Abstract
AIM: To investigate the indications and outcomes of liver transplantation for hepatic epithelioid hemangioendothelioma (HEHE).
METHODS: Between 1989 and August 2013, in the Department of General, Transplant, and Liver Surgery, Medical University of Warsaw, 1306 orthotopic liver transplantations (OLTx) were performed, including 72 retransplantations. Unresectable HEHE was an indication for OLTx in 10 patients (0.8% of primary OLTx), the mean age of the patients was 40.5 ± 13.3 years (range 23-65 years), and the male-to-female ratio was 2:8. Kaplan-Meier survival analysis in HEHE, hepatocellular carcinoma (HCC), and other OLTx recipients groups was performed. The differences in mortality were compared using the χ2 test. A P-value < 0.05 indicated statistical significance.
RESULTS: No concomitant liver disease was found in any patient. There was no neoadjuvant chemotherapy or radiotherapy. Liver function test results were normal in most of the patients. The levels of alpha-fetoprotein, carcinoembryonic antigen, and carbohydrate antigen 19-9 were normal. In immunohistochemical staining, the neoplastic cells were positive for factor VIII-related antigen, CD31, and CD34, which are endothelial cell markers, and negative for cytokeratin 19, cytokeratin 7, and HepPar-1. Nine patients were alive without tumor recurrence. One patient died 2 mo after OLTx due to septic complications. No morbidity was observed. Maximum follow-up was 11.4 years, with a minimum of 1 mo. The cumulative survival rate at the end of follow-up in HEHE patients was 87.5% compared with 54.3% in the HCC group and 76.3% in the other OLTx recipients group (χ2 test = 1.784, df = 2, P = 0.409).
CONCLUSION: Unresectable HEHE, without extrahepatic metastases is an excellent indication for liver transplantation. Long-term survival is very good and much better than in HCC patients and the entire group of OLTx patients.
Keywords: Hemangioendothelioma, Liver transplantation, Liver malignancies, Transplantation results, Transplantation indications
Core tip: Epithelioid hemangioendothelioma (EHE) of the liver (hepatic EHE, HEHE) is a very rare tumor of mesenchymal origin. It typically occurs in female patients aged 20-40 years. HEHE is resistant to chemotherapy. Unresectable tumor, limited to the liver, may be a good indication for liver transplantation. The aim of this paper was to analyze the indications and outcomes of liver transplantation for HEHE.
INTRODUCTION
Hemangioendothelioma (epithelioid hemangioendothelioma, EHE) is a rare tumor of endothelial and connective tissue origin, resembling hemangioma. This type of tumor was first described by Weiss and Enzinger[1]. Its incidence does not exceed 1 case per million[2]. EHEs are found in soft tissue and internal organs. The most commonly affected organ is the liver (hepatic EHE, HEHE). Other localizations such as the lungs, peritoneum, spleen, bones, brain, meninges, breast, heart, head, and stomach have been described in the literature[3-7].
The mechanisms underlying the development of HEHE are not known. Possible risk factors include oral contraception, toxicity associated with vinyl chloride or asbestos exposure, excessive alcohol consumption, liver injuries, viral hepatitis, and other chronic liver diseases[8].
Numerous recent studies also suggest genetic mechanisms underlying the development of EHE. Specific chromosomal translocation t(1;3)(p36.3;q25) is typical for this type of tumor[9-11].
HEHE is typically diagnosed in young females aged 20-40 years. The prognosis without treatment is poor. Most patients present with disseminated disease, which usually involves both lobes of the liver. Currently there are several methods of treatment available, including surgery (liver resection and transplantation), chemotherapy, transarterial chemoembolization (TACE), radiotherapy, and radiofrequency ablation (RFA). Multifocal unresectable HEHE may be an indication for OLTx[12].
The aim of this study was to assess the outcomes of OLTx in patients with HEHE.
MATERIALS AND METHODS
Between 1989 and August 2013, 1306 orthotopic liver transplantations (OLTx) in adults including 72 re-transplantations (re-OLTx) were performed in the Department of General, Transplant, and Liver Surgery, Medical University of Warsaw. Unresectable HEHE was an indication for OLTx in 10 patients. The mean age of patients was 40.5 ± 13.3 years (range 23-65 years), and the male-to-female was ratio 2:8.
Statistical analysis
We compared Kaplan-Meier distributions of the time to death between HEHE and hepatocellular carcinoma (HCC) groups and with the general liver transplant population, using the χ2 test. The differences were considered statistically significant when the P-value was < 0.05.
