate for CK30 [5]. The pegylated POD (PEG-POD) was quantified by Coomassie stain of a Tris-HCl gel (Bio-Rad) using a c-POD standard curve. Both CK30 and TAT are not within the detectable limits of Coomassie stain, and so protein concentration was performed using BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA) using the un-pegylated peptide to create a standard curve. DNA was compacted by diluting the plasmid in water to a final concentration of 0.2 µg/µl and added dropwise to PEG-POD or PEG-TAT with a final ratio 1.8 nmol peptide: 2 µg of DNA (0.466 pmol pCAGLuc or 0.367 pmol pCAGLacZ), vortexing to mix. The nanoparticles were dialysed three times in 5% dextrose using Biomax 10K centrifugal filter (Ultrafree, Millipore Corp., Billerica, MA, USA) and stored at 4 °C. PEG-CK30 particles were compacted following a previously described protocol [5,6], but briefly, 0.9 ml of DNA (200 µg/ml) was added to 0.1 ml of 7.1 mg/ml PEG-CK30 in 0.1 ml aliquots over approximately 2 min at 25 °C. The compacted DNA was dialysed overnight into 0.9% filtered NaCl at 4 °C (Tube-O-Dialyser, G-Biosciences, St Louis, MO, USA) and concentrated by centrifugation (Ultrafree, Millipore Corp.; nominal molecular weight limit 100 kDa). Plasmid compaction for all peptides was verified by reduced mobility through a 1% agarose gel (Invitrogen), which was relieved after 15 min of incubation with 0.25% Trypsin (Invitrogen) at 37 °C. PEG-POD particles were analysed by incubation on glow-discharged Formvar copper grids (Electron Microscopy Sciences, Hatfield, PA, USA), stained with 0.4% uranyl acetate, and visualized using CM10 Transmission Electron Microscope (FEI, Hillsboro, OR, USA) using Digital Micrograph software (Gatan, Pleasanton, CA, USA). PEG-POD particles and control samples were diluted to 0.2 mg/ml and analysed at 25°C using a BI-200SM research goniometer and laser light scattering system (Brookhaven Instruments Corporation, Holtsville, NY, USA) with 532 nm laser light. Data was collected for 5 min for each sample and the mean particle diameter determined by quadratic fit using software supplied by the manufacturer.
Localization of compacted DNA in vitro
Rhodamine labeled DNA (pGeneGrip; Gene Therapy Systems, San Diego, CA, USA) was compacted with either c-POD or PEG-POD as described. Compactions were added to HER cells grown to approximately 80% confluency on Lab Tek-II chamber slides (NUNC, Thermo Fisher Scientific, Rochester, NY, USA) in serum free DMEM to a final concentration of 0−66 µg/ml and incubated at 37°C, 5% CO2 for 24 h (for each condition, n = 4). Cells were fixed with 4% formalin for 15 min at room temperature. Imaging was performed as above using an Olympus BX51 microscope (for each condition, n = 4).
PEG-peptide/DNA nanoparticle transfections in vivo
Subretinal injections were performed as above and luciferase activity assayed as for c-POD compactions. All injections were performed in a 2 µL total volume. For tail vein injections, compacted or naked DNA was injected in a total volume of 200 µl with 0.01% fast green (Thermo Fisher Scientific) using a 25-G needle (Becton Dickinson). Forty-eight hours after injection, mice were sacrificed using CO2 and perfused with 800 µl of PBS through the left ventricle. The organs were harvested, placed in 500 µl of Luciferase Cell Culture Lysis Reagent (Promega) with 20 µl/ml of a protease inhibitor cocktail (Sigma, St Louis, MO, USA) and homogenized for 20 s. Homogenates were centrifuged at 4°C for 10 min at 12000 g and assayed for luciferase activity as per manufacturer’s instructions (Promega) and protein concentration as mentioned above. Standard curve values for the luminometer were determined using QuantiLum Recombinant Luciferase (Promega). Conversion from relative light units (RLU) to pg was calculated as: luciferase (pg) = (3.696 × 10−5 × RLU) −0.0815 (R2 = 0.9999). For β-galactosidase expression, 1.2 µg of either compacted or naked pCAGLacZ was injected into the subretinal space of BALB/cJ mice. After 48 h, eyes were enucleated, fixed in 0.25% glutaraldehyde for 30 min, and washed three times in PBS for 30 min. The eyes were incubated in X-Gal solution (FisherBiotech, Fisher Scientific, Fair Lawn, NJ, USA) for 16–18 h and then rinsed in phosphate buffer (pH 7.4) for 45 min. The eyes were fixed for 24 h in 4% PFA, dehydrated, embedded, and 18-µm sections were collected. Whole eye bright-field images were taken using a Nikon C-FMC microscope (Nikon, Tokyo, Japan) prior to sectioning. For all conditions, n = 4.
Serum stability assay
Seven hundred nanograms of PEG-POD compacted or naked pCAGLuc was treated with 2.5 U or 0.25 U DNaseI (Sigma) at 37 °C for 15 min, after which 10 µg of pronase (Sigma) was added and incubated for a further 10 min at 37 °C. The samples were then run on a 1% agarose gel to check for DNA degradation.
Electroretinography
Mice were injected into the subretinal space with 1 µl of PEG-POD~Luc nanoparticles (700 ng) or 1 µl of 5% dextrose buffer and analysed by electroretinography 48 h later. After overnight dark-adaptation, the mice were anesthetized as described above, pupils dilated with 1% Tropicamide (Akorn, Inc., Lake Forest, IL, USA), and scotopic electroretinograms recorded at two different flash intensities (−10 and 1 dB) using contact lens electrodes and the UTAS system with BigShot ganzfeld (LKC Technologies, Inc., Gaithersburg, MD, USA). Five to ten flashes were averaged for the high-low intensities.
Statistical analysis
All data analyses were performed using Prism Software 4 (GraphPad Software, Inc., San Diego, CA, USA). During analysis of the data, there was shown to be a highly significant difference (F-test, p < 0.0001) between the variances of the data sets that was mean dependent. This precludes analysis by Student’s t-test, which assumes equal variances between samples [7]. The data (RLU/mg) were logarithmically transformed to create a Gaussian distribution, eliminating the variability between data set variance, and allowing use of the parametric t-test [7]. All statistical analyses were done using log-transformed data sets. Although all data points are shown, only those above an uninjected mean ±3 SD are used in the statistical analysis and to calculate the means [5].
