Abstract
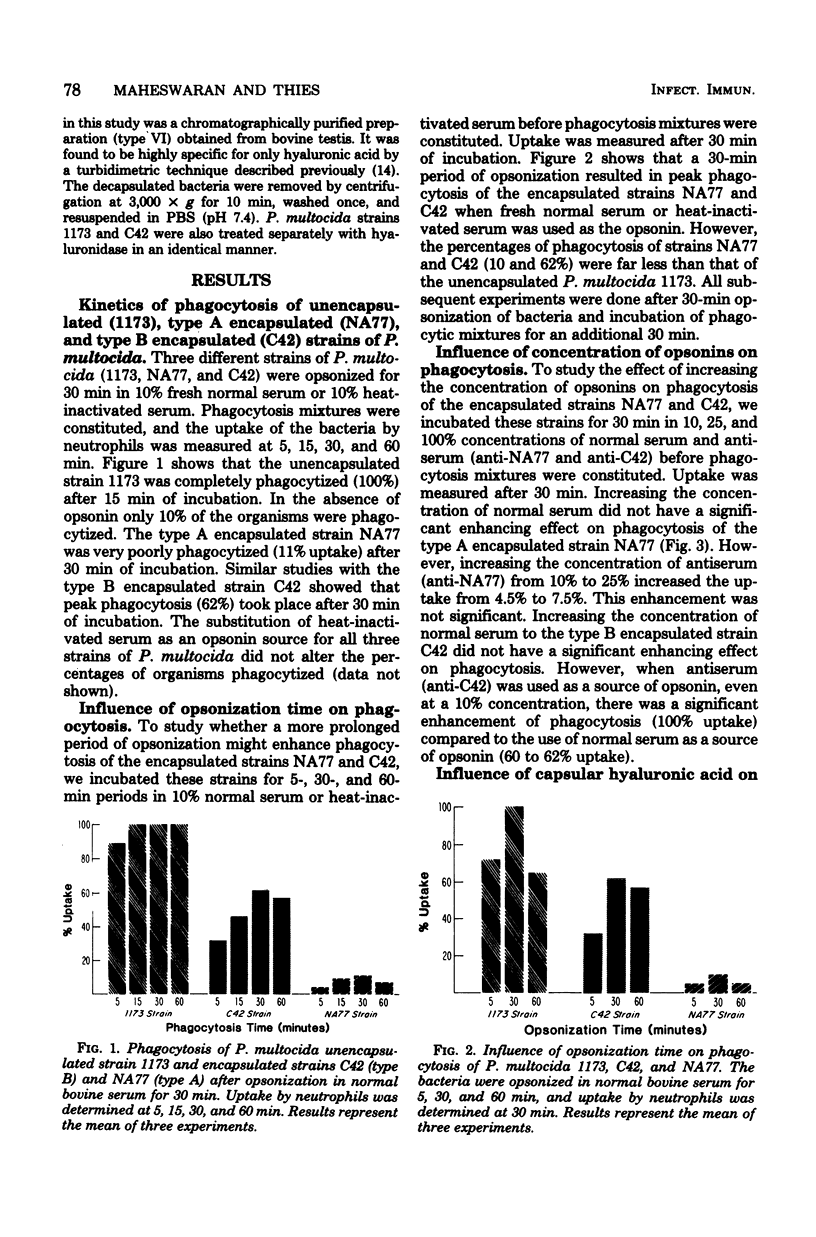
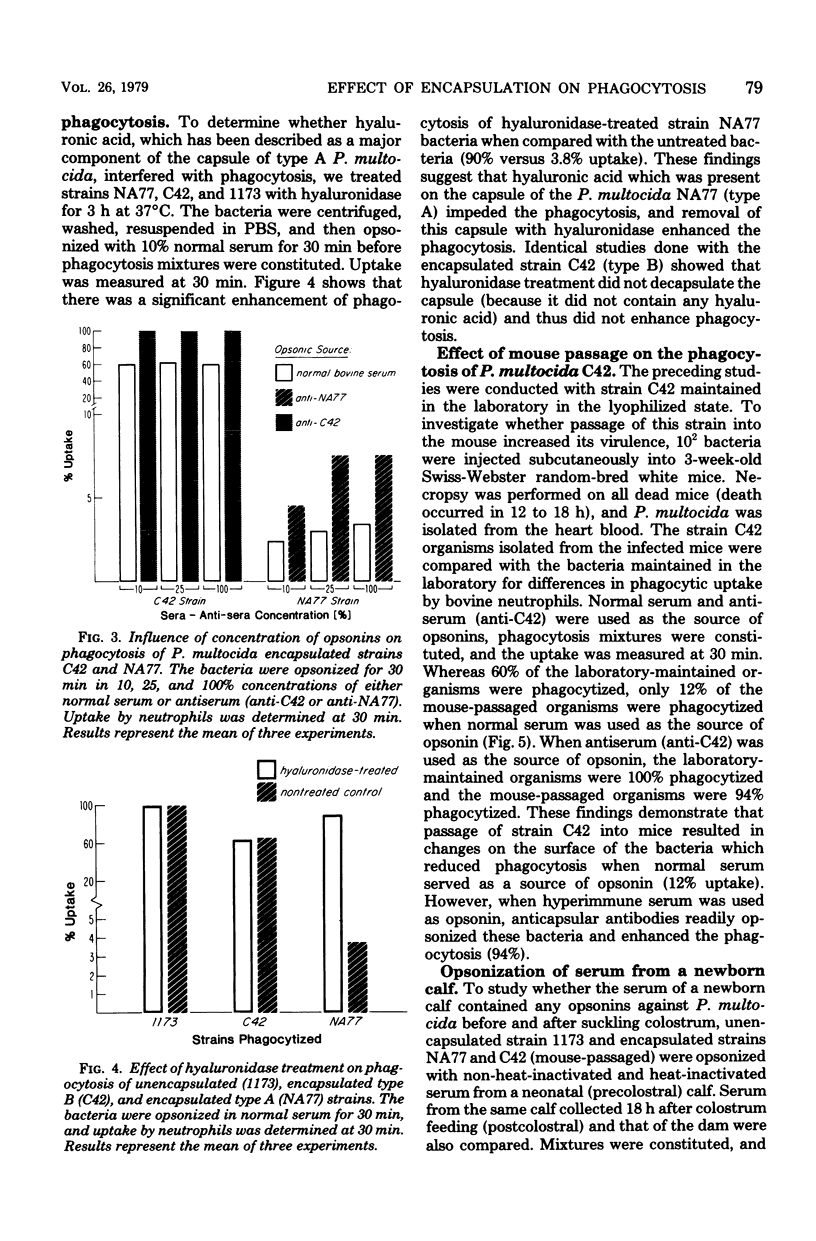
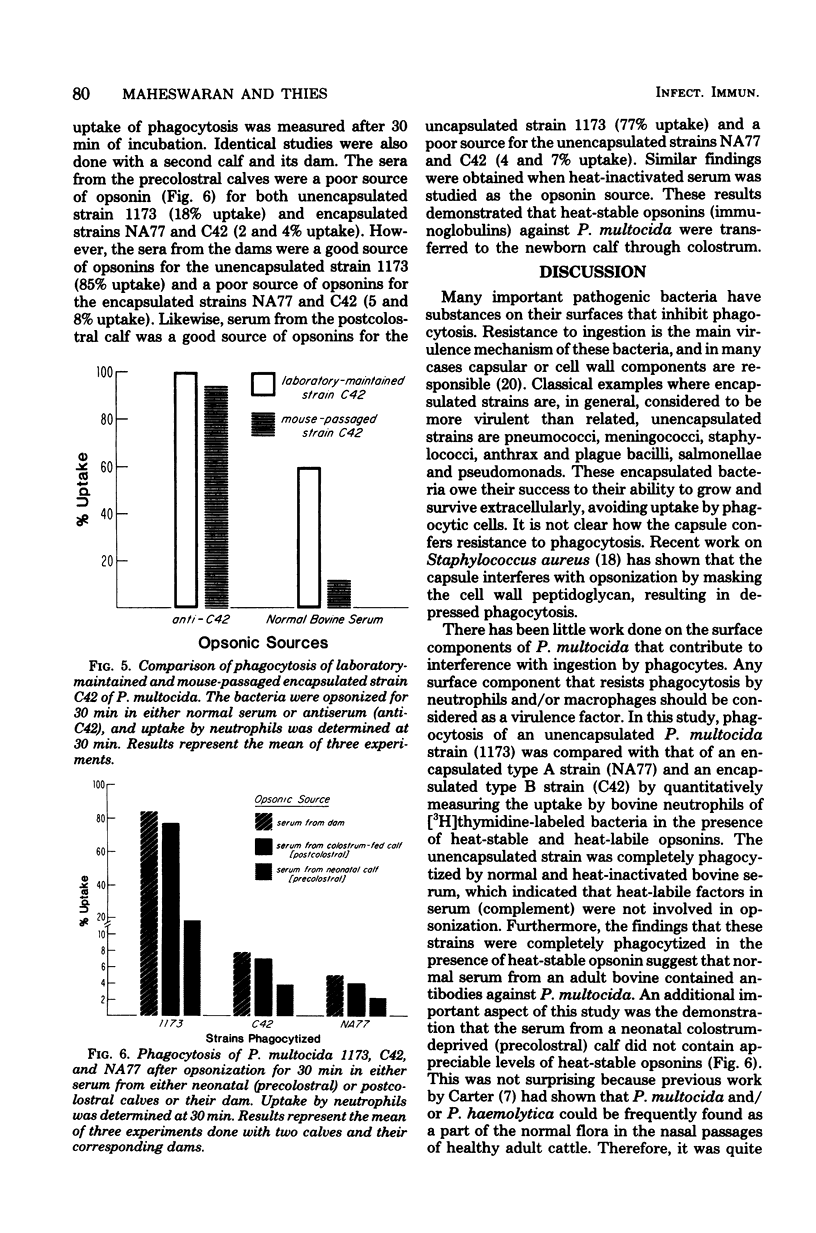
The effect of encapsulation on phagocytosis of Pasteurella multocida by bovine neutrophils was examined by using two encapsulated strains, NA77 (capsular type A) and C42 (capsular type B), and comparing them with an unencapsulated counterpart strain, 1173. The uptake of [3H]thymidine-labeled bacteria by neutrophils was quantitatively measured after incubation of the bacteria in normal bovine serum, heat-inactivated serum, and hyperimmune sera (anti-NA77 and anti-C42). Results showed that all three strains of P. multocida were ineffectively opsonized by the heat-labile serum complement system. The unencapsulated strain was completely phagocytized in the presence of heat-stable opsonins found in normal serum. Although encapsulation of strain C42 was found to interfere with opsonization by normal serum, this strain was completely phagocytized when hyperimmune serum (anti-C42) was used as the opsonin source. These results suggest that specific anticapsular antibodies found in the hyperimmune serum readily opsonized the encapsulated strain C42 and enhanced phagocytosis. The presence of a thick capsule on strain NA77 interfered with phagocytosis in the presence of normal or hyperimmune serum (anti-NA77). This interference was