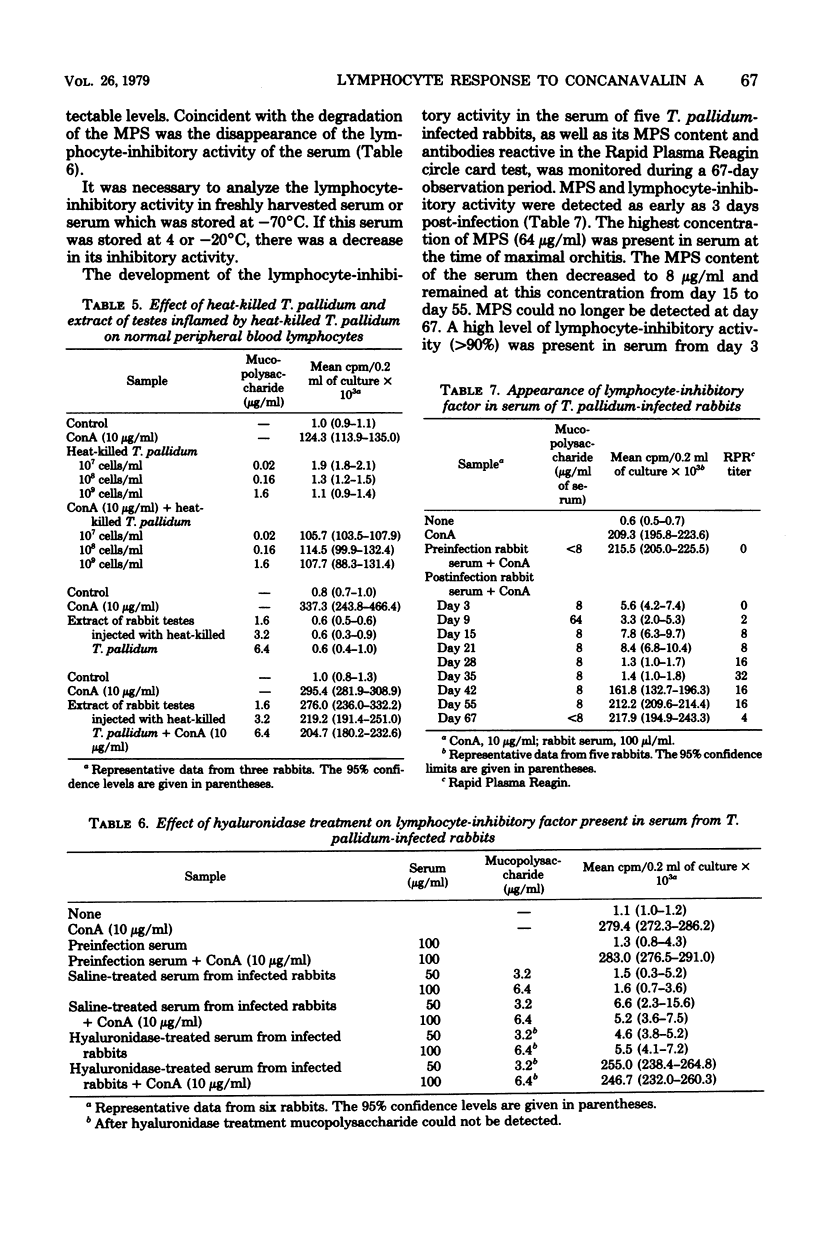
Abstract
The testicular fluid and serum from rabbits infected intratesticularly with Treponema pallidum inhibited the mitogenic response of normal rabbit peripheral blood lymphocytes to concanavalin A. Mucopolysaccharide material present in the testicular fluid and serum was associated with the lymphocyte-inhibitory activity. Degradation of the mucopolysaccharide material with hyaluronidase resulted in the loss of the inhibitory activity of testicular fluid and serum of T. pallidu-infected rabbits.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Festenstein H., Abrahams C., Bokkenheuser V. Runting syndrome in neonatal rabbits infected with Treponema pallidum. Clin Exp Immunol. 1967 May;2(3):311–320. [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Cleveland P., Johnson R. C., Miller J. N., Sykes J. A. Scanning electron microscopy of Treponema pallidum (Nichols strain) attached to cultured mammalian cells. J Bacteriol. 1977 Jun;130(3):1333–1344. doi: 10.1128/jb.130.3.1333-1344.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C., Ritzi D. M. Relationship of Treponema pallidum to acidic mucopolysaccharides. Infect Immun. 1979 Apr;24(1):252–260. doi: 10.1128/iai.24.1.252-260.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C. Surface mucopolysaccharides of Treponema pallidum. Infect Immun. 1979 Apr;24(1):244–251. doi: 10.1128/iai.24.1.244-251.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C., Wolff E. T. Mucopolysaccharide material resulting from the interaction of Treponema pallidum (Nichols strain) with cultured mammalian cells. Infect Immun. 1978 Nov;22(2):575–584. doi: 10.1128/iai.22.2.575-584.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulford K. W., Brostoff J. Leucocyte migration and cell-mediated immunity in syphilis. Br J Vener Dis. 1972 Dec;48(6):483–488. doi: 10.1136/sti.48.6.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim A. N., Streitfeld M. M. The microassay of hyaluronic acid concentration and hyaluronidase activity by capillary turbidity (CT) and capillary turbidity reduction (CTR) tests. Anal Biochem. 1973 Dec;56(2):428–434. doi: 10.1016/0003-2697(73)90208-x. [DOI] [PubMed] [Google Scholar]
- Laird S. M., Thorburn A. L. Assessment of the "luotest" in late syphillis. Br J Vener Dis. 1966 Jun;42(2):119–121. doi: 10.1136/sti.42.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levene G. M., Turk J. L., Wright D. J., Grimble A. G. Reduced lymphocyte transformation due to a plasma factor in patients with active syphilis. Lancet. 1969 Aug 2;2(7614):246–247. doi: 10.1016/s0140-6736(69)90010-5. [DOI] [PubMed] [Google Scholar]