RESULTS
The initial referral diagnosis was usually different from the final diagnosis of HEHE. Most commonly the lesions were described as hemangioma, metastases of unknown origin, or parasitic abscesses. In our Department, the diagnosis was based on a wedge liver biopsy obtained during diagnostic laparoscopy. In 2 patients the diagnosis was based on radiologic assessment of the lesions in the liver (computed tomography or magnetic resonance imaging). No patient had neoadjuvant chemotherapy or radiotherapy. Liver function test results were normal in most of the patients. In 4 more advanced cases mild elevation of markers of cholestasis such as alkaline phosphatase (ALP) and gamma-glutamyl transpeptidase (GGTP) was observed. In all the patients with HEHE, the levels of alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), and carbohydrate antigen 19-9 (CA19-9) were normal (Table 1).
Table 1.
Patient data before orthotopic liver transplantations
| Patient | AST (U/L) | ALT (U/L) | Bilirubin (mg/dL) | Albumin (g/dL) | GGTP (U/L) | ALP (U/L) | Creatinine (mg/dL) | Urea (mg/dL) | Fibrinogen (mg/dL) | PLT (103/μL) | INR | CEA (ng/mL) | CA 19.9 (IU/mL) | AFP (ng/mL) |
| 1 | 36 | 112 | 1.36 | 4.20 | 606 | NA | 0.88 | 48.0 | NA | 314 | NA | NA | NA | NA |
| 2 | 27 | 36 | 0.64 | 3.90 | 178 | 206 | 0.70 | 17.8 | 539 | 291 | 1.11 | 0.98 | 2.73 | 4.48 |
| 3 | 155 | 141 | 0.50 | 3.40 | 439 | 185 | 0.76 | 35.8 | 368 | 249 | NA | 1.49 | 2.00 | 2.01 |
| 4 | 19 | 20 | 0.76 | 4.70 | 35 | 81 | 0.63 | 19.0 | 236 | 150 | 0.96 | 0.8 | 7.70 | 1.6 |
| 5 | 13 | 24 | 0.71 | 4.60 | 28 | 74 | 0.99 | 47.0 | 250 | 203 | 1.01 | 1 | 0.70 | 1.3 |
| 6 | 96 | 121 | 1.33 | 4.40 | 13 | 47 | 0.75 | 20.0 | 412 | 207 | 1.10 | 1.2 | 22.90 | 1.5 |
| 7 | 56 | 86 | 0.67 | 3.29 | 297 | 331 | 0.69 | 24.0 | 332 | 199 | 1.01 | 0.8 | 18.80 | 1.9 |
| 8 | 24 | 28 | 0.69 | 4.03 | 19 | 79 | 0.73 | 25.0 | 468 | 42 | 1.02 | 1 | 4.50 | 1.1 |
| 9 | 26 | 30 | 0.85 | 4.14 | 78 | 87 | 0.89 | 25.0 | 356 | 100 | 1.10 | 5.9 | 12.30 | 3.1 |
| 10 | 33 | 32 | 0.61 | 3.50 | 321 | 329 | 0.98 | 37.4 | 593 | 290 | 0.93 | 0.71 | 25.80 | 2.2 |
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; GGTP: Gamma-glutamyl transpeptidase; ALP: Alkaline phosphatase; AFP: Alpha-fetoprotein; CEA: Carcinoembryonic antigen; CA19-9: Carbohydrate antigen 19-9; PLT: Platelets; INR: International normalized ratio; NA: Not avaible.
No concomitant liver disease was found in any patients. The symptoms were usually nonspecific, with mild epigastric or right upper quadrant pain or discomfort. Sometimes fatigue or weight loss was reported (Table 2). The severity of the disease symptoms increase as the disease progressed, with increasing numbers and size of the lesions.
Table 2.
Patient symptoms
| Patient | Symptoms |
| 1 | NA |
| 2 | NA |
| 3 | NA |
| 4 | Abdominal pain or discomfort; fatigue |
| 5 | None |
| 6 | NA |
| 7 | Abdominal pain or discomfort |
| 8 | NA |
| 9 | Abdominal pain or discomfort; fatigue; weight loss; hepatomegaly; dyspnea |
| 10 | Abdominal pain or discomfort |
NA: Not available.
Macroscopically, cross-sections of the resected liver showed multifocal non-encapsulated, yellowish-white tumors that involved both lobes, ranging in diameter from 0.2 to 18 cm. Subcapsular tumors showed typical umbilication.
