Abstract
AIM: To evaluate the associations of serum folate level with development, invasiveness and patient survival of gastric cancer.
METHODS: In this nested case-control study, patients with newly diagnosed gastric cancer undergoing gastrectomy were enrolled, and patients receiving chemotherapy prior to surgery, with other concurrent malignancy, or of the aboriginal and alien populations were excluded. In total, 155 gastric cancer patients and 149 healthy controls were enrolled for determination of serum folate levels and their correlation with gastric cancer. Using the median value of serum folate computed among the overall population as the cutoff value, the associations between serum folate and gastric cancer in all cases and different age and gender subgroups were analyzed by multivariate logistic regression analysis. In the patient cohort of gastric cancer, receiver-operating characteristic analyses were performed to calculate the best cutoff values of serum folate, and the associations between serum folate levels and clinicopathological features were further analyzed by multivariate regression analysis. Survival analyses were conducted using the Cox proportional hazards model.
RESULTS: The mean serum folate level was significantly lower in gastric cancer patients than that in controls (3.71 ± 0.30 ng/mL vs 8.00 ± 0.54 ng/mL, P < 0.01), and folate levels were consistently lower in gastric cancer patients regardless of age and gender (all P < 0.01). Using the median serum folate value as the cutoff value, low serum folate was significantly associated with gastric cancer risk in the whole population (OR = 19.77, 95%CI: 10.54-37.06, P < 0.001) and all strata (age < 60 years OR = 17.39, 95%CI: 7.28-41.54, age ≥ 60 years (OR = 21.67, 95%CI: 8.27-56.80), males (OR = 17.95, 95%CI: 7.93-40.62), and females (OR = 20.95, 95%CI: 7.66-57.31); all P < 0.001. In the patient cohort of gastric cancer, the respective cutoff values showed that low serum folate levels were significantly associated with serosal invasion (OR = 2.54, 95%CI: 1.23-5.23), lymphatic invasion (OR = 2.23, 95%CI: 1.17-4.26), and liver metastasis (OR = 6.67, 95%CI: 1.28-34.91) of gastric cancer (all P < 0.05). Serum folate level below 1.90 ng/mL was associated with poor patient survival (HR = 1.84, 95%CI: 1.04-3.27, P < 0.05) in univariate analysis.
CONCLUSION: Lower serum folate levels were significantly associated with gastric cancer development and invasive phenotypes. The role of folate depletion in gastric cancer invasion warrants further study.
Keywords: Folic acid, Folate, Plasma, Metastasis, Invasion
Core tip: Low folate status is involved in the development of gastric cancer, but the role of folate in invasiveness of gastric cancer remains unclear. In addition, although folate levels in blood may reflect the degree of folate depletion, an association between blood folate status and gastric cancer has not been established. In this case-control study, we found lower serum folate was significantly associated with gastric cancer development. Besides, in the patient cohort of gastric cancer, lower serum folate was significantly associated with invasive phenotypes such as serosal invasion, lymphatic invasion and liver metastasis. These findings warrant further study.
INTRODUCTION
Gastric cancer remains the fourth most common cancer and the second leading cause of cancer mortality worldwide, and the median survival in patients with advanced (stages III-IV) gastric cancer is generally less than one year[1-3]. Due to poor treatment response in advanced gastric cancer, detection of early-stage gastric cancer can effectively improve outcomes[4]. However, early diagnosis remains a challenge in most countries due to invasive characteristic of gastroscopy and a lack of practical screening biomarkers, and most patients already suffer from advanced tumor when gastric cancers are newly diagnosed[5]. In addition, the optimal degree of lymph node dissection for gastric cancer is still matter of debate, and inaccurate pre-operative image studies of lymph node metastasis frequently result in over- or incomplete resection[6]. New biomarkers to screen for and predict the invasiveness of gastric cancer may provide novel ways to resolve the dilemma and are therefore urgently needed[7,8].
Folate is involved in biological methylation reactions and nucleotide biosynthesis, and depletion of folate can result in global DNA hypomethylation, DNA damage, impaired DNA repair and altered proto-oncogene/tumor suppressor gene expressions[9,10]. Folate supplementation was recently reported to significantly increase global DNA methylation and reduce mucosal inflammation and dysplasia in a Helicobacter pylori-infected mouse model, and gastric cancer may be prevented by high folate intake[11]. Although the results of studies on dietary folate intake and risk of gastric cancer were inconsistent[12-14], gene 677CT polymorphism of methylenetetrahydrofolate reductase (MTHFR), a key enzyme in the metabolism of folate, was associated with increased risk of gastric cancer for individuals with low folate status[15,16]. Moreover, folate concentrations were reported to be significantly lower in gastric cancer tissues than in gastritis tissues, and hypomethylation and overexpression of c-myc were correlated with lower folate concentrations in tissues[17]. Increasing evidence suggests low folate status is involved in the development of gastric cancer, but the role of folate in gastric cancer invasiveness remains unclear.
