Abstract
AIM: To investigate the molecular mechanisms of miRNA in advanced gastric cancers (AGCs) before and after cytoreductive surgery (CRS) + hyperthermic intraperitoneal chemotherapy (HIPEC).
METHODS: A miRNA microarray containing human mature and precursor miRNA sequences was used to compare expression profiles in serum samples of 5 patients with AGC before and after CRS + HIPEC. The upregulation of miR-218 was confirmed by real-time reverse transcription polymerase chain reaction and its expression was analyzed in SGC7901 gastric cancer cells.
RESULTS: miRNA microarray chip analysis found that the level of miR-218 expression was upregulated more than 8 fold after CRS + HIPEC. Furthermore, miR-218 increased gastric cancer cell chemosensitivity to cisplatin in vitro and inhibited gastric cell tumor growth in nude mice in vivo (0.5 vs 0.78, P < 0.05).
CONCLUSION: Our results indicated that targeting miR-218 may provide a strategy for blocking the development of gastric cancer and reverse the multi-drug resistance of gastric cell lines.
Keywords: Advanced gastric cancer, Cytoreductive surgery, Hyperthermic intraperitoneal chemotherapy, miR-218, Multi-drug resistance, MicroRNA
Core tip: MicroRNAs (miRNAs) are short single-stranded RNAs associated with gene regulation at the transcriptional and translational levels. miRNA up- or down regulation has been linked to cancer development. We analyzed miRNA expression in advanced gastric cancers before and after cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) to gain further understanding of the molecular mechanisms of CRS + HIPEC. Furthermore, we described the regulation and function of miR-218 in gastric cancer emphatically. Our results indicated that targeting miR-218 may provide a strategy for blocking the development of gastric cancer and can reverse the multi-drug resistance of gastric cell lines.
INTRODUCTION
Gastric cancer is the second leading cause of cancer death in the world, with often a poor prognosis due to the advanced stage of the disease at diagnosis. The peritoneum is the most common site of metastasis in patients with gastric cancer[1]. Although surgery and chemotherapy can be curative for early-stage disease, the prognosis for patients with advanced disease is poor. In China, advanced gastric cancer (AGC) is the third leading cause of cancer deaths, with 300000 deaths per year[2]. In addition to hematogenous and lymphatic metastasis, patients with AGC may have free cancer cells dissemination in the peritoneal cavity, leading to peritoneal carcinomatosis (PC). PC may be present in 5%-20% of patients undergoing gastrectomy of AGC. The prognosis and survival of patients with gastric originated PC are extremely poor[3,4].
2010 French guidelines designated cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) as the standard method for treating colorectal PCs[5], and evaluated the effectiveness of CRS + HIPEC treatment for other primary tumors, including gastric cancer, ovarian, and sarcoma[6-8]. CRS + HIPEC can reduce visible tumor burden and HIPEC can eradicate micro-metastases[9-12], improving outcomes in patients with AGC of PC[13].
HIPEC was performed by our self-developed “BR-TRGII type high-precision hyperthermic perfusion intraperitoneal treatment system” with precise ± 0.2 °C temperature control, ± 5% flow control accuracy, and an automatic cooling function.
Patients completed the first session of HIPEC in the operating room under general anesthesia. At the first session, 5-fluorine and cisplatin were added into the 0.9% sodium chloride (3500-5000 mL) as perfusion liquid. HIPEC was then performed for 60 min with a velocity of 450-600 mL/min and an inflow temperature of 43 ± 0.2 °C.
Several non-randomized comparative studies have suggested that CRS + HIPEC is superior to CRS alone in patients with AGC[14,15]. Although CRS + HIPEC is an accepted method of treatment, its exact molecular mechanisms have not been fully elucidated.
MicroRNAs (miRNAs) are small non-coding RNA molecules with gene regulation capabilities[16,17]. Mature miRNAs bind to 3’ untranslated regions of target mRNA, leading to the silencing of mRNA. These miRNAs have been found to regulate complicated biological behaviors, including cell proliferation, differentiation, and death. Specific miRNAs have been found to be associated with various types of cancer, including miR-34a, miR-21, miR-16, and miR-92a in breast cancer[18-20]; miR-101 in gastric cancer[21,22]; miR-130a, miR-203, miR-205, and miR-21 in prostate cancer[23,24]; miR-182 in melanoma[25]; and miR-92b and miR-9/9* in brain tumors[26]. In contrast, few miRNAs have been shown to be associated with AGCs after CRS and HIPEC. The importance of miRNAs in cancer was highlighted by the discovery that more than 50% of miRNA target cancer-associated genomic regions or fragile sites, including lung, breast, brain, liver, colon cancer, and leukemia[27,28].
Since the molecular mechanisms of miRNA expression in AGCs treated with CRS + HIPEC remain elusive, further research is needed to examine the contribution of miRNAs to the mechanisms of carcinogenesis and their association with patient prognosis. We therefore utilized miRNA microarray chips to analyze the expression profiles of miRNAs in five patients with AGC being treated with CRS + HIPEC. We found that miR-218 was upregulated by more than 8 fold, which was confirmed by polymerase chain reaction (RT-PCR). These findings indicate the molecular basis of CRS + HIPEC effects in patients with AGC.