Microscopically, the tumors had a high cellular growth pattern with infiltrative margins. Histopathological examination demonstrated a variable cellularity - the cellular components were dominant in the peripheral region, while central areas of the nodules showed both necrosis and sclerosis and some were calcified. In the more cellular areas, tumor cells were arranged in solid cords and nests, mimicking epithelioid or histiocytoid cells. In other, less cellular, areas having a sclerotic matrix, single neoplastic cells had irregular cytoplasmic processes. Epithelioid cells were round in shape and had an eosinophilic abundant cytoplasm and vesicular nuclei. Dendritic cells displayed spindle morphology with interdigitating processes. Characteristically, tumors consisted of spindle-shaped cells and signet ring cell-like structures with intracytoplasmic lumina that contained erythrocytes. Architectural features, such as an intravascular or intrasinusoidal growth pattern characteristic of a neoplasm were observed. Microvascular invasion was found in 2 cases. The parenchymal architecture of the liver was preserved. In immunohistochemical staining, the neoplastic cells were positive for factor VIII-related antigen, endothelial cell markers CD31 and CD34, and negative for cytokeratin 19, cytokeratin 7, and HepPar-1 (Figure 1 and Table 3).
Figure 1.
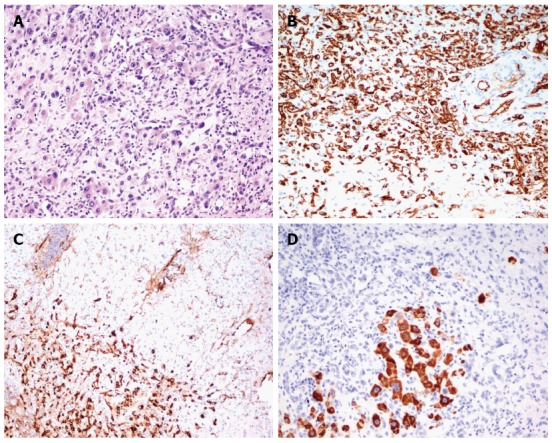
Hepatic epithelioid hemangioendothelioma microscopic staining. A: Hematoxylin and eosin staining × 20; B: CD34 staining × 20; C: Factor VIII staining × 10; D: HepPar1 staining × 20.
Table 3.
Histopathological and immunological characteristics of the tumors
| Patient | Lymph node metastases | Lesions (n)max diameter | Carcinoma cells microembolisms | CD31 | CD34 | Factor VIII | CK7 | MiB | PCNA | Hep | CK19 |
| 1 | + | Multi focal 60 mm | + | + | + | + | - | - | + | - | NA |
| 2 | - | Multi focal 180 mm | NA | NA | + | + | - | NA | NA | - | NA |
| 3 | + | Multi focal 55 mm | + | NA | + | NA | - | NA | NA | - | - |
| 4 | NA | 6 tumors 27 mm | NA | + | + | NA | NA | NA | NA | NA | NA |
| 5 | - | 9 tumors 30 mm | NA | + | + | +/- | - | NA | NA | - | NA |
| 6 | NA | 2 tumors 30 mm | NA | NA | + | + | NA | NA | NA | NA | NA |
| 7 | - | Multi focal 70 mm | NA | + | + | NA | NA | NA | NA | - | - |
| 8 | - | Multi focal 30 mm | NA | NA | + | NA | NA | NA | NA | NA | NA |
| 9 | NA | Multi focal 40 mm | NA | + | + | + | NA | NA | NA | - | - |
| 10 | - | Multi focal 20 mm | NA | + | + | + | NA | NA | NA | NA | - |
NA: Not available.
The follow-up for the HEHE group ranged from 1 mo to 11.4 years. There was no tumor-related mortality. One patient died 2 mo after OLTx due to septic complications not related to the nature of the HEHE. When this case of tumor-unrelated death was excluded from the analysis, the long-term survival in HEHE patients was 100%. No morbidity was observed. The remaining 9 patients are alive without any tumor recurrence symptoms.
Among all patients after OLTx (n = 1234), 3 groups were defined for the purpose of survival analysis: patients with HEHE (n = 10); patients with HCC (n = 155), which is the most common tumor considered an indication for OLTx; and the remaining group of OLTx recipients (n = 1069).
Kaplan-Meier analysis showed a 3-year cumulative survival rate of 87.5% for the HEHE group, 80.1% for the HCC group, and 83.5% in the remaining OLTx recipients group.
The cumulative survival rate at the end of the follow-up was 87.5% in the HEHE patients, compared with 54.3% in the HCC group and 76.3% in the other OLTx recipients group (χ2 test = 1.784, df = 2, P = 0.409) (Figure 2).
Figure 2.
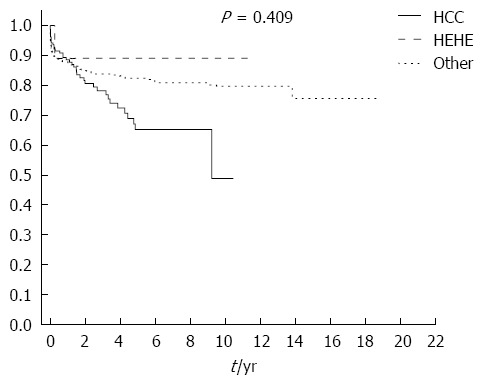
Kaplan-Meier survival analysis. Kaplan-Meier survival analysis in the hepatic epithelioid hemangioendothelioma (HEHE), hepatocellular carcinoma (HCC), and other orthotopic liver transplantations recipients groups.