due to the presence of hyaluronic acid which was a major component of the capsule. Treatment of this encapsulated strain with hyaluronidase decapsulated the bacteria. Bacteria treated in this way were almost completely phagocytized (90%) in the presence of heat-stable opsonins. The exact mechanism by which the capsule of P. multocida NA77 interfered with phagocytosis was not demonstrated; perhaps the slimy nature of the hyaluronic acid makes the phagocytic act difficult by changing the physiochemical surface properties, or it may prevent opsonization.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARKULIS S. S. Biochemical properties of a virulent and an avirulent strain of group A hemolytic Streptococcus. Ann N Y Acad Sci. 1960 Nov 21;88:1034–1053. doi: 10.1111/j.1749-6632.1960.tb20095.x. [DOI] [PubMed] [Google Scholar]
- Bond R. E., Donahue J. M., Olson L. D. Colony features of Pasteurella multocida and their use in diagnosing fowl cholera in turkeys. Avian Dis. 1970 Feb;14(1):24–28. [PubMed] [Google Scholar]
- CARTER G. R., BYRNE J. L. A serological study of the hemorrhagic septicemia Pasteurella. Cornell Vet. 1953 Apr;43(2):223–230. [PubMed] [Google Scholar]
- CARTER G. R. Studies on Pasteurella multocida. I. A hemagglutination test for the identification of serological types. Am J Vet Res. 1955 Jul;16(60):481–484. [PubMed] [Google Scholar]