Results
Pegylated POD compacts DNA to form discrete nanoparticles
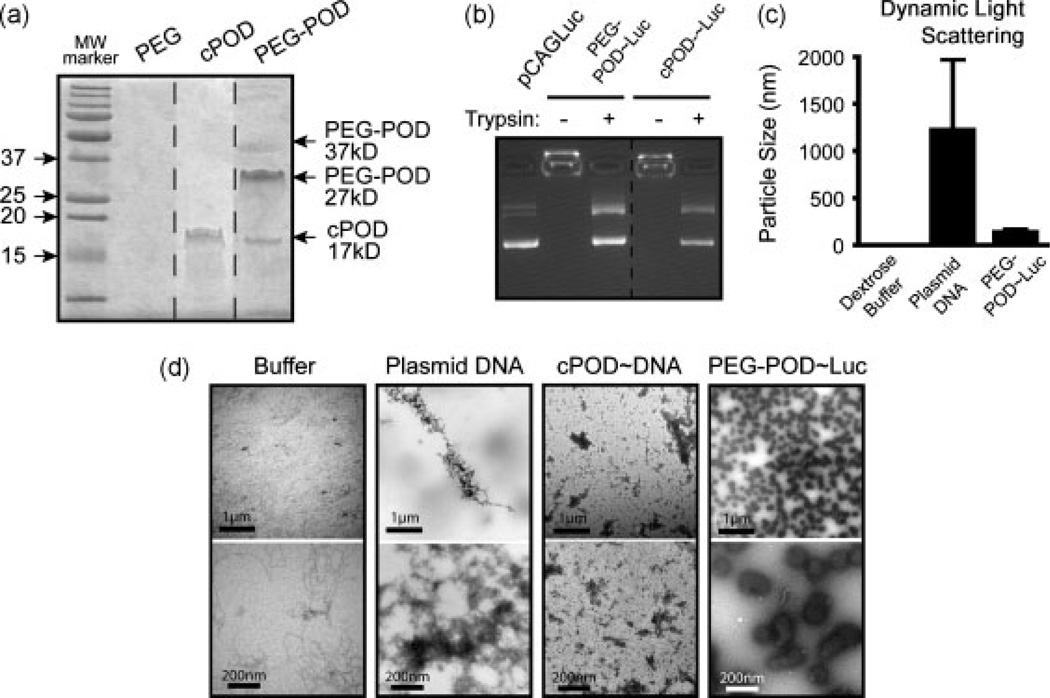
We functionalized the free sulfhydryl bond on the cysteine (c) of c-POD with a 10 kDa PEG-maleimide group. Although the molecular weight of c-POD after synthesis was determined by mass spectroscopy to be the predicted 3.5 kDa, we observed a unique band corresponding to approximately 17.5 kDa on a Coomassie stained gel, suggesting that c-POD forms pentamers in solution (Figure 1a). Functionalization of this 17.5-kDa product with 10-kDa PEG generated a gel shift to the expected 27.5-kDa protein (Figure 1a). A minor product was also detected at 37 kDa, suggesting that some c-POD pentamers may be functionalized by two 10-kDa PEG molecules (Figure 1a). Similar to c-POD [1], pegylated POD (PEG-POD) compacted DNA and prevented its migration in an agarose gel (Figure 1b). DNA from such complexes could be readily released by incubation of the PEG-POD/DNA complex with trypsin (Figure 1b). The PEG-POD/DNA complexes were suspended in 5% dextrose buffer for all experiments.
Figure 1.
PEG-POD and c-POD can both compact DNA but PEG-POD compacts DNA to form small, discrete nanoparticles. (a) cPOD is an approximately 3.5 kDa peptide that migrates at approximately 17.5 kDa as a pentamer. The addition of a 10 kDa PEG increased the molecular weight to primarily a 27.5 kDa protein, with higher species also seen. (b) Both c-POD and pegylated c-POD (PEG-POD) are able to compact DNA and retard movement during electrophoresis. Compaction is relieved upon degradation of the peptide with trypsin. (c) DLS indicated a mean average particle radius of 136 ± 27.2 nm for PEG-POD~Luc nanoparticles, 1.6 ± 0 nm for buffer alone, and 1221 ± 753.4 nm for the pCAGLuc plasmid alone. DLS shown as mean ± SD. (d) Transmission electron microscopy revealed that PEG-POD compacted DNA particles were smaller and more spherical and homogeneous in size than uncompacted plasmid DNA, which formed large irregular aggregates, or c-POD compacted DNA. Size bar, top = 1 µm, bottom = 200 nm
Closer examination of particle size by dynamic light scattering (DLS) revealed a size range of 136 ± 27.2 nm. By contrast, the controls (i.e. buffer or uncompacted DNA) were determined to be 1.6 ± 0 nm and 1221 ± 753.4 nm, respectively (Figure 1c). The large standard deviation for DNA is not surprising given that DLS is primarily accurate for spherical particles and may be detecting rotational as well as translational motion of the plasmid DNA. Visualization of PEG-POD complexed with pCAGLuc plasmid DNA (PEG-POD~Luc) by transmission electron microscopy revealed relatively homogeneous 100–175 nm spherical nanoparticles (Figure 1d). In conclusion, PEG-POD compacts DNA and forms discrete nanoparticles in solution.
Relative to c-POD, PEG-POD nanoparticles do not aggregate and are resistant to DNAseI
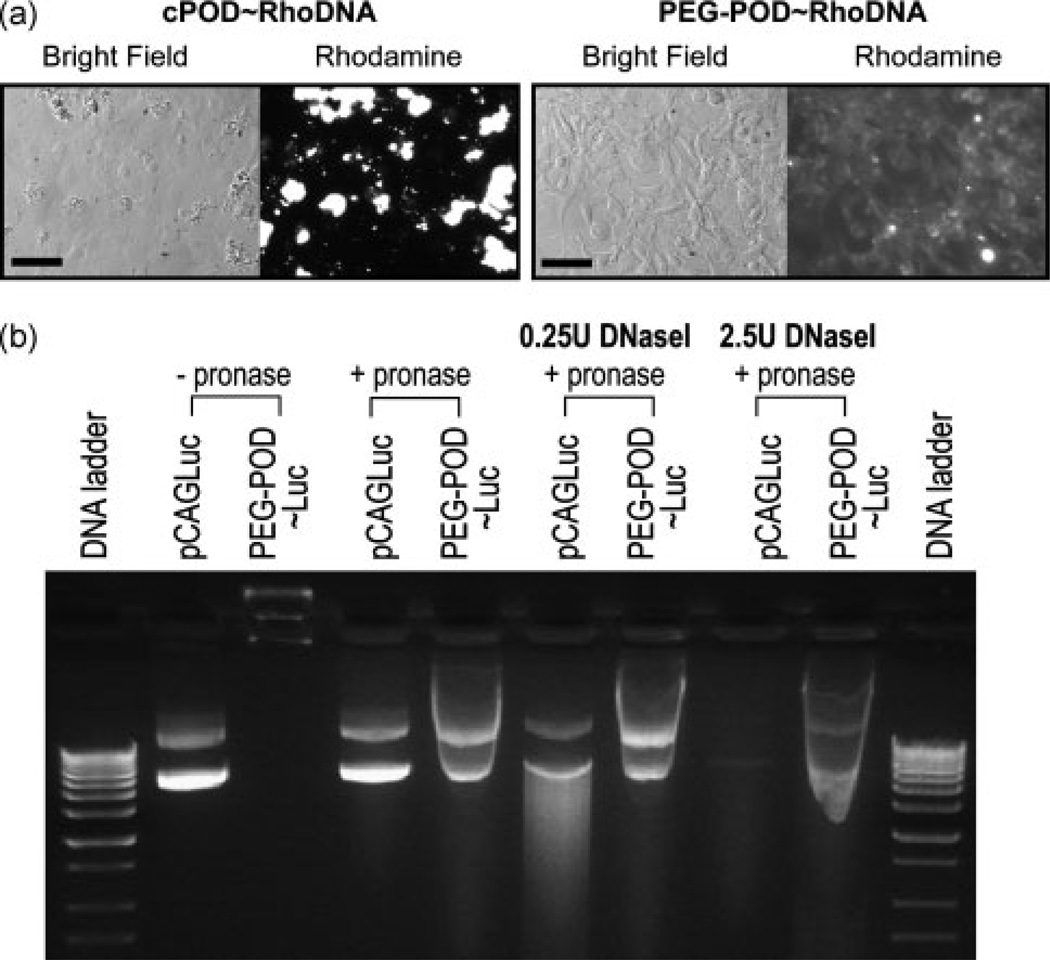
One key difference between actively dividing and quiescent cells in the context of gene delivery is that, during mitosis, the nuclear membrane disintegrates and allows easier access of large molecules such as DNA into the nucleus. The intact nuclear membrane in post-mitotic cells in vivo is considered to be a significant barrier to nonviral gene transfer [8]. We compacted rhodamine-labeled plasmid DNA using c-POD and found that, even at the level of light-microscopy, large c-POD/DNA aggregates with a size in the range 10–100 µm were readily visible when incubated with HER 911 cells in culture (Figure 2a). Unlike c-POD, PEG-POD did not form large aggregates when complexed with DNA and incubated with HER cells (Figure 2a).