Although folate levels in blood may reflect the degree of folate depletion, an association between blood folate status and gastric cancer has not been established. In a study of advanced gastric cancer, plasma folic acid concentration was lower in patients with total genomic DNA hypomethylation than that in patients with normal methylation[18]. Tan et al[19] observed that the mean serum folate concentration of gastric cancer cases was significantly lower than that of controls. However, Vollset et al[20] reported a null association between plasma folate levels and gastric cancer risk, but a mean duration of 3.3 years between blood donation and cancer diagnosis could have biased the observed results. The serial changes of serum folate were not reported when patients developed gastric cancer, and further studies to verify lower blood folate in gastric cancer patients are needed.
To our knowledge, the associations between serum folate levels, invasive phenotypes and patient survival of gastric cancer have not been evaluated. Therefore, we conducted a study to determine whether serum folate level could be clinically associated with development, invasiveness and patient survival of gastric cancer.
MATERIALS AND METHODS
Study subjects
In this case-control study, we used blood samples collected from individuals participating in two national projects conducted from January 1998 to April 2006 for the investigation of risk factors of gastric cancer in Taiwan[21,22]. Patients with newly diagnosed gastric cancer undergoing gastrectomy were enrolled, and patients receiving chemotherapy prior to surgery, with other concurrent malignancy, or of the aboriginal and alien populations were excluded. Control blood samples were obtained from individuals who visited health examination clinics with minimal gastritis or normal appearance of the gastric mucosa on gastroscopic examination. Informed consents were obtained from all subjects and/or guardians on a voluntary basis, and all patient-derived specimens were collected under protocols approved by the Institutional Review Boards (IRBs) of the parent institutions (IRB No. C07199). All the study subjects who fulfilled the inclusion and exclusion criteria and signed the informed consents were recruited.
In total, we studied 155 patients with gastric cancer, for whom complete clinical data, including tumor stage, degree of tumor invasion and presence of metastasis, and a serum sample were available. All recruited patients had been followed up for at least 5 years. The controls were matched by age (within 5 years) and date of blood collection (± 3 mo), and 149 cases were recruited. In addition, status of Helicobacter pylori (H. pylori) infection was determined by Giemsa staining of gastric tissue biopsy or by serum H. pylori antibody test (INOVA Diagnostics, San Diego, CA).
Measurement of serum folate levels
Blood samples were obtained after overnight fasting, and serum folate concentrations were measured by microbiological assay[23]. Cryopreserved Lactobacillus casei (NCIB 10463) culture were thawed into reconstituted assay medium in a water bath at 37 °C, and then added to bulk assay medium at a concentration of 200 μL/100 mL. Sodium ascorbate (0.5%) solution was used to dilute serum samples 1:20. Standard solution of folate (500 pg/mL) was made by diluting stock standards in sodium ascorbate. Assay microorganism was added, and the plates were sealed and incubated at 37 °C in the dark for 42 h after adding disinfectant. Then they were read at 570 nm and serum folate concentrations were calculated.
Statistical analysis
The discrete variables are presented as number and percentage (%); continuous variables are presented as mean and standard error. The demographic data of patients and controls were compared using the χ2 test and Student’s t-test. To avoid possible confounding effects of age and gender, multivariate logistic regression analyses were conducted to evaluate the associations between serum folate levels, gastric cancer risk, and clinicopathological features. Using the median value of serum folate computed among the overall population of cases and control as the cutoff value, the associations between serum folate and gastric cancer in all cases and different age and gender subgroups were analyzed. In the patient cohort of gastric cancer, we considered that the cutoff values in various clinicopathological features should be different, so nonparametric receiver-operating characteristics (ROC) analyses were plotted for determining the best cutoff values of serum folate in various clinicopathological features. Using the respective cutoff values, the associations between serum folate levels and clinicopathological features were analyzed. Furthermore, to determine whether serum folate concentration was associated with patients’ outcomes, survival analyses were conducted using the Cox proportional hazards model. Data were analyzed using SPSS, version 11.0 (SPSS Inc., Chicago, Illinois, United States). Nonparametric ROC analyses were performed via the STATA program, version 8.0 (Stata Corporation, College Station, Texas, United States).
RESULTS
Association between serum folate levels and gastric cancer
Demographic characteristics are summarized in Table 1. Control subjects were on average about 5 years younger than gastric cancer cases. There were no differences in gender distribution or H. pylori infection status between the two groups. The mean serum folate level was significantly lower in gastric cancer patients compared to that in controls (3.71 ± 0.30 ng/mL vs 8.00 ± 0.54 ng/mL, P < 0.01). Since gastric cancer patients were slightly older than controls, we further analyzed folate concentrations based on age and gender, and found folate levels were consistently lower in gastric cancer patients regardless of age and gender (all P < 0.01).
Table 1.