DISCUSSION
EHE is a very rare tumor. The low incidence precludes any randomized clinical study for the assessment of the best means of treatment. The available publications usually present a retrospective assessment of a single center experience and most of the papers are single case reports. The prognosis depends on the organ involved and, for a hepatic localization, the overall outcome is poor. The disease follows an unpredictable course, which is another confounding factor in the development of a treatment strategy. Disease progression may vary widely from mild with long-term survival[13,14] to severe with rapid deterioration[15,16]. Cases of spontaneous complete remission of HEHE have also been published[17]. There are no reliable predictive factors allowing for the assessment of prognosis. Well known tumor-related factors such as mitotic index, allowing for prediction of malignant potential, did not show any predictive value in cases of HEHE and are considered of low value in clinical practice.
In most of the cases, the disease is asymptomatic or the symptoms are only mild. The most commonly reported symptoms include abdominal pain or right subcostal discomfort related to hepatomegaly. Generalized cachexia or jaundice is less commonly present[18]. Similar symptoms were also observed in the current study patients. Most of the patients presented with normal blood tests. There were increased ALP and GGTP levels present in some cases but the levels of tumor markers such as CEA, CA19-9, and AFP were normal. These results are typical for HEHE[19].
All the studied HEHE tumors had typical pathology and immunohistochemical pattern with positive factor VIII staining and positive endothelial markers CD31 (platelet endothelial cell adhesion molecular 1) and CD34 (human hematopoietic progenitor cell antigen)[20,21].
In 2006, Mehrabi et al[22] published a meta-analysis of studies published between 1984 and 2005 that included 402 cases of HEHE. Treatment details and outcomes were available for 286 cases. It was the largest meta-analysis of HEHE studies. At the time of diagnosis, most patients presented with symptoms of the disease, including, most commonly, right epigastric pain, hepatomegaly, and weight loss. Most of the patients had a multifocal involvement of both lobes of the liver. Extrahepatic disease was present in 36.6% of the patients. The most commonly encountered extrahepatic localizations were the lungs (8.5%), lymph nodes (7.7%), peritoneum (6.1%), bones (4.9%), spleen (3.2%), and diaphragm (1.6%). The most common method of treatment according to the meta-analysis was OLTx (44.8% of cases). Limited extrahepatic focal lesions were not considered an absolute contraindication to OLTx. The outcomes of OLTx are shown in Table 3. In the meta-analysis, 1-year disease-free survival was 81.3% (Table 4). The disease recurrence rate was 27% regardless of the treatment method used. Most of the HEHE liver recurrence was observed more than 2 years after transplantation. There was no recurrence in the study group. In the meta-analysis of Mehrabi et al[22], 21% of patients received radiotherapy or systemic chemotherapy or TACE. There is no consensus on preferable treatment, no criteria for introducing specific medications into a treatment plan, and no prospective studies evaluating their efficacy. In non-randomized observational studies, several drugs such as thalidomide, doxorubicin, 5-fluorouracil, and vincristine led to a reduction in lesion size or improvement in general status. TACE seems to be an acceptable bridge treatment in patients awaiting liver transplantation, similar to patients with HCC. There are single reports of successful use of interferon alpha 2b in the treatment of extrahepatic disease. Limited experience with radiotherapy does not provide enough data to assess its clinical significance. Moreover, in most of the radiotherapy cases, this method was used in combination with chemotherapy. In patients receiving chemotherapy or radiotherapy, 5-year survival was 30%. Liver resection was performed in 9.4% of the patients in the meta-analysis of Mehrabi et al[22]. Radical liver resection might be a treatment of choice, but usually the disease is locally advanced at the time of diagnosis and the patients do not qualify for surgery. Palliative resection is not recommended for this type of tumor due to a high risk of progression and recurrence after surgery[23]. The prognosis after liver resection is independent of the presence of extrahepatic lesions; therefore, extrahepatic disease should not be considered a contraindication to surgical intervention. Mehrabi et al[22] reported that 25% of patients with HEHE did not receive any treatment at all. In most of these cases, 1-year survival was less than 50%. However, there are cases of spontaneous regression. The 5-year survival rate was less than 5%. The longest reported survival exceeded 27 years after the diagnosis of HEHE. Mehrabi et al[22] suggested an algorithm to guide selection of the best treatment for patients with HEHE. Histopathological confirmation of the tumor is followed by the assessment of surgical resectability and the presence of extrahepatic disease. Liver resection should be considered an option, if possible. In patients with bilobar intrahepatic tumor spread without an involvement of other viscera, liver transplantation is the treatment of choice. Adjuvant chemotherapy, neoadjuvant TACE, chemotherapy, and radiotherapy are all available treatment options for cases of extrahepatic lesions.