To further define the function of miR-218, it was upregulated in human gastric cancer cells (SGC7901), and cell vitality and chemosensitivity were then studied.
MATERIALS AND METHODS
Patients
Five patients with gastric PC, 4 men, and 1 woman, aged 46-75 years (median, 50.3 ± 1.6 years) were recruited from January to June 2012 (Table 1) and treated with CRS + HIPEC. Eligibility criteria included the following: (1) no prior chemotherapy or chemotherapy administered more than 6 mo ago; (2) an Eastern Cooperative Oncology Group performance score of 0-2; (3) adequate bone marrow, hepatic, and renal functions; and (4) 18-75 years of age. All diagnoses of AGC were confirmed histologically. Pre- and post-operative serum samples were collected from the 5 patients. All patients provided written informed consent, and the study protocol was approved by the ethics committee of Guangzhou Medical College and was in accordance with the Helsinki Declaration on ethical principles for medical research involving human subjects.
Table 1.
Clinical background of 5 patients with advanced gastric cancer
| Age | Sex | TNM stage | Histology of tumor | Drug regimen of HIPEC | Duration of HIPEC (min) | ECOG | HIPEC temperature (°C) | Time of surgery (h) | Following time (yr) and results |
| 49 | Male | T4aN1M0 | Poorly-differentiated adenocarcinoma | 5-Fu cisplatin | 60 | 1 | 43 | 3 | 1, alive |
| 56 | Male | T3N2M0 | Moderately-differentiated adenocarcinoma | 5-Fu cisplatin | 60 | 2 | 43 | 3.5 | 1.5, alive |
| 67 | Female | T4N2M0 | Poorly-differentiated adenocarcinoma | 5-Fu cisplatin | 60 | 0 | 43 | 3.8 | 1, dead |
| 53 | Male | T3N1M0 | Poorly-differentiated adenocarcinoma | 5-Fu cisplatin | 60 | 1 | 43 | 4 | 1.2, dead |
| 62 | Male | T4bN0M0 | Moderately-differentiated adenocarcinoma | 5-Fu cisplatin | 60 | 2 | 43 | 3 | 0.5, alive |
ECOG: Eastern Cooperative Oncology Group; HIPEC: Hyperthermic intraperitoneal chemotherapy.
CRS and HIPEC
All CRS and HIPEC procedures were performed by the same team of surgical oncologists and anesthesiologists. Abdominal exploration was performed according to previously reported methods[29]. The volume and character of ascites were also recorded. After evaluation, patients were scheduled for CRS and HIPEC[30]. HIPEC used to manage peritoneal surface malignancies was in accordance with the standard protocol of our center. Hyperthermic perfusion was performed with 4 L of heated saline supplied with 60 mg of cisplatin and 1000 mg of 5-FU. Human serum samples were collected from the 5 AGC patients 1 h before and 1 h after CRS + HIPEC, which were quickly frozen in liquid nitrogen and stored at -80 °C.
RNA isolation
Total RNA was isolated from samples using TRIzol kits (Invitrogen, Carlsbad, CA, United States) according to the manufacturer’s protocol. Yield and quality of RNA were assessed by ultraviolet absorbance at 260 nm and 280 nm.
miRNA microarrays
miRNA was isolated from the total RNA sample using mirVana miRNA Isolation Kits (Ambion Inc., Austin, TX, United States). The miRNA quantity was assessed by a fluorometer (Bio-Rad, Hercules, CA, United States) and by 1% formaldehyde agarose gel electrophoresis. Expression of miRNAs was assayed using microRNA chips (Exiqon, Vedbaek, Denmark). Serum samples were sent to KangChen-Biotech (Shanghai, China) for array hybridization, background subtraction, and normalization. Total RNA (5 μg) was labeled with Cy3 modified RNA linker at the 3’-end using T4 RNA ligase. Briefly, the reaction was carried out in 3 μL volumes at 37 °C for 30 min containing 2.0 μL RNA, 1.0 μL of CIP buffer, and CIP (Exiqon) mixture. The reaction was terminated by heating at 80 °C for 3 min. Images were analyzed with Genepix Pro 6.0 (Axon). Data were then collected from three independent experiments and miRNA with at least 2-fold changes were selected for further analysis.
Two step real-time RT-PCR
Real-time quantitative PCR was performed using SYBR Green PCR kits (SYBR biopars, GUASNR, Iran). The reaction was carried out in a 20 μL volume mixture, containing 300 nM of each primer (forward 5’-AAGACACCCTGGACGAAGCC-3’, reverse 5’-ACAACCAGAGTCCACCGGCG-3’), 10 ng of cDNA.
DNA was amplified with an initial denaturation at 94 °C for 3 min, followed by 35 cycles of 94 °C for 15 s and 59.8 °C for 15 s (gain set at 10 for SYBR Green). Each experiment was carried out in triplicate. The amplification efficiency was assessed based on the slope of a linear regression model. Ten-fold dilutions of each cDNA were used as a PCR template.