Figure 2.
PEG-POD compacted DNA nanoparticles have reduced aggregation and increased protection against DNaseI. (a) Rhodamine-labeled DNA was complexed with c-POD or PEG-POD and added to HER cells in culture and observed after 24 h. c-POD/DNA complexes form large aggregates in cell culture while PEG-POD/Rhodamine labeled DNA nanoparticles showed a diffuse punctate pattern across cells. Scale bar = 50 µm. (b) In the absence of pronase, plasmid migration was inhibited by the compaction of the PEG-POD~Luc nanoparticles. Nanoparticle DNA was released following a 10-min pronase incubation. Treatment with a low (0.25 U) or high (2.5 U) dose of DNaseI led to partial and complete digestion of pCAGLuc, respectively. However, PEG-POD nanoparticles remained largely intact under both conditions
Serum nuclease activity significantly decreases the half-life of plasmid DNA. DNA injected intravenously into mice is degraded within 10 min [9], making protection from degradation an important property for genes to be successfully delivered in vivo by intravenous injection. DNaseI is an endonuclease found in human serum and many tissues in the body [10] and it has been shown that serum nuclease activity is primarily derived from DNaseI [11]. Hence, DNaseI was utilized to test the potential stability of pegylated POD/DNA nanoparticles in serum. At both a low (0.25 U) and high (2.5 U) concentration of DNaseI, plasmid DNA compacted with PEG-POD showed substantially less degradation than naked DNA (Figure 2b). The addition of pronase led to the release of the nanoparticle-associated DNA. We conclude that PEG-POD/DNA complexes are protected from DNaseI degradation and therefore have potentially increased serum stability.
c-POD can deliver DNA in vitro but not in vivo
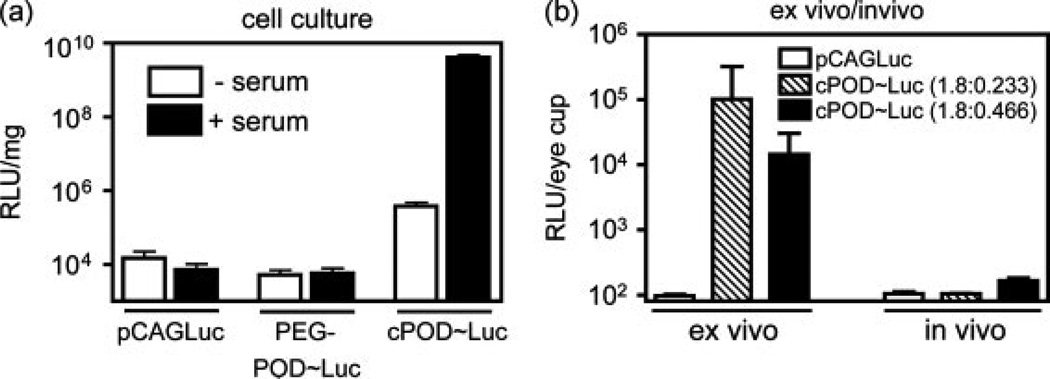
Previously, we have shown that c-POD [cysteine-POD (i.e. unpegylated POD)] can compact nucleic acids and deliver DNA to mitotic HER cells in vitro [1]. To extend this observation to post-mitotic cells and to quantify functional recombinant protein levels, we transfected HER cells with a c-POD/pCAGLuc polyplex containing a plasmid expressing luciferase under a chicken β-actin promoter (pCAGLuc) (Figure 3a). Luciferase expression was measured after 48 h in both mitotic cells and nonmitotic (serum-starved) cells. In dividing cells, c-POD increased gene expression relative to plasmid DNA by 5.8 × 105-fold (p < 0.005). c-POD complexed DNA had a 1.09 × 105-fold increase in gene expression in dividing cells over nondividing cells (p < 0.005). However, in nondividing cells, transfection with c-POD/DNA was only 25.8-fold greater than DNA only (p < 0.05). Although PEG-POD~Luc nanoparticles had less aggregation than c-POD/DNA complexes, they showed a concomitant lack of transfection in vitro relative to DNA alone in either mitotic or nonmitotic cells (p > 0.05). Hence, we conclude that actively mitotic cells are more amenable to transduction by c-POD/DNA complexes than nonmitotic cells and that PEG-POD complexes are ineffective in increasing the efficiency of transduction compared to DNA alone in vitro. In the case of c-POD transduction, however, we did not rule out the possibility that serum itself, and not cell division, enhances transduction.
Figure 3.
c-POD can deliver genes in vivo but not in vivo. (a) HER cells were transfected with a c-POD/pCAGLuc complex with or without prior serum-starvation and analysed after 48 h to quantify protein levels. Although expression was increased upon c-POD compaction in serum-starved cells (p < 0.05), expression was still significantly less than that in dividing cells (p < 0.005) (n = 3). (b) Compaction with c-POD significantly increased luciferase expression relative to plasmid alone ex vivo when delivered to primary RPE/sclera explants at both a ratio of 1.8 nmol c-POD to 0.233 pmol (n = 8, p < 0.0001) and 0.466 pmol pCAGLuc (n = 3, p < 0.0001). in vivo assay for transfection by subretinal injection of 0.2 µg of compacted DNA showed no significant increase in luciferase expression relative to plasmid alone (n = 4). Luciferase activity is presented as the mean ± SEM
To extend these observations to primary cells, explants of the posterior murine eye cup were incubated ex vivo with c-POD/pCAGLuc polyplex for 48 h, which resulted in significant transfection relative to DNA alone in the RPE/sclera at both a ratio of 1.8 nmol c-POD to 0.233 pmol (RLU = 1.0 × 105 ± 7.7 × 104; p < 0.0001) and 0.466 pmol pCAGLuc (RLU = 1.4 × 104 ± 9.1 × 103; p < 0.0001) (Figure 3b) but not in the retina (data not shown). However, when these same experiments were performed in murine eyes in vivo, no significant luciferase activity (p > 0.05) was detected above background using either compaction (Figure 3b). Hence, we conclude that whereas c-POD can compact and deliver DNA to mitotic cells in culture and primary cells in explants ex vivo, it fails to do so in vivo.