Table 4.
Hepatic epithelioid hemangioendothelioma treatment results summary
| Ref. | Year (analyzed period) | Source | Patients |
Survival percentage |
||||||
| OLTx | Liver resection | Chemio radio therapy | TACE | No treatment | All | |||||
| Recent report | 2013 (1989-2013) | Single center | 10 | 3-yr | 87.5% | - | - | - | - | - |
| Rodrigue et al[25] | 2008 | UNOS | 110 | 1-yr | 80% | - | - | - | - | - |
| 3-yr | 68% | |||||||||
| 5-yr | 64% | |||||||||
| Lerut et al[24] | 2007 | ELTR | 59 | 1-yr | 93% | - | - | - | - | - |
| 5-yr | 83% | |||||||||
| 10-yr | 72% | |||||||||
| Mehrabi et al[22] | 2006 (1984-2005) | Meta-analysis | 286 | 1-yr | 96% | 100% | 72% | - | 40% | 83.4% |
| 3-yr | 80% | 87% | 49% | 12% | 55.8% | |||||
| 5-yr | 54.5% | 75% | 30% | 4.5% | 41.1% | |||||
| Wang et al[26] | 2012 (2004-2011) | Single center | 33 | 3-yr | - | 74.1% | - | 81.6% | - | 73.3% |
| Grotz et al[27] | 2010 (1984-2007) | Single center | 30 | 1-yr | 91% | 100% | 57% | - | 57% | - |
| 3-yr | 73% | 86% | 43% | 43% | ||||||
| 5-yr | 73% | 86% | 29% | 29% | ||||||
| 10-yr | 42.2% | 86% | - | - | ||||||
| Nudo et al[28] | 2008 (1991-2005) | Multi center | 11 | 5-yr | 82% | - | - | - | - | - |
OLTx: Orthotopic liver transplantations; TACE: Transarterial chemoembolization; UNOS: United Network for Organ Sharing; ELTR: European Liver Transplant Registry.
Lerut et al[24], published data from the European Liver Transplant Registry (ELTR) presenting outcomes of liver transplantation in 59 patients with HEHE and an average follow-up of 78.5 mo. One-third of patients underwent some type of intervention before OLTx. In 17% of patients, extrahepatic lesions were found before OLTx or during the transplantation. Intrahepatic dissemination was present in 96% of the patients. Survival data is shown in Table 3. Recurrence-free survival observed at 1 year, 5 years, and 10 years of follow-up was 90%, 82%, and 64%, respectively. Tumor recurrence was found in 23.7% of patients at an average of 49 mo after OLTx. Recurrence-related mortality was 15.3%. Vascular involvement had a significant influence on the long-term prognosis. Survival was not affected by lymph node metastases, prior treatment, nor the presence of extrahepatic disease[24].
Rodriguez et al[25], in a study based on the United Nations for Organ Sharing database, presented data of 110 patients after OLTx performed between 1987 and 2005. The authors reported survival only slightly inferior to the results from the ELTR database (Table 4)[25].
Wang et al[26] reported the outcomes of 33 patients with HEHE who underwent various interventions other than OLTx. Liver resection was performed in 17 patients, TACE in 12 patients, and liver resection followed by TACE in 3 patients. Only 1 patient had OLTx performed in this group. There was no significant difference in 3-year survival between the treatment groups (Table 4). The authors selected several factors influencing treatment outcome. Older age, clinical symptoms, and elevated CA 19-9 were associated with poorer survival rates. The patients with symptoms (n = 17; P = 0.001, hazard ratio = 86.5) (12 with abdominal pain or discomfort, 3 with chest pain, 1 with weight loss, and 1 with jaundice) had poorer overall survival. The presence of symptoms was validated as the only significant independent prognostic factor (P = 0.012) by multivariate analysis[26].