Cell culture
A human gastric cancer cell line SGC7901 was cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS and 1% penicillin/streptomycin at 37 °C in 5% CO2.
Generation of SGC7901 cells with stable expression of miR-218
SGC7901 cells were first transfection with pCR-miR-218 or pCR-miR-NC, and then selected in 1 mg/mL G418 (Invitrogen) supplied complete DMEM. miR-218 or miR-NC stable overexpressed cells were obtained after 3-4 wk of selection. The chemosensitivity of these cells were evaluated using a WST-1 kit (Roche) according to the manufacturer’s instruction at the indicated times. Each experiment was carried out in triplicate.
Anchorage-independent colony formation assay
The soft agar colony formation assay was used to monitor anchorage-independent growth. Briefly, agar plates were prepared using 1% agarose (Sigma, Saint Louis, MI, United States). Plates were stored at 4 °C and pre-warmed in 37 °C before use. Top agar (0.5%) containing 103 cells were then added onto the well. After 3-4 wk of incubation, colonies were assessed after methanol fixation and 0.1% crystal violet staining. Colonies with a diameter greater than 2 mm were counted. Each experiment was carried out in triplicate.
In vitro assay of chemosensitivity to cisplatin
6 × 103 cells were first seeded in each well of 96-well plates. After overnight incubation, cisplatin was added into each well, resulting in the final concentration of 0 to 256 μmol/L. Cell viability was determined using WST-1 kit after 72 h. IC50 was obtained from three independent experiments. Cisplatin at twice the concentration of IC50 were used in the following studies.
In vivo tumorigenesis study
Twenty four BALB/c nude mice (female, 6-9 wk, Guangzhou Laboratory Animal Center, Chinese Academy of Sciences, Guangzhou, China) were used in the study. Mice were maintained in a special pathogen-free (SPF) house with 12 h alternating light and dark cycles, and were given adequate nutrition and water ad libitum. 6 × 106 of cells suspended in FBS-free DMEM were injected into each side of the posterior flank of nude mice subcutaneously. Mice were sacrificed and tumors were collected 30 days after implantation. Tumor volume was calculated as follows: volume = 0.5 × length × width2. Experimental protocols were reviewed and approved by the Animal Ethics Committee of Guangzhou Medical College.
Statistical analysis
Quantitative data were presented as mean ± SD. Comparisons between the quantitative data were made using the t test. SPSS 16.0 was used in the statistical analysis and P < 0.05 was considered statistically significant.
RESULTS
miRNA expression profiles in sera of patients with AGC before and after CRS + HIPEC
miRNA expression profiles of paired serum samples of 5 AGC patients before and after CRS + HIPEC were analyzed by miRCURY™ bead-based flow LNA microarray platform, using 5S RNA for normalization. The miRNA expression patterns differed significantly (Figure 1). Of the miRNAs assayed, miR-218 was upregulated by more than 8-fold (Table 2).
Figure 1.
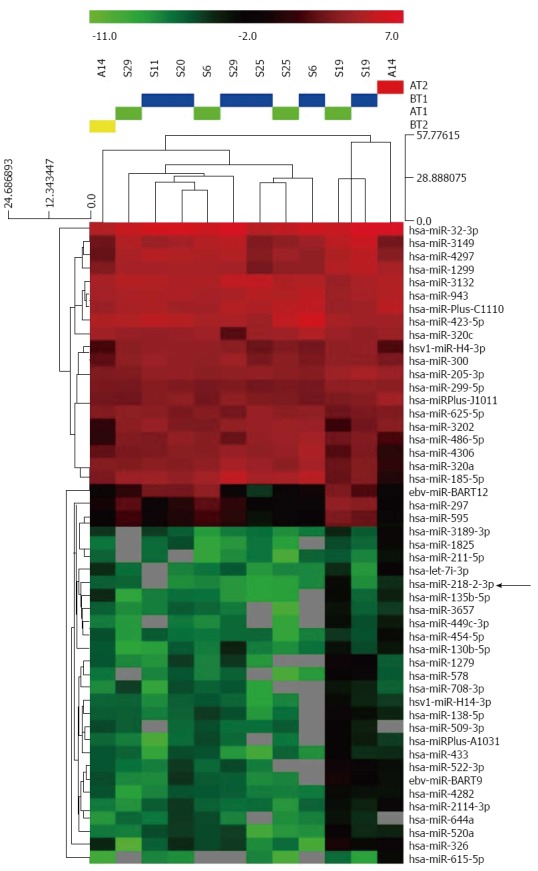
Hierarchical clustering of microRNA in advanced gastric cancer serum samples. Advanced gastric cancer (AGC) serum samples were clustered according to the expression profile of 86 differentially-expressed microRNAs (miRNAs) of the paired serum samples of 5 AGC patients collected 1 h before and 1 h after cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy. Samples were in columns and miRNAs in rows. The P values for these miRNAs were less than 0.05 in AGC serum samples.
Table 2.