PEG-POD~Luc nanoparticles can deliver genes to the RPE in vivo
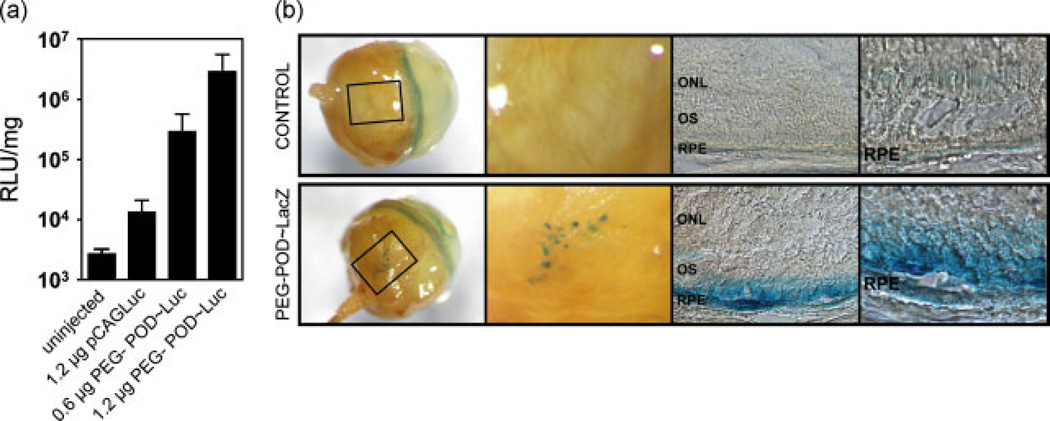
PEG-POD~Luc nanoparticles were injected into the subretinal space of 6–8-week-old male BALB/cJ mice and assayed for luciferase activity after 48 h. Luciferase activity was normalized to protein concentration (Figure 4a). Transfection using PEG-POD nanoparticles containing 1.2 µg of DNA (2.88 × 106 ± 9.58 × 105 RLU/mg) exhibited expression levels significantly greater than 1.2 µg of DNA alone (1.35 × 104 ± 3.69 × 103 RLU/mg; p < 0.0001) or uninjected controls (2.73 × 103 ± 2.60 × 102 RLU/mg; p < 0.0001). Expression of DNA only was significantly higher than uninjected controls (p < 0.05). When 0.6 µg of PEG-POD nanoparticles were injected in the same volume, we continued to observe significantly greater expression (2.92 × 105 ± 1.37 × 105 RLU/mg) relative to uninjected controls (p < 0.001) or 1.2 µg of naked DNA (p < 0.05). However, there was a significant, 9.8-fold reduction in mean luminescence when 0.6 µg of DNA was injected relative to the injection of 1.2 µg of PEG-POD nanoparticles (p < 0.05). Similar results were obtained when C57BL/6J mice were utilized instead of BALB/cJ (data not shown). We conclude that PEG-POD nanoparticles can efficiently deliver transgenes to cells in vivo relative to DNA alone and that this property is dose-dependent at the doses examined here.
Figure 4.
PEG-POD~Luc nanoparticles can transfect RPE ocular cells in vivo after subretinal injection in a dose-dependent manner. (a) PEG-POD compacted DNA nanoparticles were injected into the subretinal space of 6–8-week-old BALB/cJ mice and analysed 48 h after injection. PEG-POD~Luc nanoparticles (1.2 µg) exhibited expression levels significantly higher than 1.2 µg of DNA alone (p < 0.0001) or uninjected controls (p < 0.0001). Half the dose (0.6 µg of PEG-POD nanoparticles) showed a significant increase in expression relative to uninjected controls (p < 0.001) and 1.2 µg of naked DNA (p < 0.05), but a significant reduction compared to 1.2 µg of PEG-POD nanoparticles (p < 0.05). For uninjected, DNA only and 0.6 µg of POD, n = 4, and, for 1.2 µg of POD, n = 8. Data are presented as the mean ± SEM. (b) PEG-POD~LacZ nanoparticles (1.2 µg) were injected into the subretinal space of BALB/cJ mice and expression assayed 48 h after injection. Analysis showed punctate patches of LacZ staining in the RPE, visible through the sclera and choroid. Sections show that expression is localized in the RPE, although some staining was visible in the outer segments of retinas injected with the PEG-POD nanoparticles. No β-galactosidase expression was detected in any of the control eyes. A representative buffer injected eye is shown. In both test and control eyes, a ring of background LacZ stain was observed at the corneal/scleral junction (arrow). OS, outer segments; ONL: outer nuclear layer
Recombinant luciferase (Promega) was used to generate a standard curve for the RLU values (R2 = 0.9999). The in vivo expression of naked pCAGLuc plasmid produced 0.418 ± 0.136 pg/mg, 0.6 µg of PEG-POD~Luc produced 10.75 ± 5.07 pg/mg and 1.2 µg of PEG-POD~Luc produced 106.2 ± 35.42 pg/mg luciferase protein.
To localize transgene expression, we synthesized PEG-POD nanoparticles compacting a green fluorescent protein (GFP)-expressing plasmid under the chicken β-actin promoter. GFP was not detected in any tissue following subretinal injection (data not shown). To increase the sensitivity of our assay, nanoparticles containing a β-galactosidase (pCAGLacZ) transgene were synthesized and 1.2 µg was injected into the subretinal space of 6–8-week-old BALB/cJ mice. Eyes were harvested and stained for β-galactosidase activity after 48 h (Figure 4b). LacZ staining was consistent with expression occurring predominantly in the RPE cell monolayer. However, because attempts to identify nonpigmented RPE by antibody and lectin staining were incompatible with the fixative conditions required for X-Gal staining, we cannot exclude the possibility of transduction of other cell types, such as infiltrating cells of the immune system located on or near the RPE. The retinas of some mice also contained LacZ stain in the outer segments but not in the inner segments or outer nuclear layer. We hence conclude that staining in the outer segments was likely a result of the leakage of β-galactosidase stain from the RPE cells, a phenomenon previously observed during LacZ stain of RPE transfection [12]. Control eyes were either uninjected or injected with the same volume of uncompacted DNA, PEG-POD luciferase nanoparticles, PEG-POD alone, or buffer. No β-galactosidase stain was detected in any of the control eyes beyond background levels (data not shown).
PEG-POD~Luc nanoparticles do not cause toxicity by functional analysis
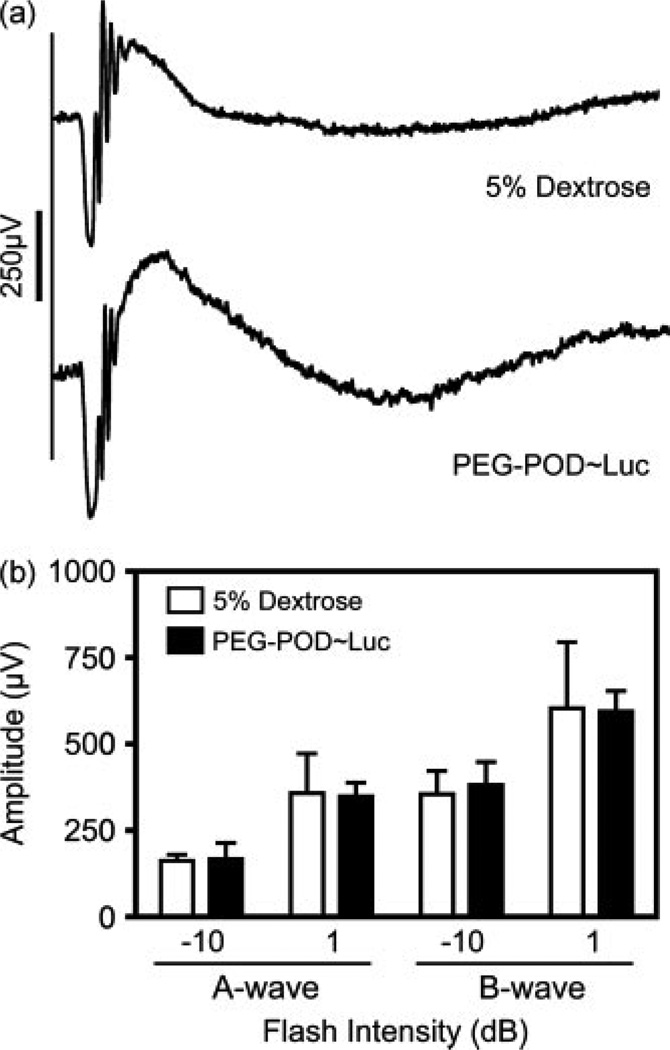
To examine the toxicity of the PEG-POD~Luc nanoparticles in vivo, we injected either PEG-POD~Luc nanoparticles or buffer alone into the subretinal space of C57BL/6J mice. After 48 h, electroretinograms were recorded at −10 and 1 dB. Both the PEG-POD~Luc and buffer-injected eyes had normal electroretinograms with defined A- and B-waves (representative examples at 1 dB flash intensity are presented in Figure 5a). The waves were analysed for A and B-wave amplitude and plotted by flash intensity (Figure 5b). Amplitudes were compared between buffer and nanoparticle-injected eyes for A-wave at −10 dB (164.5 ± 9.1, 169.7 ± 46.4), the B-wave at −10 dB (361.1 ± 56.7, 350.7 ± 39.8), the a-wave at 1 dB (357.7 ± 33.4, 384.1 ± 66.9), and the b-wave at 1 dB (606.4 ± 95.5, 597.8 ± 58.1). There was no significant difference between the nanoparticle- and buffer-injected eyes under any of the conditions examined (p > 0.05).