Demographic data and folate concentrations of gastric cancer patients and controls
| Case (n = 155) | Control (n = 149) | P value1 | |
| Age (yr) | 62.02 ± 1.14 | 57.21 ± 0.86 | < 0.01 |
| Gender n (%) | |||
| Male | 88 (56.8) | 90 (60.4) | NS |
| Female | 67 (43.2) | 59 (39.6) | |
| H. pylori infection n (%) | |||
| No | 83 (53.5) | 78 (52.3) | NS |
| Yes | 72 (46.5) | 71 (47.7) | |
| Folate (ng/mL) | 3.71 ± 0.30 | 8.00 ± 0.54 | < 0.01 |
| Male | 3.12 ± 0.34 | 7.34 ± 0.74 | < 0.01 |
| Female | 4.49 ± 0.54 | 9.01 ± 0.76 | < 0.01 |
| Age ≥ 60 | 3.70 ± 0.42 | 9.87 ± 1.17 | < 0.01 |
| Age < 60 | 3.72 ± 0.43 | 6.78 ± 0.43 | < 0.01 |
All P values ≥ 0.05 were considered to be statistically non-significant (NS).
Using the median value of serum folate (4.38 ng/mL) computed among the overall population of cases and control as the cutoff value, the adjusted odds ratio (OR) for detecting the occurrence of gastric cancer was 19.77 (95%CI: 10.54-37.06) (Table 2). In addition, serum folate level lower than 4.38 ng/mL was found to be consistently associated with higher gastric cancer risk in all strata (age < 60 years, age ≥ 60 years, males, and females; all P < 0.001). No significant interaction of other variables was observed.
Table 2.
Association between serum folate level and occurrence of gastric cancer n (%)
|
Folate |
Odds ratio1 |
|||
| > 4.38 ng/mL | ≤ 4.38 ng/mL | 95%CI | P value | |
| All cases | ||||
| Control (n = 149) | 119 (79.9) | 30 (20.1) | 1 | |
| Gastric cancer (n = 155) | 33 (21.3) | 122 (78.7) | 19.77 (10.54-37.06) | < 0.001 |
| Age < 60 (yr) | ||||
| Control (n = 90) | 70 (77.8) | 20 (22.2) | 1 | |
| Gastric cancer (n = 59) | 10 (16.9) | 49 (83.1) | 17.39 (7.28-41.54) | < 0.001 |
| Age ≥ 60 (yr) | ||||
| Control (n = 59) | 49 (83.1) | 10 (16.9) | 1 | |
| Gastric cancer (n = 96) | 23 (24.0) | 73 (76.0) | 21.67 (8.27-56.80) | < 0.001 |
| Male | ||||
| Control (n = 90) | 68 (75.6) | 22 (24.4) | 1 | |
| Gastric cancer (n = 88) | 14 (15.9) | 74 (84.1) | 17.95 (7.93-40.62) | < 0.001 |
| Female | ||||
| Control (n = 59) | 51 (86.4) | 8 (13.6) | 1 | |
| Gastric cancer (n = 67) | 19 (28.4) | 48 (71.6) | 20.95 (7.66-57.31) | < 0.001 |
Age and gender were adjusted by multivariate regression analysis.
Association between serum folate levels and invasiveness of gastric cancer
To further assess the value of using serum folate levels for detecting invasiveness among gastric cancer patients, we calculated the best cutoff values of various invasive phenotypes by ROC analyses. Using the cutoff values for multivariate regression analyses, the adjusted ORs for detecting the invasiveness of gastric cancer were obtained, as shown in Table 3. Serum folate ≤ 2.61 ng/mL was significantly associated with serosal invasion (OR = 2.54, 95%CI: 1.23-5.23) and lymphatic invasion (OR = 2.23, 95%CI: 1.17-4.26). Serum folate ≤ 1.90 ng/mL was significantly correlated with liver metastasis (OR = 6.67, 95%CI: 1.28-34.91) (all P < 0.05).
Table 3.
Associations between serum folate levels and clinicopathological features of gastric cancer n (%)
|
Folate |
Odds ratio1 |
|||
| > 2.61 ng/mL | ≤ 2.61 ng/mL | 95%CI | P value2 | |
| Stage | ||||
| Early (n = 28) | 14 (50.0) | 14 (50.0) | 1 | |
| Advanced (n = 127) | 54 (42.5) | 73 (57.5) | 1.38 (0.60-3.14) | NS |
| Serosal invasion | ||||
| Absent (n = 43) | 26 (60.5) | 17 (39.5) | 1 | |
| Present (n = 112) | 42 (37.5) | 70 (62.5) | 2.54 (1.23-5.23) | < 0.05 |
| Lymph node metastasis | ||||
| Absent (n = 51) | 26 (51.0) | 25 (49.0) | 1 | |
| Present (n = 104) | 42 (40.4) | 62 (59.6) | 1.54 (0.78-3.02) | NS |
| Venous invasion | ||||
| Absent (n = 90) | 42 (46.7) | 48 (53.3) | 1 | |
| Present (n = 65) | 26 (40.0) | 39 (60.0) | 1.32 (0.69-2.52) | NS |
| Lymphatic invasion | ||||
| Absent (n = 74) | 40 (54.1) | 34 (45.9) | 1 | |
| Present (n = 81) | 28 (34.6) | 53 (65.4) | 2.23 (1.17-4.26) | < 0.05 |
| Liver metastasis | (> 1.9 ng/mL) | (≤ 1.90 ng/mL) | ||
| Absent (n = 146) | 104 (71.2) | 42 (28.8) | 1 | |
| Present (n = 8) | 2 (25.0) | 6 (75.0) | 6.67 (1.28-34.91) | <0.05 |
Age and gender were adjusted by multivariate regression analysis;
All P values ≥ 0.05 were considered to be statistically non-significant (NS).