Grotz et al[27] presented outcomes of 30 patients with HEHE who received treatment at the Mayo Clinic between 1984 and 2007. Liver resection was performed in 11 patients, OLTx in 11 patients, chemotherapy in 5 patients, and the remaining 3 patients received no intervention. The survival rates were similar to the studies reported previously (Table 4). The authors suggested that liver resection may be a suitable solution in patients with a solitary lesion limited to the liver, and OLTx may be offered to patients with multiple lesions in the liver (> 10 lesions and involvement of > 4 segments). Both methods resulted in comparable outcomes regarding survival and recurrence rate. According to the authors from the Mayo Clinic, the extrahepatic lesions (present in 37% of the patients) should not be considered a contraindication to surgery, because they did not influence the outcomes in the study group. Chemotherapy was not effective in this group of patients. Grotz et al[27] presented a treatment protocol based on selected risk factors. The authors focused on the type of intrahepatic dissemination of the tumor, the number of segments involved, the number and size of the lesions, and the presence of extrahepatic disease. Liver resection or RFA of the tumors was suggested in cases with a maximum of 10 lesions measuring not more than 10 cm in diameter and involving not more than 4 segments of the liver. In the other cases, OLTx was recommended. Extrahepatic lesions were not considered a contraindication to any form of surgical intervention, but chemotherapy and metastasectomy were strongly suggested. The authors also recommended liver resection or RFA in the case of local recurrence in the liver[27].
Nudo et al[28], in a Canadian multicenter study, presented treatment outcomes of 11 patients. Four patients received adjuvant therapy for HEHE before OLTx [interferon therapy (n = 1), splenectomy (n = 1), adriamycin therapy (n = 1), and surgical resection (n = 1)]. There was a 36.4% recurrence rate of HEHE during follow-up (on average, 25 mo from OLTx). Two patients with local recurrence underwent liver resection. In the 2 remaining cases of recurrence, radiotherapy or pegylated interferon was administered[28].
Cardinal et al[29] analyzed results of 25 patients who received treatment during 1976-2007 and found that the mean survival time was longer in the OLTx group (172 mo) compared with the TACE group (83 mo), but this difference did not reach statistical significance.
Liver resection should be considered the treatment of choice in patients with resectable HEHE. However, a substantial group of patients present with locally advanced disease that is initially unresectable. The results of the present study show that OLTx is a valid and effective method of treatment in patients with unresectable HEHE. The survival rates of patients after OLTx for HEHE were superior to survival rates of patients with HCC who underwent OLTx. Moreover, the survival of HEHE patients was better than the survival of patients with OLTx for other indications. The small number of patients in the HEHE group compared with the other groups of patients has to be considered, since it may lead to a significant statistical bias. The outcomes presented in the current study are comparable to the best outcomes published in the literature. The authors of the current study suggest following a reasonable qualification protocol that enables selection of an appropriate treatment for a specific patient. In the study group, all of the patients presented with the disease limited to the liver, which might positively influence the outcomes, but most of the authors do not consider extrahepatic lesions a contraindication to OLTx. The use of multiple methods of treatment may result in some benefits for survival related to the presumed synergistic effect of combined surgery and chemotherapy, radiotherapy, or TACE. Further studies are necessary to refine treatment protocols in HEHE, but it may be impossible to produce good quality evidence due to the very low incidence of the tumor.
COMMENTS
Background
Epithelioid hemangioendothelioma (EHE) is a rare tumor of endothelial and connective tissue origin. EHEs are found in soft tissue and internal organs. The most commonly affected organ is the liver. Hepatic EHE (HEHE) is typically diagnosed in young females aged 20-40 years. Most patients present with disseminated disease, which usually involves both lobes of the liver. The treatment options include surgery (liver resection and transplantation), chemotherapy, transarterial chemoembolization (TACE), radiotherapy, and radiofrequency ablation. Multifocal unresectable HEHE may be an indication for orthotopic liver transplantation (OLTx).
Research frontiers
Adequate selection for OLTx is of utmost importance among patients with liver tumors. The comparison of outcomes in OLTx for HEHE with outcomes in patients with other tumors, especially hepatocellular carcinoma, provides a good basis for decision-making in this type of indication for transplantation.
Applications
The results of the present study show that OLTx is a valid and effective method of treatment in patients with unresectable HEHE. A reasonable qualification protocol that enables selection of an appropriate treatment for a specific patient is needed. The use of multiple methods of treatment may result in some benefits for survival related to the presumed synergistic effect of combined surgery and chemotherapy, radiotherapy, or TACE. Further studies are necessary to refine treatment protocols in HEHE but it may be impossible to produce good quality evidence due to the very low incidence of the tumor.
Peer review
The authors described a series of 10 HEHE cases that were candidates for liver transplantation. As reported by the authors, the outcome was very good compared to HCC and other liver diseases as a whole. In general, the article is interesting and represent the experience of a single center, adding a new experience to other published reports, which were well-discussed by the authors.