Three-fold upregulation of microRNAs in advanced gastric cancer tumor serums
| MicroRNA | Raising multiples | P value |
| miR-218 | 8.419 | 0.004 |
| miR-135b | 5.317 | 0.003 |
| miR-135a | 4.788 | 0.016 |
| miR-433 | 4.401 | 0.025 |
| miR-409 | 4.389 | 0.039 |
| miR-96 | 4.280 | 0.050 |
| miR-647 | 4.113 | 0.067 |
| miR-376a | 3.940 | 0.003 |
| miR-4326 | 3.899 | 0.045 |
| miR-1306 | 3.705 | 0.021 |
| miR-937 | 3.699 | 0.028 |
| miR-326 | 3.661 | 0.007 |
| miR-214 | 3.500 | 0.001 |
| miR-377 | 3.403 | 0.009 |
| miR-661 | 3.397 | 0.005 |
| miR-3678 | 3.235 | 0.011 |
| miR-632 | 3.226 | 0.035 |
| miR-154 | 3.140 | 0.028 |
| miR-134 | 3.119 | 0.027 |
Real-time PCR validation of microarray results
Results of the microarray were further validated by quantitative RT-PCR, including the results of miR-218, miR-135a, miR-409, and miR-96. The RT-PCR results were consistent with the microarray data (Figure 2).
Figure 2.
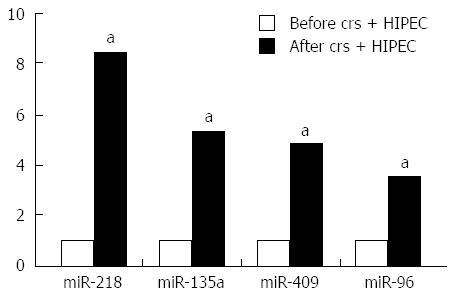
Upregulation of microRNA after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy treatment. aP < 0.05 vs control.
Upregulation of miR-218 suppressed in the proliferation of gastric cancer
MiR-218 was reported to be downregulated in gastric cancers[31] and was upregulated after CRS + HIPEC. To explore the roles of miR-218 in drug resistance, SGC7901 gastric cell line with a stable overexpression of miR-218 were established by transfecting miR-218 plasmids and then undergoing cisplatin-based selection. Meanwhile, the SGC7901/miR-NC cell line was established as a control. The overexpression of miR-218 in SGC7901/miR-218 cells was confirmed by RT-PCR (Figure 3C). Cell growth was measured using WST-1 kit at indicated time points.
Figure 3.
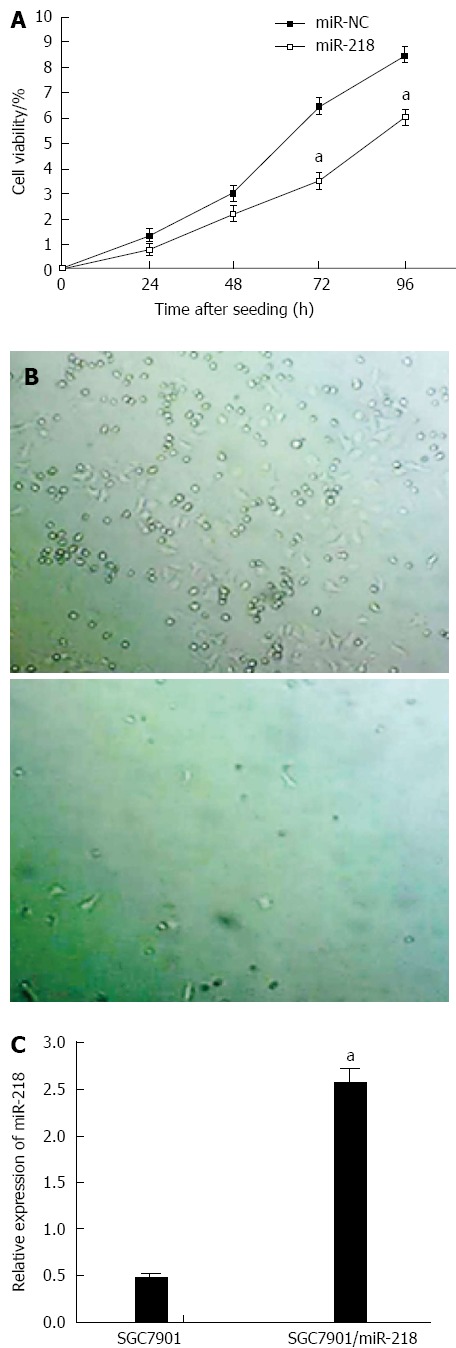
Upregulation of miR-218 inhibited growth of gastric cancers. A: The upregulation of miR-218 inhibited SGC7901 (human gastric cancer cells) cell growth. Cell viability was measured using a WST-1 kit at indicated time points. Data are presented as mean ± SD from three independent experiments performed in sextuple; B: miR-218 overexpression reduced colony formation of SGC7901 cells. 103 cells were mixed with agarose and seeded in 9-well plates for two weeks; C: The expression levels of miR-218 in the parental SGC7901 and the SGC7901/miR-218 stable cell line. Data are presented as mean ± SD. aP < 0.05 vs control.
The results showed that upregulation of miR-218 markedly inhibited the proliferation of SGC7901 cells (Figure 3A). The anchorage independent colony formation assay also demonstrated the same results (Figure 3B).