- Musher D. M., Schell R. F., Jones R. H., Jones A. M. Lymphocyte transformation in syphilis: an in vitro correlate of immune suppression in vivo? Infect Immun. 1975 Jun;11(6):1261–1264. doi: 10.1128/iai.11.6.1261-1264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musher D. M., Schell R. F., Knox J. M. In vitro lymphocyte response to Treponema refringens im human syphilis. Infect Immun. 1974 Apr;9(4):654–657. doi: 10.1128/iai.9.4.654-657.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S., Baseman J. B., Folds J. D. Selective response of lymphocytes from Treponema pallidum-infected rabbits to mitogens and Treponema reiteri. Infect Immun. 1977 Feb;15(2):417–422. doi: 10.1128/iai.15.2.417-422.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S., Folds J. D., Baseman J. B. Depression of lymphocyte response to concanavalin A in rabbits infected with Treponema pallidum (Nichols strain). Infect Immun. 1976 Jul;14(1):320–322. doi: 10.1128/iai.14.1.320-322.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S., Folds J. D., Baseman J. B. Selective in vitro response of thymus-derived lymphocytes from Treponema pallidum-infected rabbits. Infect Immun. 1977 Dec;18(3):603–611. doi: 10.1128/iai.18.3.603-611.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICE F. A. Chondroitin sulfate and hyaluronic acid in syphilomas of cortisone-treated rabbits. Science. 1956 Aug 10;124(3215):275–275. doi: 10.1126/science.124.3215.275. [DOI] [PubMed] [Google Scholar]
- SCOTT V., DAMMIN G. J. Hyaluronidase and experimental syphilis. III. Metachromasia in syphilitic orchitis and its relationship to hyaluronic acid. Am J Syph Gonorrhea Vener Dis. 1950 Nov;34(6):501–514. [PubMed] [Google Scholar]
- SCOTT V., DAMMIN G. J. Morphologic and histochemical sequences in syphilitic and in tuberculous orchitis in the rabbit. Am J Syph Gonorrhea Vener Dis. 1954 May;38(3):189–202. [PubMed] [Google Scholar]
- Sølling J., Sølling K., Jacobsen K. U., Olsen S., From E. Circulating immune complexes in syphilitic nephropathy. A case report. Br J Vener Dis. 1978 Feb;54(1):53–56. doi: 10.1136/sti.54.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware J. L., Folds J. D., Baseman J. B. Serum of rabbits infected with Treponema pallidum (Nichols) inhibits in vitro transformation of normal rabbit lymphocytes. Cell Immunol. 1979 Feb;42(2):363–372. doi: 10.1016/0008-8749(79)90201-6. [DOI] [PubMed] [Google Scholar]
- Wicher V., Wicher K. In vitro cell response of Treponema pallidum-infected rabbits. I. Lymphocyte transformation. Clin Exp Immunol. 1977 Sep;29(3):480–486. [PMC free article] [PubMed] [Google Scholar]
- Wicher V., Wicher K. In vitro cell response of Treponema pallidum-infected rabbits. II. Inhibition of lymphocyte response to phytohaemagglutinin by serum of T. pallidum-infected rabbits. Clin Exp Immunol. 1977 Sep;29(3):487–495. [PMC free article] [PubMed] [Google Scholar]
- Wright D. J., Grimble A. S. Why is the infectious stage of syphilis prolonged? Br J Vener Dis. 1974 Feb;50(1):45–49. doi: 10.1136/sti.50.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigler J. A., Jones A. M., Jones R. H., Kubica K. M. Demonstration of extracellular material at the surface of pathogenic T. pallidum cells. Br J Vener Dis. 1976 Feb;52(1):1–8. doi: 10.1136/sti.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]