Association between serum folate levels and patient survival
The survival curves for patients with different serum folate levels are compared in Figure 1. A folate level lower than 1.90 ng/mL was associated with poor survival (P = 0.03). Cox proportional hazard model analyses showed that advanced stage (P < 0.001), serosal invasion (P < 0.001), lymph node metastasis (P < 0.001), lymphatic invasion (P < 0.001), venous invasion (P < 0.001), liver metastasis (P < 0.001), and serum folate level lower than 1.90 ng/mL (P = 0.036) were associated with poor overall survival (Table 4). Multivariate analysis showed that only serosal invasion (P < 0.001) and liver metastasis (P < 0.001) were independent risk factors for poor overall survival.
Figure 1.
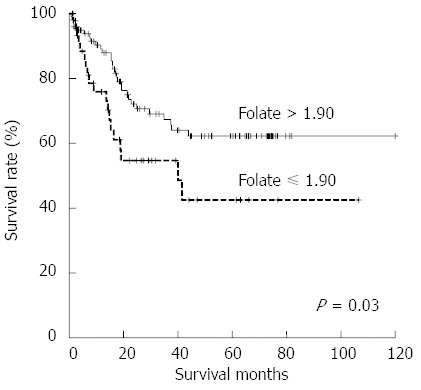
Survival curves for patients with different serum folate levels. The survival rate for those with lower folate levels was significantly lower compared with those with higher folate levels (P = 0.03).
Table 4.
Univariate analysis of mortality predictors in gastric cancer patients
| Hazard ratio | 95%CI | P value1 | |
| Age | |||
| > 60 vs ≤ 60 | 1.27 | 0.97-1.67 | NS |
| Gender | |||
| Male vs female | 1.29 | 0.98-1.69 | NS |
| Stage | |||
| Advanced vs early | 7.78 | 3.96-15.26 | < 0.001 |
| Depth of invasion | |||
| Serosal vs non-serosal invasion | 3.96 | 2.67-5.89 | < 0.001 |
| Lymph node metastasis | |||
| Yes vs no | 2.47 | 1.78-3.43 | < 0.001 |
| Lymphatic invasion | |||
| Yes vs no | 2.17 | 1.53-3.07 | < 0.001 |
| Venous invasion | |||
| Yes vs no | 2.45 | 1.73-3.46 | < 0.001 |
| Liver metastasis | |||
| Yes vs no | 6.01 | 4.04-8.93 | < 0.001 |
| Folate | |||
| ≤ 1.90 vs > 1.90 ng/mL | 1.84 | 1.04-3.27 | 0.036 |
All P values ≥ 0.05 were considered to be statistically non-significant (NS).
DISCUSSION
Previous studies have shown that low folate status is involved in the development of gastric cancer[15-17], but the role of folate in the invasiveness of gastric cancer is still unclear. To our knowledge, this is the first study to investigate whether serum folate could be clinically associated with invasiveness of gastric cancer. We found low serum folate levels were significantly associated with various invasive phenotypes such as serosal invasion, lymphatic invasion and liver metastasis. The findings of our study suggest that folate depletion may play a role in the development of gastric cancer, but the effect of folate depletion in gastric cancer invasion warrants further investigation.
Although folate supplementation has been shown to be effective in preventing global loss of methylation in a mouse model of gastric cancer development[11], further study will be required to determine whether folate depletion and subsequent perturbed hypomethylation directly contribute to tumor invasion and metastasis. In a recent study, Wang et al[24] found that folate deficiency could enhance invasiveness of colon cancer cells by activation of Shh signaling through promoter hypomethylation and cross actions with the NF-κB pathway. However, the effect of folate deficiency on methyl group metabolism and methylation is highly complex, and is influenced by cell type, stage of transformation, and genetic variations at specific sites in the genome[25-28]. For example, in animal studies of colorectal cancer, although folate showed chemoprotective effects against carcinogenesis, it also appeared to promote progression of cancer once neoplastic foci had been established[29-31]. The mechanisms of folate deficiency that contribute to the invasiveness of gastric cancer have yet to be fully elucidated.