MiR-218 increased chemosensitivity of gastric cancer cells to cisplatin via its target mTOR inhibitor
To investigate whether miR-218 plays an important role in the drug resistance of gastric cancer, gastric tumor cell lines, SGC7901/miR-NC, SGC7901/miR-218, or SGC7901/miR-218 using mTOR inhibitor rapamycin and different concentrations of cisplatin (0 to 256 μmol/L) for 72 h (Figure 4A). Cell viability was measured using the WST-1 kit. The results showed that overexpression of miR-218 increased sensitivity of SGC7901 cells to cisplatin, while rapamycin can further enhance chemosensitivity. Cisplatin at 10 μmol/L showed a remarkable inhibition of cell proliferation (Figure 4B).
Figure 4.
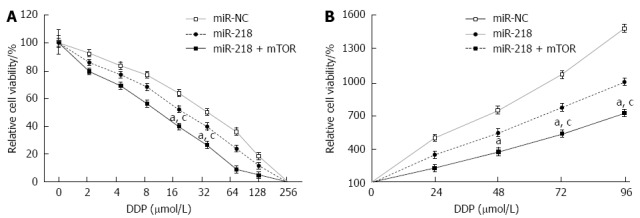
miR-218 increased chemosensitivity to cisplatin could be accelerated by overexpression of mTOR. Cells were transfected with miR-NC, or miR-218 with or without mTOR cDNA. Cells were exposed to cisplatin for further detection 10 h after transfection. A: Tumor cells proliferation assay of different cisplatin concentrations. 6 × 103 cells were seeded in 96-well plates and incubated with different concentration of cisplatin for 72 h. Data are presented as mean ± SD from three independent experiments performed in sextuple; B: Cell proliferation in the presence of 12 μmol/L cisplatin. Data are presented as mean ± SD from three independent experiments performed in sextuple. aP < 0.05 vs control; cP < 0.05 vs miR-218 + mTOR.
MiR-218 impaired in vivo tumor growth
The chemosensitization role of miR-218 was further explored using ectopic transplantation in a nude mice model. Stable cell lines, SGC7901/miR-218, and SGC7901/miR-NC were injected into nude mice subcutaneously. Tumor growth in the miR-218 group was reduced significantly when compared to the control group (Figure 5A and B). The tumor weights of the xenograft in the miR-218 group were greater than those in the miR-NC group (Figure 5C). These data indicated the suppressor role of miR-218 in gastric cancer.
Figure 5.
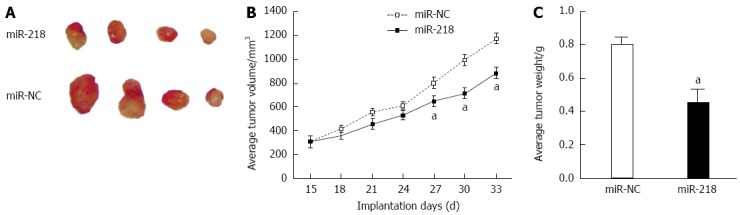
Upregulation of miR-218 inhibited gastric tumor growth in vivo. Cells stably expressing miR-218 or miR-NC were incubated in Dulbecco's modified Eagle’s medium and subcutaneously injected into each side of the posterior flank of nude mice (n = 24). Thirty-three days after injection, mice were sacrificed and tumors were removed. A: Tumor volume at 33 d; B: Tumor volumes were detected every three days from the time they were obvious; C: Average tumor weights. aDenoted statistical significance between the two groups of miR-218 and control. aP < 0.05 vs control.
DISCUSSION
PC of gastrointestinal or primary origin is considered invariably fatal, with only palliative treatment possible. To date, no standard treatment has been developed for PC from AGC, although the original trial of CRS + HIPEC resulted in a mean overall survival of 7.2 ± 4.6 mo with acceptable morbidity[32]. This new treatment modality has gradually gained acceptance in many countries. A meta-analysis of 13 randomized controlled trials found that HIPEC showed marked improvements of survival in patients with AGC when compared with current treatments[33]. As a result, CRS + HIPEC was recommended as the optimal treatment for AGC by a panel of international experts[34]. Nevertheless, this treatment modality remains controversial, and additional high-quality clinical studies are still needed in order to prove its effectiveness.
Changes in miRNA expression have been found to contribute to the initiation and progression of cancer. The relationship between miRNAs and tumors has suggested that miRNAs may be altered by treatment in patients with AGC. We therefore analyzed paired serum samples from patients with AGC that were obtained before and after CRS + HIPEC. We found that miR-218 was upregulated by more than 8 fold after CRS + HIPEC. Further analysis using computer-aided algorithms (TargetScan and PicTar software) predicted that more than 30 genes were potential targets of miR-218, some of which are associated with multi-drug resistance (MDR). Thus, together with previous studies[35-36], our bioinformatics results suggest that miR-218 may be altered by HIPEC.