Figure 5.
PEG-POD nanoparticles are nontoxic by functional analysis. Subretinal injections were performed in 6–8-week-old C57BL/6J males with either PEG-POD~Luc nanoparticles or buffer alone. Electroretinography was performed at −10 and 1 dB flash intensities 48 h post-injection. (a) Representative tracings at 1 dB from mice after 48 h post-injection. (b) A- and B-wave amplitudes at −10 and 1 dB. There was no significant difference between nanoparticle- and buffer-injected eyes under any of the test conditions (p > 0.05). Data are presented as the mean ± SEM (n = 4)
Intravenous delivery of PEG-POD~Luc
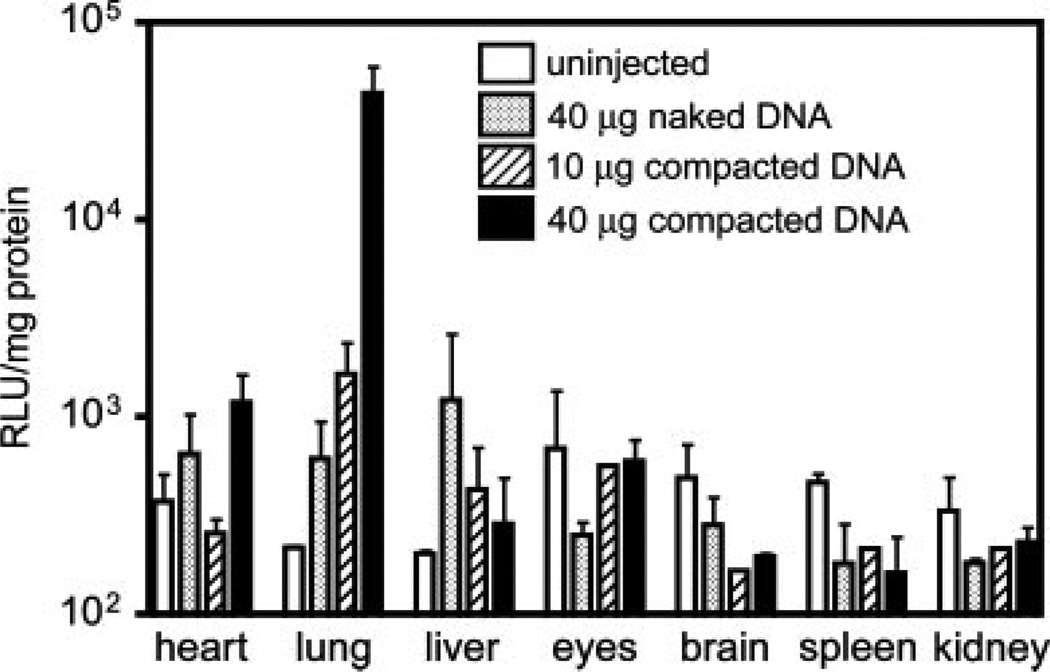
Because PEG-POD protects DNA from DNaseI digestion, we wished to examine whether this protection conferred an increase in transfection during intravenous delivery, and specifically whether ocular tissues could be transduced by this route of administration (Figure 6). When 40 µg of PEG-POD~Luc was delivered intravenously via tail vein injection, the levels of luciferase in the lung (4.445 × 104 ± 8082 RLU/mg) were significantly greater than when the same amount of DNA was injected alone (623.7 ± 139.1 RLU/mg; p < 0.0001). Delivery of 10 µg of PEG-POD~Luc also resulted in a significant increase in luciferase activity (1670 ± 430.5 RLU/mg; p < 0.05) over 40 µg of plasmid DNA but was significantly lower than 40 µg of PEG-POD~Luc (p < 0.0001), indicating a dose-dependence at the concentrations examined. A number of other tissues were tested in a similar manner throughout the body, with no significant difference in transfection between experimental conditions (p > 0.05). There was a trend towards an inverse relationship between lung and liver transfection (Figure 6), which could indicate that PEG-POD compaction potentially increases lung transduction via decreased clearance and uptake by the liver. Although ocular transfection was not observed, gene therapy has broad application in many tissues outside the eye and there have been a number of efforts to isolate vectors that can deliver DNA by intravenous delivery.
Figure 6.
PEG-POD~Luc nanoparticles have increased transfection in vivo following intravenous injection. BALB/cJ mice were injected with 150 µl of buffer containing either 40 µg of pCAGLuc, 10 µg of PEG-POD~Luc, or 40 µg of PEG-POD~Luc. Luciferase expression in the lung increased significantly above pCAGLuc when either 10 µg of PEG-POD~Luc (p < 0.05) or 40 µg of PEG-POD~Luc was injected (p < 0.0001). Increasing the dose of PEG-POD~Luc injected from 10 µg to 40 µg also resulted in a significant increase in luciferase expression (p < 0.0001). For 40 µg of naked DNA, n = 6; for 10 µg of PEG-POD~Luc, n = 3; and, for 40 µg of PEG-POD~Luc, n = 4. RLU/mg is presented as the mean ± SEM
PEG-POD transfection relative to PEG-TAT and PEG-CK30
We examined the relative gene transfer efficiency between PEG-POD and two additional cell penetrating peptides: HIV TAT [13] and CK30, a 30-mer polylysine [14]. Using the same protocol, these latter peptides were pegylated to produce PEG-TAT and PEG-CK30. PEG-TAT has been shown to compact DNA and deliver it to cells in culture [15,16] but, to our knowledge, has not been used on its own for the successful delivery of plasmid DNA in vivo. Various PEG-TAT:DNA ratios were generated and compaction of DNA was assessed by gel electrophoresis. PEG-TAT was shown to fully compact plasmid DNA at 1.8 nmol peptide: 2 µg of DNA (data not shown), the same ratio used for PEG-POD. The PEG-TAT nanoparticles were synthesized using the same protocol as PEG-POD nanoparticles. PEG-CK30 nanoparticles have been made previously and documented to show efficacy in vivo [5,6,17] and hence the ratio and protocol outlined in those studies was followed [5], yielding fully compacted DNA that was released using trypsin digestion (data not shown).