Few studies have investigated the association between blood folate status and gastric cancer development, and the usefulness of blood folate as a biomarker is still a subject of debate. A Chinese case-control study showed that gastric cancer patients had a significantly lower serum folate level than that of controls, but a European study found there was no significant difference in plasma folate level between pre-gastric cancer patients and the controls[19,20]. However, in that European study, the blood samples were collected 3.3 years (mean, with a range of 0.4-7.13 years) before diagnosis of gastric cancer, and this discrepancy could be biased by the time interval between blood donations and cancer diagnosis. In addition, other risk factors may be also involved in the discrepancy between Chinese and European populations. For example, in a recent meta-analysis[32], the gene polymorphism in thymidylate synthase, an important enzyme involved in folate metabolism, was associated with an increased risk of gastroesophageal cancer among Asians, but not among Caucasians. However, the interaction between other risk factor and blood folate in different populations warrants further evaluation. In the present study of Chinese population, we confirmed that the serum folate level was significantly lower in patients than that in controls, and low serum folate was significantly associated with increased gastric cancer risk regardless of age and gender. According to the observations of previous studies and our results, decreased folate levels can be found after the development of gastric cancer. Further studies to confirm the causal relationship between folate depletion and gastric cancer evolution will be crucial.
The association between folate and outcomes of gastric cancer has rarely been investigated, although MTHFR 677TT carriers with low folate and vitamin B12 intakes were reported to have the lowest survival rate in a cohort study of gastric cancer[33]. The present investigation is the first to report that a low serum folate level was associated with poor patient survival in univariate analysis, but the association became insignificant in multivariate analysis. Although low serum folate level was not associated with prognosis in this study, outcome analysis could be biased by different strategies and clinical conditions in cancer treatment. Large-scale well-controlled prospective studies will be needed to determine the value of serum folate level as a prognostic biomarker.
Decreased serum folate levels may be caused by poor intake of folate-rich foods, impaired folate absorption, impaired folate metabolism or rapid folate consumption. Interestingly, the role of dietary folate intake in the development of gastric cancer has been reported in several studies, with controversial results[12-14]. Larsson et al[12] conducted a meta-analysis and found significant heterogeneity, especially among different geographic regions. The estimated relative risks for subjects with the highest dietary folate intake relative to the lowest dietary folate intake were 0.68 (95%CI: 0.58-0.80) for studies conducted in the United States, 1.15 (95%CI: 0.91-1.45) for European studies, and 0.89 (95%CI: 0.40-1.96) for studies conducted elsewhere. The disparity in these results may be due to complex host-environment interactions. Moreover, a variety of conditions, including defects in the uptake system, gastrointestinal diseases, drug interaction, and hypochlorhydria may also affect the normal intestinal folate absorption process[34,35]. Gastric cancer arises by a multi-stage process, and severity of atrophic gastritis is correlated to the risk of progression to gastric cancer[36]. Although low serum folate in patients with gastric cancer may be caused by disturbed folate absorption due to severe underlying atrophic gastritis, the possible mechanisms involved in folate depletion may be complicated and should be further clarified.
The association between H. pylori infection and folate depletion has been evaluated in previous studies. In a recent meta-analysis[35], no significant association between H. pylori infection and folate levels was observed, and eradication of H. pylori infection had no effect on serum folate levels. In this case-control study, the impact of H. pylori infection on serum folate levels was also not significant. However, the proportions of patients with H. pylori infection were similar in the gastric cancer group and the matched control group, and the prevalence of H. pylori infection in the gastric cancer group may be underestimated due to H. pylori eradication therapy and lower sensitivity of H. pylori test in severe atrophic gastritis[37]. Further study may help to clarify the effect of H. pylori infection on folate levels among gastric cancer patients.
There are several limitations to this study. First, although selection bias could not be completely excluded, we enrolled study subjects in a multi-center setting. This should help minimize the selection bias. Second, despite the fact that a causal relationship between folate depletion and gastric cancer evolution could not be determined in this clinical study; we provided important clues for further study. Third, although the controls were matched by age (within 5 years) in the present study, gastric cancer patients were significantly older than the controls. Using multivariate regression and subgroup analyses, we didn’t observe significant correlation with age. Fourth, some important information could not be fully obtained in this retrospective study; for example, body mass index (BMI). Even though the mean BMI values of study subjects with available data were not significantly different between the gastric cancer group and the control group, we did not present the findings because BMI data for some subjects were missing. A prospective study could provide a more complete description of study population. Finally, although we did not analyze other environmental factors, such as cigarette smoking or alcohol consumption in this retrospective study, associations between lower blood folate levels and these environmental factors in gastric cancer patients were not reported in previous studies[19]. Further prospective control studies are needed to clarify the effects of other risk factors.