- Carter G. R. Improved hemagglutination test for identifying type A strains of Pasteurella multocida. Appl Microbiol. 1972 Jul;24(1):162–163. doi: 10.1128/am.24.1.162-163.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter G. R. Pasteurellosis: Pasteurella multocida and Pasteurella hemolytica. Adv Vet Sci. 1967;11:321–379. [PubMed] [Google Scholar]
- Grewal A. S., Rouse B. T., Babiuk L. A. Mechanisms of resistant of herpesviruses: comparison of the effectiveness of different cell types in mediating antibody-dependent cell-mediated cytotoxicity. Infect Immun. 1977 Mar;15(3):698–703. doi: 10.1128/iai.15.3.698-703.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie L. E. The bovine respiratory disease complex. Can Vet J. 1974 Sep;15(9):233–242. [PMC free article] [PubMed] [Google Scholar]
- Maheswaran S. K., Johnson G. H., Pomeroy B. S. Studies on Pasteurella multocida. I. The capsular polysaccharides from turkey isolates. Avian Dis. 1973 Oct-Dec;17(4):705–716. [PubMed] [Google Scholar]
- Maheswaran S. K., Thies E. S., Dua S. K. Studies on Pasteurella multocida. III. In vitro assay for cell-mediated immunity. Avian Dis. 1976 Apr-Jun;20(2):332–341. [PubMed] [Google Scholar]
- Orskov F. Virulence factors of the bacterial cell surface. J Infect Dis. 1978 May;137(5):630–633. doi: 10.1093/infdis/137.5.630. [DOI] [PubMed] [Google Scholar]