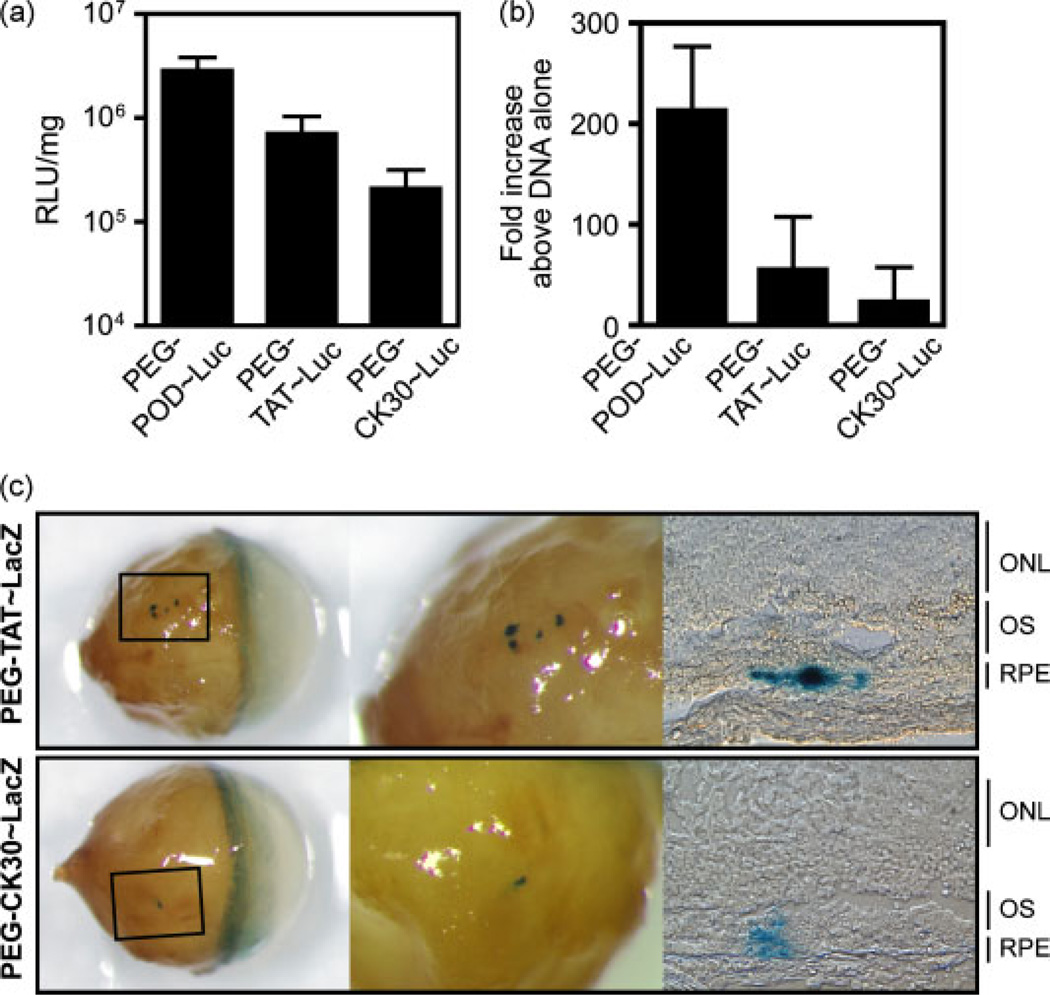
BALB/cJ mice were injected into the subretinal space with 1.2 µg of either PEG-TAT~Luc or PEG-CK30 ~Luc and the eyes were harvested 48 h later (Figure 7a). PEG-TAT~Luc expression (7.12 × 105 ± 3.24 × 106 RLU/mg) was significantly greater than 1.2 µg of DNA alone (p < 0.05) or uninjected controls (p < 0.005). PEG-CK30 ~Luc showed the lowest expression of the three cell penetrating peptides (CPPs) (2.09 × 105 ± 1.08× 105 RLU/mg) but was still significantly greater than 1.2 µg of DNA alone (p < 0.05) or uninjected controls (p < 0.005). Of all the injections, only one PEG-CK30 ~Luc injection fell below the uninjected control mean RLU/mg ±3 SD and thus this single injection was excluded from our statistical analysis. PEG-POD nanoparticles showed a significant 16.4-fold increase in the mean relative to PEG-CK30 (p < 0.005). PEG-TAT nanoparticles were not significantly different than those formed by either PEG-POD or PEG-CK30 (p < 0.05). Injections in C57BL/6J using nanoparticles formed with the three peptides exhibited similar RLU values and significance as those in BALB/cJ (data not shown). These results collectively indicate that all three cell-penetrating peptides significantly increase transfection in vivo relative to DNA alone. PEG-POD increased expression of plasmid DNA by 215-fold (±63), PEG-TAT by 56.52-fold (±21.33) and PEG-CK30 by 24.73-fold (±15.30) relative to the same amount of DNA injected alone (Figure 7b). However, PEG-POD appears to be the most efficient peptide in the case of subretinal DNA delivery.
Figure 7.
PEG-POD nanoparticles can transfect RPE cells more efficiently than PEG-TAT and PEG-CK30. (a) PEG-TAT~Luc was significantly increased compared to 1.2 µg of DNA alone (p < 0.05) and uninjected controls (p < 0.005). PEG-CK30 ~Luc showed the lowest expression of the three CPPs but was still significantly increased above 1.2 µg of DNA alone (p < 0.05) and uninjected controls (p < 0.005). PEG-POD nanoparticles showed a significant increase in transfection compared to PEG-CK30 (p < 0.005). PEG-TAT nanoparticles were not significantly different from those formed by either PEG-POD or PEG-CK30 (p < 0.05). (b) Fold increase of nanoparticle injections relative to 1.2 µg of naked pCAGLuc. For 1.2 µg of TAT and CK30, n = 6. Data are presented as the mean ± SEM. (c) PEG-TAT and PEG-CK30 LacZ nanoparticles (1.2 µg) were injected into the subretinal space of BALB/cJ mice and expression assayed 48 h post-injection, as in Fig. 4b. Similar to PEG-POD~LacZ, both nanoparticles transduce the RPE cell layer
Nanoparticles containing a β-galactosidase (pCAGLacZ) transgene were synthesized using each of the pegylated CPPs and injected into the subretinal space of 6–8-week-old BALB/cJ mice. Eyes were harvested and stained for β-galactosidase activity after 48 h. Although all three cell penetrating peptides were able to primarily transduce the RPE, the most robust staining was seen by PEG-POD~LacZ (Figure 4b), followed by PEG-TAT~LacZ and minimal staining using PEG-CK30 ~LacZ (Figure 7c).