Footnotes
P- Reviewer: Akarsu M, Galvao FHF, Sira MM, Rodriguez-Peralvarez M S- Editor: Gou SX L- Editor: Cant MR E- Editor: Wang CH
References
- 1.Weiss SW, Enzinger FM. Epithelioid hemangioendothelioma: a vascular tumor often mistaken for a carcinoma. Cancer. 1982;50:970–981. doi: 10.1002/1097-0142(19820901)50:5<970::aid-cncr2820500527>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Hertl M, Cosimi AB. Liver transplantation for malignancy. Oncologist. 2005;10:269–281. doi: 10.1634/theoncologist.10-4-269. [DOI] [PubMed] [Google Scholar]
- 3.Tiu CM, Chou YH, Wang HT, Chang T. Epithelioid hemangioendothelioma of spleen with intrasplenic metastasis: ultrasound and computed-tomography appearance. Comput Med Imaging Graph. 1992;16:287–290. doi: 10.1016/0895-6111(92)90032-5. [DOI] [PubMed] [Google Scholar]
- 4.Lee KC, Ng WF, Chan JK. Epithelioid haemangioendothelioma presenting as a gastric polyp. Histopathology. 1988;12:335–337. [PubMed] [Google Scholar]
- 5.Ellis GL, Kratochvil FJ. Epithelioid hemangioendothelioma of the head and neck: a clinicopathologic report of twelve cases. Oral Surg Oral Med Oral Pathol. 1986;61:61–68. doi: 10.1016/0030-4220(86)90204-5. [DOI] [PubMed] [Google Scholar]
- 6.Marchiano D, Fisher F, Hofstetter S. Epithelioid hemangioendothelioma of the heart with distant metastases. A case report and literature review. J Cardiovasc Surg (Torino) 1993;34:529–533. [PubMed] [Google Scholar]
- 7.Kamath SM, Nagaraj HK, Mysorekar VV. Hepatic and adrenal hemangioendothelioma-a case report. J Clin Diagn Res. 2013;7:2583–2584. doi: 10.7860/JCDR/2013/6808.3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishak KG, Goodman ZD, Stocker JT. Epithelioid haemangioendothelioma. In: Ishak KG, Goodman ZD, Stocker JT, editors. Tumors of the liver and intrahepatic bile ducts. Washington, DC: Armed Forces Institute of Pathology; 2001. pp. 282–293. [Google Scholar]
- 9.Mendlick MR, Nelson M, Pickering D, Johansson SL, Seemayer TA, Neff JR, Vergara G, Rosenthal H, Bridge JA. Translocation t(1; 3)(p36.3; q25) is a nonrandom aberration in epithelioid hemangioendothelioma. Am J Surg Pathol. 2001;25:684–687. doi: 10.1097/00000478-200105000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Errani C, Zhang L, Sung YS, Hajdu M, Singer S, Maki RG, Healey JH, Antonescu CR. A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. Genes Chromosomes Cancer. 2011;50:644–653. doi: 10.1002/gcc.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woelfel C, Liehr T, Weise A, Langrehr J, Kotb WA, Pacyna-Gengelbach M, Katenkamp D, Petersen I. Molecular cytogenetic characterization of epithelioid hemangioendothelioma. Cancer Genet. 2011;204:671–676. doi: 10.1016/j.cancergen.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Marino IR, Todo S, Tzakis AG, Klintmalm G, Kelleher M, Iwatsuki S, Starzl TE, Esquivel CO. Treatment of hepatic epithelioid hemangioendothelioma with liver transplantation. Cancer. 1988;62:2079–2084. doi: 10.1002/1097-0142(19881115)62:10<2079::aid-cncr2820621002>3.0.co;2-j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishak KG, Sesterhenn IA, Goodman ZD, Rabin L, Stromeyer FW. Epithelioid hemangioendothelioma of the liver: a clinicopathologic and follow-up study of 32 cases. Hum Pathol. 1984;15:839–852. doi: 10.1016/s0046-8177(84)80145-8. [DOI] [PubMed] [Google Scholar]
- 14.Makhlouf HR, Ishak KG, Goodman ZD. Epithelioid hemangioendothelioma of the liver: a clinicopathologic study of 137 cases. Cancer. 1999;85:562–582. doi: 10.1002/(sici)1097-0142(19990201)85:3<562::aid-cncr7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi Y, Inagaki K, Hirota S, Yoshikawa T, Ikawa H. Epithelioid hemangioendothelioma with marked liver deformity and secondary Budd-Chiari syndrome: pathological and radiological correlation. Pathol Int. 1999;49:547–552. doi: 10.1046/j.1440-1827.1999.00906.x. [DOI] [PubMed] [Google Scholar]