In the present study, we focused on the effect of miR-218 on AGC metastasis and found that miR-218 was a tumor suppressor in AGC metastasis. Furthermore, miR-218 increased chemosensitivity to cisplatin in vitro and in vivo by inducing apoptosis. Cisplatin is currently used in the treatment of many types of advanced cancer, including gastric cancer, due to the broadest-spectrum anticancer capability[37]. Currently, the most effective systemic treatment for metastatic gastric cancer consists of cisplatin-based combination chemotherapy[38]. However, chemoresistance is still the most major and frequent obstacle to effective treatment[39]. Our data show that miR-218 overexpression can reverse the MDR of cisplatin and can increase the chemosensitivity of gastric cancer cells. When compared to the mTOR inhibitor rapamycin, the effect is better than miR-218 alone. Therefore, miR-218 may be used as a biomarker to confirm the extensiveness of tumor resection and to evaluate the efficacy of HIPEC.
The key finding in our study is that miR-218 suppressed the proliferation of tumor cells and inhibited the mTOR signaling pathway in gastric cancer. In addition, upregulation of miR-218 increased chemosensitivity of gastric cancer cells to cisplatin via its target mTOR.
In conclusion, we have identified several miRNAs whose expression was affected before and after CRS + HIPEC in AGC. The upregulation of miR-218 can inhibit AGC cell invasion and metastasis, as well as further reverse the MDR of cisplatin. The results indicate that restoration of miR-218 may be a rational therapeutic strategy for the treatment of AGC in the future. Importantly, differential miRNA expression patterns before and after CRS + HIPEC provide a solid basis for further validation, including functional studies to identify other potential oncogenic or tumor suppressor miRNAs in AGC.
COMMENTS
Background
Gastric cancer is the second leading cause of cancer death in the world, with often a poor prognosis due to the advanced stage of the disease at diagnosis.
Research frontiers
2010 French guidelines designated cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) as the standard method for treating colorectal PCs, and evaluated the effectiveness of CRS + HIPEC treatment for other primary tumors, including gastric cancer, ovarian, and sarcoma.
Applications
To further define the function of miR-218, it was upregulated in human gastric cancer cells (SGC7901), and the cell vitality and chemosensitivity were then studied.
Peer review
This is an interesting study with both in vivo and in vitro data. The discovery of miR-218 was based on microarray results; hence the clinical significance is good.
Footnotes
Supported by The PhD Start-up Funds of Guangzhou Medical College, Guangdong Province, China, No. 2012C66 and No. 2012C69; Guangdong Province Natural Science Fund, No. S2013010016662; and the National Natural Science Foundation of China, No. 81201932 and No. 81372493
P- Reviewer: Chen RF, Fang Y, George V, Spisni E S- Editor: Qi Y L- Editor: Rutherford A E- Editor: Wang CH
References
- 1.Shim JH, Ko KJ, Yoo HM, Oh SI, Jeon DJ, Jeon HM, Park CH, Song KY. Morbidity and mortality after non-curative gastrectomy for gastric cancer in elderly patients. J Surg Oncol. 2012;106:753–756. doi: 10.1002/jso.23121. [DOI] [PubMed] [Google Scholar]
- 2.Zhang XL, Shi HJ, Cui SZ, Tang YQ, Ba MC. Prospective, randomized trial comparing 5-FU/LV with or without oxaliplatin as adjuvant treatment following curative resection of gastric adenocarcinoma. Eur J Surg Oncol. 2011;37:466–472. doi: 10.1016/j.ejso.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 3.Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL, Faure JL, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88:358–363. doi: 10.1002/(sici)1097-0142(20000115)88:2<358::aid-cncr16>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 4.Gill RS, Al-Adra DP, Nagendran J, Campbell S, Shi X, Haase E, Schiller D. Treatment of gastric cancer with peritoneal carcinomatosis by cytoreductive surgery and HIPEC: a systematic review of survival, mortality, and morbidity. J Surg Oncol. 2011;104:692–698. doi: 10.1002/jso.22017. [DOI] [PubMed] [Google Scholar]
- 5.Golse N, Bakrin N, Passot G, Mohamed F, Vaudoyer D, Gilly FN, Glehen O, Cotte E. Iterative procedures combining cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal recurrence: postoperative and long-term results. J Surg Oncol. 2012;106:197–203. doi: 10.1002/jso.23062. [DOI] [PubMed] [Google Scholar]