Discussion
The present study aimed to develop POD as a nonviral gene delivery vector for use in vivo. Compaction with CPPs has been shown to increase transfection in post-mitotic cells and may do so by facilitating passage into the nucleus through the nuclear membrane pore [6,16]. Although most efficient in dividing cells, the presence of luciferase in serum starved cells indicates that POD is able to facilitate low levels of transfection in post-mitotic cells. However, c-POD was unable to transduce cells in vivo upon injection into the subretinal space. The lack of efficient transduction using c-POD/DNA complexes in vivo could be a result of differences in the extracellular environment, including physiologic salt and saline conditions, that can cause aggregation and sequester the nanoparticles [3]. The addition of a PEG moiety (PEGylation) to c-POD resulted in small, discrete, spherical nanoparticles without aggregation in vitro. PEGylation of polycation-nucleic acid complexes, including those made with polyethylenimine (PEI), has been shown to stabilize complex size and prevent aggregation, resulting in smaller particles as measured by DLS, electron microscopy, and cell culture staining [3], which are properties observed in PEG-POD nanoparticles. PEGylation has been shown to lead to an overall reduction in positive surface charge and a subsequent decrease in transfection efficiency in vitro [3], which could explain why PEG-POD compacted DNA was unable to transfect cells in vitro. Interestingly, the same peptide/DNA ratio was used to compact DNA for both c-POD and PEG-POD complexes, indicating that the net charge of the complexes is similar. Studies using PEI have shown a strong correlation between particle size and transfection efficiency in cell culture, implying that large complexes may be able to fall out of solution in culture to transfect cells, a condition not applicable for use in vivo [3,18].
For POD nanoparticles, the change in particle structure after PEGylation translated into significantly higher transfection efficiency in vivo. The mean expression of the luciferase transgene using PEG-POD nanoparticles was 106.3 pg/mg (215-fold increase above DNA alone) with a maximum expression of 282.4 pg/mg, although a significant decrease was observed at the 1-week versus 48-h time point (data not shown). There is evidence that this level of expression is sufficient for physiologic rescue. For example, biodegradable microspheres releasing an estimated 707 pg of glial-derived neurotrophic factor over 2 months have been shown to rescue the DBA/2J spontaneous glaucoma model [19], and encapsulated fibroblasts secreting 68.7 ± 2.3 pg human fibroblast growth factor 2 over 24 h locally rescued the outer nuclear layer in a rat model of photoreceptor degeneration [20].
Although we were unable to express significant levels of protein to produce GFP at levels of detection by fluorescence microscopy, the use of quantitative luciferase expression revealed expression levels higher with the CPPs PEG-POD and PEG-TAT relative to PEG-CK30, which has been previously reported to express substantial levels of GFP in the retina [17]. Though our results appear to contrast with the results obtained in similar experiments performed with PEG-CK30 in ocular tissues [17], there are some discrepancies between PEG-CK30 GFP localization in the eye and the results obtained from viral studies discussed elsewhere [21]. Hence, further investigation is warranted to understand the underlying mechanism of these differences. Our results using PEG-POD in the eye were comparable to the previously reported transfection efficiency obtained using PEG-CK30 nanoparticles in the lung (186 pg/mg luciferase, an approximate 26-fold increase above DNA only control) [5,22], whose application is currently in clinical trials for cystic fibrosis via administration by inhalation [23].
All three pegylated peptides showed transfection primarily in the RPE. Although LacZ staining was visible in the outer segments of retinas injected with PEG-POD nanoparticles, the spread of LacZ stain has been observed during RPE transfection [12], and a lack of stain in the inner segments further indicates that it was not generated in the photoreceptors. Injection into the subretinal space exposes the nanoparticles to both the RPE and the outer segments of the photoreceptors. Although the phagocytic nature of the RPE might be expected to facilitate transfection, the RPE cells additionally have a larger surface area than the outer segments, and so would be exposed to a higher number of nanoparticles per cell. However, we cannot rule out the possibility of photoreceptor tranduction occurring below the threshold of sensitivity of the LacZ assay. Analysis of the LacZ stain by this assay indicates that PEG-CK30 does not transfect photoreceptors, as previously reported using a GFP reporter gene [17].
Targeting the RPE for transduction for rescue of retinal degeneration using growth factors could be a logical choice for sustained therapy applicable to the heterogeneous etiologies of many ocular diseases. Transduction of the RPE has been previously demonstrated using a variety of other vectors. Lipofectamine 2000 has been shown to deliver plasmid DNA into the RPE, although with significant toxicity to photoreceptors [12]. Use of NeuroPorter demonstrated significant transfection of the RPE without any significant damage [12]. Intravenous injection of poly (lactide-co-glycolide) nanoparticles functionalized with transferrin and RGD-peptide have been shown to transfect the RPE in mice with laser-induced damage [24].
In addition to subretinal delivery, PEG-POD-mediated plasmid stability indicated a potential use in intravenous administration. PEG-POD was found to deliver the luciferase transgene preferentially to the lung with a mean expression of 1.56 pg/mg using 40 µg of DNA. Studies using PEI have shown levels of approximately 35 pg/mg 48 h after 125 µg of complexed DNA was injected intravenously [25]. Mice injected hydrodynamically via the tail vein using 10 µg of DNA showed approximately 20 pg/mg of expression when harvested after 8 h [26]. One hundred micrograms of PEG-CK30 nanoparticles administered by inhalation resulted in 186.39 pg/mg luciferase expression [5]. Interestingly, TAT/DNA complexes injected intravenously in mice showed a decreased expression in tissue relative to DNA injected alone [15]. Although intravenous delivery of cationic peptide nanoparticles appears to increase plasmid DNA stability in the serum, transfection is still less than that achieved using other nonviral techniques.
There are a number of different strategies to overcome the limitations of nonviral gene therapy. Lipids compact DNA and increase the affinity for the plasma membrane and thus intracellular uptake. Intravenous delivery of transferrin-targeted liposomes appears to transfect cells throughout the eye in an organ-specific manner using a photoreceptor specific promoter [27]. A number of different polymers have also been explored. HIV Tat is a CPP that has been previously shown to compact DNA and deliver transgenes to cells in culture [15,16]. Delivery of FGF-2 transgene using K8/JTS-1, an aphipathic polylysine analog, delayed photoreceptor degeneration after 60 days in the Royal College of Surgeons rat [28]. Although unproven in the eye, a number of other nonviral approaches have been developed. For example, organically modified silica nanoparticles have been shown to transfect neurons in the brain at levels comparable to virus [29]. Yet there have been very few examples of rescue using these DNA delivery strategies.
Although PEG-POD and other CPP-mediated gene delivery methods need to be improved, the isolation of candidate molecules is an important step towards the development of nonviral gene delivery. In the present study, we demonstrate that nanoparticles composed of PEG-POD, PEG-TAT or PEG-CK30 complexed with pCAGLacZ all show expression in the RPE. Currently, nonviral gene therapy is a relatively new approach and the potential gene delivery reagents are still being developed. These findings indicate that cationic peptides, and specifically pegylated cell penetrating peptides such as PEG-POD, are promising candidates for gene delivery.
Note added in proof: Recently, we have shown that the POD sequence can be used to deliver recombinant protein to intraocular tissues and skin [30].
Acknowledgements
This study was supported by grants to R.K.-S. from the National Institutes of Health/National Eye Institute (EY014991 and EY013887), The Foundation for Fighting Blindness, The Ellison Foundation, the Virginia B Smith Trust, and grants to the Department of Ophthalmology at Tufts University from the Lions Eye Foundation and Research to Prevent Blindness. We would also like to thank David Kaplan at Tufts University for his assistance in performing the dynamic light scattering experiments.