The present study demonstrated several important findings: first, lower serum folate was observed in gastric cancer patients. Second, we found strong relationships between lower serum folate and various invasive phenotypes, including serosal invasion, lymphatic invasion and liver metastasis in gastric cancer. Third, serum folate level was demonstrated to be a potentially useful biomarker of gastric cancer progression in terms of occurrence, serosal invasion, lymphatic invasion and liver metastasis, but further studies are needed to confirm that serum folate is an important biomarker for gastric cancer. In conclusion, our study findings suggest that folate depletion may play a role in the development and invasiveness of gastric cancer, but the effect of folate depletion in gastric cancer invasion warrants further study.
COMMENTS
Background
Treatment response in advanced gastric cancer is usually poor, but detection of early-stage gastric cancer can effectively improve outcomes. Early diagnosis remains a challenge, and most patients already suffer from advanced tumor when gastric cancers are newly diagnosed. In addition, inaccurate pre-operative image studies of lymph node metastasis frequently result in over- or incomplete resection. New biomarkers to screen for and predict the invasiveness of gastric cancer are urgently needed.
Research frontiers
Folate is involved in biological methylation reactions and nucleotide biosynthesis, and depletion of folate can result in carcinogenesis. Folate supplementation was recently reported to significantly increase global DNA methylation and reduce mucosal inflammation and dysplasia, and gastric cancer may be prevented by high folate intake. Increasing evidence suggests low folate status is involved in the development of gastric cancer, but the role of folate in gastric cancer invasiveness remains unclear.
Related publications
An association between blood folate status and gastric cancer has not been established. The mean serum folate concentration of gastric cancer cases was significantly lower than that of controls in a Chinese study, but a null association between plasma folate levels and gastric cancer risk was reported in a European study. The associations between serum folate levels, invasive phenotypes and patient survival of gastric cancer have not been evaluated.
Innovations and breakthroughs
In this case-control study, authors found lower serum folate was significantly associated with gastric cancer development. Besides, in the patient cohort of gastric cancer, lower serum folate was significantly associated with invasive phenotypes such as serosal invasion, lymphatic invasion and liver metastasis. However, serum folate level was not associated with patient survival.
Applications
Serum folate level was demonstrated to be a potentially useful biomarker of gastric cancer progression in terms of occurrence, serosal invasion, lymphatic invasion and liver metastasis, but further studies are needed to confirm that serum folate is an important biomarker for gastric cancer.
Terminology
Folate is a naturally occurring form of the vitamin B that can be found in food. Depletion of folate can result in global DNA hypomethylation, DNA damage, impaired DNA repair and altered proto-oncogene/tumor suppressor gene expressions.
Peer review
This is valuable research which addresses an important topic. In this case-control study, the serum folate level was significantly lower in gastric cancer patients than that in controls, and folate levels were consistently lower in gastric cancer patients regardless of age and gender. In the patient cohort of gastric cancer, low serum folate levels were significantly associated with invasive phenotypes of gastric cancer. Findings of this study suggest that folate depletion may play a role in the development and invasiveness of gastric cancer, but the effect of folate depletion in gastric cancer invasion warrants further investigation.
Footnotes
Supported by National Science Council, Executive Yuan, No. NSC-96-2314-B-075A-007, No. NSC100-2628-B005-002MY4, No. NSC101-2320-B-005-006-MY3 and No. NSC 101-2911-I-005-301; and the ATU plan of the Ministry of Education, Taiwan
P- Reviewer: Gallus S, Massironi S, Solga SF, Tu H S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
References
- 1.Roukos DH. Current status and future perspectives in gastric cancer management. Cancer Treat Rev. 2000;26:243–255. doi: 10.1053/ctrv.2000.0164. [DOI] [PubMed] [Google Scholar]