- 6.Cotte E, Glehen O, Mohamed F, Lamy F, Falandry C, Golfier F, Gilly FN. Cytoreductive surgery and intraperitoneal chemo-hyperthermia for chemo-resistant and recurrent advanced epithelial ovarian cancer: prospective study of 81 patients. World J Surg. 2007;31:1813–1820. doi: 10.1007/s00268-007-9146-8. [DOI] [PubMed] [Google Scholar]
- 7.Glehen O, Gilly FN, Arvieux C, Cotte E, Boutitie F, Mansvelt B, Bereder JM, Lorimier G, Quenet F, Elias D. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2010;17:2370–2377. doi: 10.1245/s10434-010-1039-7. [DOI] [PubMed] [Google Scholar]
- 8.Helm CW, Randall-Whitis L, Martin RS, Metzinger DS, Gordinier ME, Parker LP, Edwards RP. Hyperthermic intraperitoneal chemotherapy in conjunction with surgery for the treatment of recurrent ovarian carcinoma. Gynecol Oncol. 2007;105:90–96. doi: 10.1016/j.ygyno.2006.10.051. [DOI] [PubMed] [Google Scholar]
- 9.Ba MC, Cui SZ, Lin SQ, Tang YQ, Wu YB, Wang B, Zhang XL. Chemotherapy with laparoscope-assisted continuous circulatory hyperthermic intraperitoneal perfusion for malignant ascites. World J Gastroenterol. 2010;16:1901–1907. doi: 10.3748/wjg.v16.i15.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yonemura Y, Kawamura T, Bandou E, Takahashi S, Sawa T, Matsuki N. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br J Surg. 2005;92:370–375. doi: 10.1002/bjs.4695. [DOI] [PubMed] [Google Scholar]
- 11.Glehen O, Schreiber V, Cotte E, Sayag-Beaujard AC, Osinsky D, Freyer G, François Y, Vignal J, Gilly FN. Cytoreductive surgery and intraperitoneal chemohyperthermia for peritoneal carcinomatosis arising from gastric cancer. Arch Surg. 2004;139:20–26. doi: 10.1001/archsurg.139.1.20. [DOI] [PubMed] [Google Scholar]
- 12.Mori T, Fujiwara Y, Sugita Y, Azama T, Ishii T, Taniguchi K, Yamazaki K, Takiguchi S, Yasuda T, Yano M, et al. Application of molecular diagnosis for detection of peritoneal micrometastasis and evaluation of preoperative chemotherapy in advanced gastric carcinoma. Ann Surg Oncol. 2004;11:14–20. doi: 10.1007/BF02524340. [DOI] [PubMed] [Google Scholar]
- 13.Scaringi S, Kianmanesh R, Sabate JM, Facchiano E, Jouet P, Coffin B, Parmentier G, Hay JM, Flamant Y, Msika S. Advanced gastric cancer with or without peritoneal carcinomatosis treated with hyperthermic intraperitoneal chemotherapy: a single western center experience. Eur J Surg Oncol. 2008;34:1246–1252. doi: 10.1016/j.ejso.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Fujimoto S, Takahashi M, Mutou T, Kobayashi K, Toyosawa T, Isawa E, Sumida M, Ohkubo H. Improved mortality rate of gastric carcinoma patients with peritoneal carcinomatosis treated with intraperitoneal hyperthermic chemoperfusion combined with surgery. Cancer. 1997;79:884–891. doi: 10.1002/(sici)1097-0142(19970301)79:5<884::aid-cncr3>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 15.Hall JJ, Loggie BW, Shen P, Beamer S, Douglas Case L, McQuellon R, Geisinger KR, Levine EA. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for advanced gastric cancer. J Gastrointest Surg. 2004;8:454–463. doi: 10.1016/j.gassur.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 17.Claverie JM. Fewer genes, more noncoding RNA. Science. 2005;309:1529–1530. doi: 10.1126/science.1116800. [DOI] [PubMed] [Google Scholar]
- 18.Hui C, Yujie F, Lijia Y, Long Y, Hongxia X, Yong Z, Jundong Z, Qianyong Z, Mantian M. MicroRNA-34a and microRNA-21 play roles in the chemopreventive effects of 3,6-dihydroxyflavone on 1-methyl-1-nitrosourea-induced breast carcinogenesis. Breast Cancer Res. 2012;14:R80. doi: 10.1186/bcr3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rivas MA, Venturutti L, Huang YW, Schillaci R, Huang TH, Elizalde PV. Downregulation of the tumor-suppressor miR-16 via progestin-mediated oncogenic signaling contributes to breast cancer development. Breast Cancer Res. 2012;14:R77. doi: 10.1186/bcr3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nilsson S, Möller C, Jirström K, Lee A, Busch S, Lamb R, Landberg G. Downregulation of miR-92a is associated with aggressive breast cancer features and increased tumour macrophage infiltration. PLoS One. 2012;7:e36051. doi: 10.1371/journal.pone.0036051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carvalho J, van Grieken NC, Pereira PM, Sousa S, Tijssen M, Buffart TE, Diosdado B, Grabsch H, Santos MA, Meijer G, et al. Lack of microRNA-101 causes E-cadherin functional deregulation through EZH2 up-regulation in intestinal gastric cancer. J Pathol. 2012;228:31–44. doi: 10.1002/path.4032. [DOI] [PubMed] [Google Scholar]
- 22.He XP, Shao Y, Li XL, Xu W, Chen GS, Sun HH, Xu HC, Xu X, Tang D, Zheng XF, et al. Downregulation of miR-101 in gastric cancer correlates with cyclooxygenase-2 overexpression and tumor growth. FEBS J. 2012;279:4201–4212. doi: 10.1111/febs.12013. [DOI] [PubMed] [Google Scholar]