References
- 1.Johnson LN, Cashman SM, Kumar-Singh R. Cell-penetrating peptide for enhanced delivery of nucleic acids and drugs to ocular tissues including retina and cornea. Mol Ther. 2008;16:107–114. doi: 10.1038/sj.mt.6300324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis PB, Cooper MJ. Vectors for airway gene delivery. Aaps J. 2007;9:E11–E17. doi: 10.1208/aapsj0901002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mishra S, Webster P, Davis ME. PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. Eur J Cell Biol. 2004;83:97–111. doi: 10.1078/0171-9335-00363. [DOI] [PubMed] [Google Scholar]
- 4.Fallaux FJ, Kranenburg O, Cramer SJ, et al. Characterization of 911: a new helper cell line for the titration and propagation of early region 1-deleted adenoviral vectors. Hum Gene Ther. 1996;7:215–222. doi: 10.1089/hum.1996.7.2-215. [DOI] [PubMed] [Google Scholar]
- 5.Ziady AG, Gedeon CR, Miller T, et al. Transfection of airway epithelium by stable pegylated poly L-lysine DNA nanoparticles in vivo . Mol Ther. 2003;8:936–947. doi: 10.1016/j.ymthe.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Liu G, Li D, Pasumarthy MK, et al. Nanoparticles of compacted DNA transfect postmitotic cells. J Biol Chem. 2003;278:32578–32586. doi: 10.1074/jbc.M305776200. [DOI] [PubMed] [Google Scholar]
- 7.Moore DS, McCabe GP, Duckworth WM, Alwan L. The Practice of Business Statistics. 2nd edn. New York: WH Freeman; 2008. [Google Scholar]
- 8.Dean DA, Strong DD, Zimmer WE. Nuclear entry of nonviral vectors. Gene Ther. 2005;12:881–890. doi: 10.1038/sj.gt.3302534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawabata K, Takakura Y, Hashida M, et al. The fate of plasmid DNA after intravenous injection in mice: involvement of scavenger receptors in its hepatic uptake. Pharm Res. 1995;12:825. doi: 10.1023/a:1016248701505. [DOI] [PubMed] [Google Scholar]
- 10.Love J, Hewitt RR. The relationship between human serum and human pancreatic DNAse I. J Bio Chem. 1979;254:12588. [PubMed] [Google Scholar]
- 11.Takeshita H, Nakajima T, Mogi K, et al. Rapid quantification of DNase I activity in one-microliter serum samples. Clin Chem. 2004;50:446–448. doi: 10.1373/clinchem.2003.025304. [DOI] [PubMed] [Google Scholar]
- 12.Kachi S, Oshima Y, Esumi N, et al. Nonviral ocular gene transfer. Gene Ther. 2005;12:843–851. doi: 10.1038/sj.gt.3302475. [DOI] [PubMed] [Google Scholar]
- 13.Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 14.Shen WC, Ryser HJ. Conjugation of poly L-lysine to albumin and horseradish peroxidase: a novel method of enhancing the cellular uptake of proteins. Proc Natl Acad Sci USA. 1978;75:1872–1876. doi: 10.1073/pnas.75.4.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ignatovich IA, Dizhe EB, Pavlotskaya AV, et al. Complexes of plasmid DNA with basic domain 47–57 of the HIV-1 Tat protein are transferred to mammalian cells by endocytosis-mediated pathways. J Biol Chem. 2003;278:42625–42636. doi: 10.1074/jbc.M301431200. [DOI] [PubMed] [Google Scholar]
- 16.Rudolph C, Plank C, Lausier J, Schillinger U, Muller RH, Rosenecker J. Oligomers of the arginine-rich motif of the HIV-1 TAT protein are capable of transferring plasmid DNA into cells. J Biol Chem. 2003;278:11411–11418. doi: 10.1074/jbc.M211891200. [DOI] [PubMed] [Google Scholar]
- 17.Farjo R, Skaggs J, Quiambao AB, Cooper MJ, Naash MI. Efficient non-viral ocular gene transfer with compacted DNA nanoparticles. PLoS ONE. 2006;1:E38. doi: 10.1371/journal.pone.0000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogris M, Steinlein P, Kursa M, Mechtler K, Kircheis R, Wagner E. The size of DNA/transferrin-PEI complexes is an important factor for gene expression in cultured cells. Gene Ther. 1998;5:1425–1433. doi: 10.1038/sj.gt.3300745. [DOI] [PubMed] [Google Scholar]
- 19.Ward MS, Khoobehi A, Lavik EB, Langer R, Young MJ. Neuroprotection of retinal ganglion cells in DBA/2 J mice with GDNF-loaded biodegradable microspheres. J Pharm Sci. 2007;96:558–568. doi: 10.1002/jps.20629. [DOI] [PubMed] [Google Scholar]
- 20.Uteza Y, Rouillot JS, Kobetz A, et al. Intravitreous transplantation of encapsulated fibroblasts secreting the human fibroblast growth factor 2 delays photoreceptor cell degeneration in Royal College of Surgeons rats. Proc Natl Acad Sci USA. 1999;96:3126–3131. doi: 10.1073/pnas.96.6.3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar-Singh R. Barriers for retinal gene therapy: separating fact from fiction. Vision Res. 2008;48:1671–1680. doi: 10.1016/j.visres.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ziady AG, Gedeon CR, Muhammad O, et al. Minimal toxicity of stabilized compacted DNA nanoparticles in the murine lung. Mol Ther. 2003;8:948–956. doi: 10.1016/j.ymthe.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Konstan MW, Davis PB, Wagener JS, et al. Compacted DNA nanoparticles administered to the nasal mucosa of cystic fibrosis subjects are safe and demonstrate partial to complete cystic fibrosis transmembrane regulator reconstitution. Hum Gene Ther. 2004;15:1255–1269. doi: 10.1089/hum.2004.15.1255. [DOI] [PubMed] [Google Scholar]
- 24.Singh SR, Grossniklaus HE, Kang SJ, Edelhauser HF, Ambati BK, Kompella UB. Intravenous transferrin, RGD peptide and dual-targeted nanoparticles enhance anti-VEGF intraceptor gene delivery to laser-induced CNV. Gene Ther. 2009;16:645–659. doi: 10.1038/gt.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goula D, Benoist C, Mantero S, Merlo G, Levi G, Demeneix BA. Polyethylenimine-based intravenous delivery of transgenes to mouse lung. Gene Ther. 1998;5:1291–1295. doi: 10.1038/sj.gt.3300717. [DOI] [PubMed] [Google Scholar]
- 26.Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258–1266. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 27.Zhu C, Zhang Y, Pardridge WM. Widespread expression of an exogenous gene in the eye after intravenous administration. Invest Ophthalmol Vis Sci. 2002;43:3075–3080. [PubMed] [Google Scholar]
- 28.Neuner-Jehle M, Berghe LV, Bonnel S, et al. Ocular cell transfection with the human basic fibroblast growth factor gene delays photoreceptor cell degeneration in RCS rats. Hum Gene Ther. 2000;11:1875–1890. doi: 10.1089/10430340050129495. [DOI] [PubMed] [Google Scholar]
- 29.Bharali DJ, Klejbor I, Stachowiak EK, et al. Organically modified silica nanoparticles: a nonviral vector for in vivo gene delivery and expression in the brain. Proc Natl Acad Sci USA. 2005;102:11539–11544. doi: 10.1073/pnas.0504926102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson LN, Cashman SM, Read SP, Kumar-Singh K. Cell penetrating peptide POD mediates delivery of recombinant proteins to retina, cornea and skin. Vision Res. 2009 doi: 10.1016/j.visres.2009.08.028. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]