- 2.Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903–2909. doi: 10.1200/JCO.2005.05.0245. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.González CA, Agudo A. Carcinogenesis, prevention and early detection of gastric cancer: where we are and where we should go. Int J Cancer. 2012;130:745–753. doi: 10.1002/ijc.26430. [DOI] [PubMed] [Google Scholar]
- 5.El Abiad R, Gerke H. Gastric cancer: endoscopic diagnosis and staging. Surg Oncol Clin N Am. 2012;21:1–19. doi: 10.1016/j.soc.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Catarci M, Montemurro LA, Di Cintio A, Ghinassi S, Coppola L, Pinnarelli L, Belardi A, Koch M, Grassi GB. Lymph node retrieval and examination during the implementation of extended lymph node dissection for gastric cancer in a non-specialized western institution. Updates Surg. 2010;62:89–99. doi: 10.1007/s13304-010-0017-8. [DOI] [PubMed] [Google Scholar]
- 7.Oh SY, Kwon HC, Kim SH, Lee S, Lee JH, Graves CA, Camphausen K, Kim HJ. Prognostic significance of serum levels of vascular endothelial growth factor and insulin-like growth factor-1 in advanced gastric cancer patients treated with FOLFOX chemotherapy. Chemotherapy. 2012;58:426–434. doi: 10.1159/000345918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523–1529. doi: 10.1093/annonc/mdn169. [DOI] [PubMed] [Google Scholar]
- 9.Kim YI. Folate and DNA methylation: a mechanistic link between folate deficiency and colorectal cancer? Cancer Epidemiol Biomarkers Prev. 2004;13:511–519. [PubMed] [Google Scholar]
- 10.Duthie SJ, Narayanan S, Blum S, Pirie L, Brand GM. Folate deficiency in vitro induces uracil misincorporation and DNA hypomethylation and inhibits DNA excision repair in immortalized normal human colon epithelial cells. Nutr Cancer. 2000;37:245–251. doi: 10.1207/S15327914NC372_18. [DOI] [PubMed] [Google Scholar]
- 11.Gonda TA, Kim YI, Salas MC, Gamble MV, Shibata W, Muthupalani S, Sohn KJ, Abrams JA, Fox JG, Wang TC, et al. Folic acid increases global DNA methylation and reduces inflammation to prevent Helicobacter-associated gastric cancer in mice. Gastroenterology. 2012;142:824–833.e7. doi: 10.1053/j.gastro.2011.12.058. [DOI] [PubMed] [Google Scholar]
- 12.Larsson SC, Giovannucci E, Wolk A. Folate intake, MTHFR polymorphisms, and risk of esophageal, gastric, and pancreatic cancer: a meta-analysis. Gastroenterology. 2006;131:1271–1283. doi: 10.1053/j.gastro.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Pelucchi C, Tramacere I, Bertuccio P, Tavani A, Negri E, La Vecchia C. Dietary intake of selected micronutrients and gastric cancer risk: an Italian case-control study. Ann Oncol. 2009;20:160–165. doi: 10.1093/annonc/mdn536. [DOI] [PubMed] [Google Scholar]
- 14.Xiao Q, Freedman ND, Ren J, Hollenbeck AR, Abnet CC, Park Y. Intakes of folate, methionine, vitamin B6, and vitamin B12 with risk of esophageal and gastric cancer in a large cohort study. Br J Cancer. 2014;110:1328–1333. doi: 10.1038/bjc.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boccia S, Hung R, Ricciardi G, Gianfagna F, Ebert MP, Fang JY, Gao CM, Götze T, Graziano F, Lacasaña-Navarro M, et al. Meta- and pooled analyses of the methylenetetrahydrofolate reductase C677T and A1298C polymorphisms and gastric cancer risk: a huge-GSEC review. Am J Epidemiol. 2008;167:505–516. doi: 10.1093/aje/kwm344. [DOI] [PubMed] [Google Scholar]
- 16.Sun L, Sun YH, Wang B, Cao HY, Yu C. Methylenetetrahydrofolate reductase polymorphisms and susceptibility to gastric cancer in Chinese populations: a meta-analysis. Eur J Cancer Prev. 2008;17:446–452. doi: 10.1097/CEJ.0b013e328305a140. [DOI] [PubMed] [Google Scholar]
- 17.Weng YR, Sun DF, Fang JY, Gu WQ, Zhu HY. Folate levels in mucosal tissue but not methylenetetrahydrofolate reductase polymorphisms are associated with gastric carcinogenesis. World J Gastroenterol. 2006;12:7591–7597. doi: 10.3748/wjg.v12.i47.7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang JY, Xiao SD, Zhu SS, Yuan JM, Qiu DK, Jiang SJ. Relationship of plasma folic acid and status of DNA methylation in human gastric cancer. J Gastroenterol. 1997;32:171–175. doi: 10.1007/BF02936363. [DOI] [PubMed] [Google Scholar]
- 19.Tan W, Miao X, Wang L, Yu C, Xiong P, Liang G, Sun T, Zhou Y, Zhang X, Li H, et al. Significant increase in risk of gastroesophageal cancer is associated with interaction between promoter polymorphisms in thymidylate synthase and serum folate status. Carcinogenesis. 2005;26:1430–1435. doi: 10.1093/carcin/bgi090. [DOI] [PubMed] [Google Scholar]
- 20.Vollset SE, Igland J, Jenab M, Fredriksen A, Meyer K, Eussen S, Gjessing HK, Ueland PM, Pera G, Sala N, et al. The association of gastric cancer risk with plasma folate, cobalamin, and methylenetetrahydrofolate reductase polymorphisms in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2007;16:2416–2424. doi: 10.1158/1055-9965.EPI-07-0256. [DOI] [PubMed] [Google Scholar]