- Penn C. W., Nagy L. K. Isolation of a protective, non-toxic capsular antigen from Pasteurella multocida, types B and E. Res Vet Sci. 1976 Jan;20(1):90–96. [PubMed] [Google Scholar]
- Peterson P. K., Wilkinson B. J., Kim Y., Schmeling D., Douglas S. D., Quie P. G., Verhoef J. The key role of peptidoglycan in the opsonization of Staphylococcus aureus. J Clin Invest. 1978 Mar;61(3):597–609. doi: 10.1172/JCI108971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebers P. A., Heddleston K. L., Rhoades K. R. Isolation from Pasteurella multocida of a lipopolysaccharide antigen with immunizing and toxic properties. J Bacteriol. 1967 Jan;93(1):7–14. doi: 10.1128/jb.93.1.7-14.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. Microbial surfaces in relation to pathogenicity. Bacteriol Rev. 1977 Jun;41(2):475–500. doi: 10.1128/br.41.2.475-500.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoef J., Peterson P. K., Quie P. G. Kinetics of staphylococcal opsonization, attachment, ingestion and killing by human polymorphonuclear leukocytes: a quantitative assay using [3H]thymidine labeled bacteria. J Immunol Methods. 1977;14(3-4):303–311. doi: 10.1016/0022-1759(77)90141-7. [DOI] [PubMed] [Google Scholar]
- van Oss C. J. Phagocytosis as a surface phenomenon. Annu Rev Microbiol. 1978;32:19–39. doi: 10.1146/annurev.mi.32.100178.000315. [DOI] [PubMed] [Google Scholar]