- 16.Clements D, Hubscher S, West R, Elias E, McMaster P. Epithelioid haemangioendothelioma. A case report. J Hepatol. 1986;2:441–449. doi: 10.1016/s0168-8278(86)80055-1. [DOI] [PubMed] [Google Scholar]
- 17.Otrock ZK, Al-Kutoubi A, Kattar MM, Zaatari G, Soweid A. Spontaneous complete regression of hepatic epithelioid haemangioendothelioma. Lancet Oncol. 2006;7:439–441. doi: 10.1016/S1470-2045(06)70697-0. [DOI] [PubMed] [Google Scholar]
- 18.Läuffer JM, Zimmermann A, Krähenbühl L, Triller J, Baer HU. Epithelioid hemangioendothelioma of the liver. A rare hepatic tumor. Cancer. 1996;78:2318–2327. doi: 10.1002/(sici)1097-0142(19961201)78:11<2318::aid-cncr8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 19.Mistry AM, Gorden DL, Busler JF, Coogan AC, Kelly BS. Diagnostic and therapeutic challenges in hepatic epithelioid hemangioendothelioma. J Gastrointest Cancer. 2012;43:521–525. doi: 10.1007/s12029-012-9389-y. [DOI] [PubMed] [Google Scholar]
- 20.Choi KH, Moon WS. Epithelioid hemangioendothelioma of the liver. Clin Mol Hepatol. 2013;19:315–319. doi: 10.3350/cmh.2013.19.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gill R, O’Donnell RJ, Horvai A. Utility of immunohistochemistry for endothelial markers in distinguishing epithelioid hemangioendothelioma from carcinoma metastatic to bone. Arch Pathol Lab Med. 2009;133:967–972. doi: 10.5858/133.6.967. [DOI] [PubMed] [Google Scholar]
- 22.Mehrabi A, Kashfi A, Fonouni H, Schemmer P, Schmied BM, Hallscheidt P, Schirmacher P, Weitz J, Friess H, Buchler MW, et al. Primary malignant hepatic epithelioid hemangioendothelioma: a comprehensive review of the literature with emphasis on the surgical therapy. Cancer. 2006;107:2108–2121. doi: 10.1002/cncr.22225. [DOI] [PubMed] [Google Scholar]
- 23.Ben-Haim M, Roayaie S, Ye MQ, Thung SN, Emre S, Fishbein TA, Sheiner PM, Miller CM, Schwartz ME. Hepatic epithelioid hemangioendothelioma: resection or transplantation, which and when? Liver Transpl Surg. 1999;5:526–531. doi: 10.1002/lt.500050612. [DOI] [PubMed] [Google Scholar]
- 24.Lerut JP, Orlando G, Adam R, Schiavo M, Klempnauer J, Mirza D, Boleslawski E, Burroughs A, Sellés CF, Jaeck D, et al. The place of liver transplantation in the treatment of hepatic epitheloid hemangioendothelioma: report of the European liver transplant registry. Ann Surg. 2007;246:949–57; discussion 957. doi: 10.1097/SLA.0b013e31815c2a70. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez JA, Becker NS, O’Mahony CA, Goss JA, Aloia TA. Long-term outcomes following liver transplantation for hepatic hemangioendothelioma: the UNOS experience from 1987 to 2005. J Gastrointest Surg. 2008;12:110–116. doi: 10.1007/s11605-007-0247-3. [DOI] [PubMed] [Google Scholar]
- 26.Wang LR, Zhou JM, Zhao YM, He HW, Chai ZT, Wang M, Ji Y, Chen Y, Liu C, Sun HC, et al. Clinical experience with primary hepatic epithelioid hemangioendothelioma: retrospective study of 33 patients. World J Surg. 2012;36:2677–2683. doi: 10.1007/s00268-012-1714-x. [DOI] [PubMed] [Google Scholar]
- 27.Grotz TE, Nagorney D, Donohue J, Que F, Kendrick M, Farnell M, Harmsen S, Mulligan D, Nguyen J, Rosen C, et al. Hepatic epithelioid haemangioendothelioma: is transplantation the only treatment option? HPB (Oxford) 2010;12:546–553. doi: 10.1111/j.1477-2574.2010.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nudo CG, Yoshida EM, Bain VG, Marleau D, Wong P, Marotta PJ, Renner E, Watt KD, Deschênes M. Liver transplantation for hepatic epithelioid hemangioendothelioma: the Canadian multicentre experience. Can J Gastroenterol. 2008;22:821–824. doi: 10.1155/2008/418485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cardinal J, de Vera ME, Marsh JW, Steel JL, Geller DA, Fontes P, Nalesnik M, Gamblin TC. Treatment of hepatic epithelioid hemangioendothelioma: a single-institution experience with 25 cases. Arch Surg. 2009;144:1035–1039. doi: 10.1001/archsurg.2009.121. [DOI] [PubMed] [Google Scholar]