- 21.Wu CY, Wu MS, Chiang EP, Wu CC, Chen YJ, Chen CJ, Chi NH, Chen GH, Lin JT. Elevated plasma osteopontin associated with gastric cancer development, invasion and survival. Gut. 2007;56:782–789. doi: 10.1136/gut.2006.109868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu MS, Wu CY, Chen CJ, Lin MT, Shun CT, Lin JT. Interleukin-10 genotypes associate with the risk of gastric carcinoma in Taiwanese Chinese. Int J Cancer. 2003;104:617–623. doi: 10.1002/ijc.10987. [DOI] [PubMed] [Google Scholar]
- 23.O’Broin S, Kelleher B. Microbiological assay on microtitre plates of folate in serum and red cells. J Clin Pathol. 1992;45:344–347. doi: 10.1136/jcp.45.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang TP, Hsu SH, Feng HC, Huang RF. Folate deprivation enhances invasiveness of human colon cancer cells mediated by activation of sonic hedgehog signaling through promoter hypomethylation and cross action with transcription nuclear factor-kappa B pathway. Carcinogenesis. 2012;33:1158–1168. doi: 10.1093/carcin/bgs138. [DOI] [PubMed] [Google Scholar]
- 25.Stempak JM, Sohn KJ, Chiang EP, Shane B, Kim YI. Cell and stage of transformation-specific effects of folate deficiency on methionine cycle intermediates and DNA methylation in an in vitro model. Carcinogenesis. 2005;26:981–990. doi: 10.1093/carcin/bgi037. [DOI] [PubMed] [Google Scholar]
- 26.Chiang EP, Wang YC, Tang FY. Folate restriction and methylenetetrahydrofolate reductase 677T polymorphism decreases adoMet synthesis via folate-dependent remethylation in human-transformed lymphoblasts. Leukemia. 2007;21:651–658. doi: 10.1038/sj.leu.2404575. [DOI] [PubMed] [Google Scholar]
- 27.Sohn KJ, Jang H, Campan M, Weisenberger DJ, Dickhout J, Wang YC, Cho RC, Yates Z, Lucock M, Chiang EP, et al. The methylenetetrahydrofolate reductase C677T mutation induces cell-specific changes in genomic DNA methylation and uracil misincorporation: a possible molecular basis for the site-specific cancer risk modification. Int J Cancer. 2009;124:1999–2005. doi: 10.1002/ijc.24003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neuhouser ML, Nijhout HF, Gregory JF, Reed MC, James SJ, Liu A, Shane B, Ulrich CM. Mathematical modeling predicts the effect of folate deficiency and excess on cancer-related biomarkers. Cancer Epidemiol Biomarkers Prev. 2011;20:1912–1917. doi: 10.1158/1055-9965.EPI-10-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song J, Sohn KJ, Medline A, Ash C, Gallinger S, Kim YI. Chemopreventive effects of dietary folate on intestinal polyps in Apc+/-Msh2-/- mice. Cancer Res. 2000;60:3191–3199. [PubMed] [Google Scholar]
- 30.Song J, Medline A, Mason JB, Gallinger S, Kim YI. Effects of dietary folate on intestinal tumorigenesis in the apcMin mouse. Cancer Res. 2000;60:5434–5440. [PubMed] [Google Scholar]
- 31.Lindzon GM, Medline A, Sohn KJ, Depeint F, Croxford R, Kim YI. Effect of folic acid supplementation on the progression of colorectal aberrant crypt foci. Carcinogenesis. 2009;30:1536–1543. doi: 10.1093/carcin/bgp152. [DOI] [PubMed] [Google Scholar]
- 32.Zhou JY, Shi R, Yu HL, Zeng Y, Zheng WL, Ma WL. The association between two polymorphisms in the TS gene and risk of cancer: a systematic review and pooled analysis. Int J Cancer. 2012;131:2103–2116. doi: 10.1002/ijc.27465. [DOI] [PubMed] [Google Scholar]
- 33.Galván-Portillo MV, Oñate-Ocaña LF, Pérez-Pérez GI, Chen J, Herrera-Goepfert R, Chihu-Amparan L, Flores-Luna L, Mohar-Betancourt A, López-Carrillo L. Dietary folate and vitamin B12 intake before diagnosis decreases gastric cancer mortality risk among susceptible MTHFR 677TT carriers. Nutrition. 2010;26:201–208. doi: 10.1016/j.nut.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Said HM. Intestinal absorption of water-soluble vitamins in health and disease. Biochem J. 2011;437:357–372. doi: 10.1042/BJ20110326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lahner E, Persechino S, Annibale B. Micronutrients (Other than iron) and Helicobacter pylori infection: a systematic review. Helicobacter. 2012;17:1–15. doi: 10.1111/j.1523-5378.2011.00892.x. [DOI] [PubMed] [Google Scholar]
- 36.Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 37.Shin CM, Kim N, Lee HS, Lee HE, Lee SH, Park YS, Hwang JH, Kim JW, Jeong SH, Lee DH, et al. Validation of diagnostic tests for Helicobacter pylori with regard to grade of atrophic gastritis and/or intestinal metaplasia. Helicobacter. 2009;14:512–519. doi: 10.1111/j.1523-5378.2009.00726.x. [DOI] [PubMed] [Google Scholar]