- 23.Boll K, Reiche K, Kasack K, Mörbt N, Kretzschmar AK, Tomm JM, Verhaegh G, Schalken J, von Bergen M, Horn F, et al. MiR-130a, miR-203 and miR-205 jointly repress key oncogenic pathways and are downregulated in prostate carcinoma. Oncogene. 2013;32:277–285. doi: 10.1038/onc.2012.55. [DOI] [PubMed] [Google Scholar]
- 24.Li T, Li RS, Li YH, Zhong S, Chen YY, Zhang CM, Hu MM, Shen ZJ. miR-21 as an independent biochemical recurrence predictor and potential therapeutic target for prostate cancer. J Urol. 2012;187:1466–1472. doi: 10.1016/j.juro.2011.11.082. [DOI] [PubMed] [Google Scholar]
- 25.Segura MF, Hanniford D, Menendez S, Reavie L, Zou X, Alvarez-Diaz S, Zakrzewski J, Blochin E, Rose A, Bogunovic D, et al. Aberrant miR-182 expression promotes melanoma metastasis by repressing FOXO3 and microphthalmia-associated transcription factor. Proc Natl Acad Sci USA. 2009;106:1814–1819. doi: 10.1073/pnas.0808263106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nass D, Rosenwald S, Meiri E, Gilad S, Tabibian-Keissar H, Schlosberg A, Kuker H, Sion-Vardy N, Tobar A, Kharenko O, et al. MiR-92b and miR-9/9* are specifically expressed in brain primary tumors and can be used to differentiate primary from metastatic brain tumors. Brain Pathol. 2009;19:375–383. doi: 10.1111/j.1750-3639.2008.00184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 28.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugarbaker PH. Cytoreductive surgery and perioperative intraperitoneal chemotherapy as a curative approach to pseudomyxoma peritonei syndrome. Tumori. 2001;87:S3–S5. [PubMed] [Google Scholar]
- 30.Glehen O, Mohamed F, Gilly FN. Peritoneal carcinomatosis from digestive tract cancer: new management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol. 2004;5:219–228. doi: 10.1016/S1470-2045(04)01425-1. [DOI] [PubMed] [Google Scholar]
- 31.Li BS, Zhao YL, Guo G, Li W, Zhu ED, Luo X, Mao XH, Zou QM, Yu PW, Zuo QF, et al. Plasma microRNAs, miR-223, miR-21 and miR-218, as novel potential biomarkers for gastric cancer detection. PLoS One. 2012;7:e41629. doi: 10.1371/journal.pone.0041629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujimoto S, Shrestha RD, Kokubun M, Ohta M, Takahashi M, Kobayashi K, Kiuchi S, Okui K, Miyoshi T, Arimizu N. Intraperitoneal hyperthermic perfusion combined with surgery effective for gastric cancer patients with peritoneal seeding. Ann Surg. 1988;208:36–41. doi: 10.1097/00000658-198807000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan TD, Black D, Sugarbaker PH, Zhu J, Yonemura Y, Petrou G, Morris DL. A systematic review and meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for resectable gastric cancer. Ann Surg Oncol. 2007;14:2702–2713. doi: 10.1245/s10434-007-9487-4. [DOI] [PubMed] [Google Scholar]
- 34.Sugarbaker PH, Yu W, Yonemura Y. Gastrectomy, peritonectomy, and perioperative intraperitoneal chemotherapy: the evolution of treatment strategies for advanced gastric cancer. Semin Surg Oncol. 2003;21:233–248. doi: 10.1002/ssu.10042. [DOI] [PubMed] [Google Scholar]
- 35.Tie J, Pan Y, Zhao L, Wu K, Liu J, Sun S, Guo X, Wang B, Gang Y, Zhang Y, et al. MiR-218 inhibits invasion and metastasis of gastric cancer by targeting the Robo1 receptor. PLoS Genet. 2010;6:e1000879. doi: 10.1371/journal.pgen.1000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao C, Zhang Z, Liu W, Xiao S, Gu W, Lu H. Reduced microRNA-218 expression is associated with high nuclear factor kappa B activation in gastric cancer. Cancer. 2010;116:41–49. doi: 10.1002/cncr.24743. [DOI] [PubMed] [Google Scholar]
- 37.Emoto S, Yamaguchi H, Kamei T, Ishigami H, Suhara T, Suzuki Y, Ito T, Kitayama J, Watanabe T. Intraperitoneal administration of cisplatin via an in situ cross-linkable hyaluronic acid-based hydrogel for peritoneal dissemination of gastric cancer. Surg Today. 2014;44:919–926. doi: 10.1007/s00595-013-0674-6. [DOI] [PubMed] [Google Scholar]
- 38.Ajani JA, Buyse M, Lichinitser M, Gorbunova V, Bodoky G, Douillard JY, Cascinu S, Heinemann V, Zaucha R, Carrato A, et al. Combination of cisplatin/S-1 in the treatment of patients with advanced gastric or gastroesophageal adenocarcinoma: Results of noninferiority and safety analyses compared with cisplatin/5-fluorouracil in the First-Line Advanced Gastric Cancer Study. Eur J Cancer. 2013;49:3616–3624. doi: 10.1016/j.ejca.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Park SC, Chun HJ. Chemotherapy for advanced gastric cancer: review and update of current practices. Gut Liver. 2013;7:385–393. doi: 10.5009/gnl.2013.7.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]


