Abstract
AIM: To investigate the effect of the ‘‘minimizing tacrolimus’’ strategy on long-term survival of patients after liver transplantation (LT).
METHODS: We conducted a retrospective study of 319 patients who received LT between January 2009 and December 2011 at the First Affiliated Hospital of Zhejiang University School of Medicine. Following elimination of ineligible patients, 235 patients were included in the study. The relationship between early tacrolimus (TAC) exposure and survival period was analyzed by Kaplan Meier curves. Adverse effects related to TAC were evaluated by the χ2 test. Routine monitoring of blood TAC concentration (TC) was performed using the PRO-TracTM II Tacrolimus Elisa Kit (Diasorin, United States).
RESULTS: Of 235 subjects enrolled in the study, 124 (52.8%) experienced adverse effects due to TAC. When evaluating mean TC, the survival time of patients with a mean TC < 5 ng/mL was significantly shorter than that in the other groups (911.3 ± 131.6 d vs 1381.1 ± 66.1 d, 911.3 ± 131.6 d vs 1327.3 ± 47.8 d, 911.3 ± 131.6 d vs 1343.2 ± 83.1 d, P < 0.05), while the survival times of patients with a mean TC of 5-7, 7-10 and 10-15 ng/mL were comparable. Adverse effects due to TAC in all four groups were not significantly different. When comparing the standard deviation (SD) of TC among the groups, the survival time of patients with a SD of 2-4 was significantly longer than that in the other groups (1388.8 ± 45.4 d vs 1029.6 ± 131.3 d, 1388.8 ± 45.4 d vs 1274.9 ± 57.0 d, P < 0.05), while in patients with a SD < 2 and SD > 4, the survival time was not statistically different. Adverse effects experienced in all three groups were not statistically different. In Cox regression analysis, male patients and those with a primary diagnosis of benign disease, mean TC > 5 ng/mL and TC SD 2-4 had better outcomes.
CONCLUSION: The early ‘‘minimizing tacrolimus’’ strategy with a mean TC of 5-10 ng/mL and SD of 2-4 was beneficial in terms of long-term survival after LT.
Keywords: Tacrolimus, Liver transplantation, Outcome, Minimizing tacrolimus, Immunosuppressive drug
Core tip: Liver transplantation (LT) is a life-saving technique for patients with end-stage liver disease. Tacrolimus (TAC) is the cornerstone immunosuppressant for prevention of graft rejection in many LT centers. However, frequent TAC-related toxicities are observed and these are a major concern in LT patients. Thus, the ‘‘minimizing tacrolimus’’ strategy after LT is very important, especially in the early phase.
INTRODUCTION
Liver transplantation (LT) is a life-saving technique for patients with end-stage liver disease. The primary outcomes following LT are poor, however, improvements in surgical techniques and in donor liver protection, specifically new developments in immunosuppressive drugs have led to enhanced graft survival rates[1]. Tacrolimus (TAC), is a calcineurin inhibitor, and has become the cornerstone immunosuppressant in many LT centers in recent years[2]. Compared to cyclosporine, TAC plays a greater role in reducing acute cellular rejection (ACR) leading to better graft and overall survival in LT patients[3]. However, the narrow therapeutic index and greater pharmacokinetic variability of TAC necessitates routine monitoring of TAC blood concentration, especially in the early phase after LT[4,5]. In addition, TAC-related toxicity, mainly due to over immunosuppression, is frequent in liver transplant patients and is currently the main concern in these patients[6]. Therefore, better strategies to optimize TAC in allograft recipients are needed, these include the ‘‘minimizing tacrolimus’’ strategy, identification and validation of pharmacodynamic biomarkers, and direct drug measurement at the target sites, i.e., allograft tissue[7] and lymphocytes[8,9].
The currently recommended target range for blood tacrolimus concentration (TC) after LT is 10-15 ng/mL during the first 4-6 wk, and then gradually decreasing to 5-10 ng/mL during long-term maintenance[10-14]. The higher TAC concentration, especially during the early phase after LT, may be associated with adverse effects such as hepatocellular carcinoma[15], early renal impairment[16,17] and even death[18]. Numerous studies used lowered early TC level known as the ‘‘minimizing tacrolimus’’ strategy to avoid these over immunosuppression side effects. A meta-analysis (n = 957) compared the standard dosage of TAC (10-15 ng/mL) with a minimized dosage (5-8 ng/mL) and showed no variation in biopsy-proven rejection over a 6-week follow-up period[19]. Early exposure to a TC of 7-10 ng/mL (the initial 2 wk) following LT has been proved to be safe with regards to acute rejection and longer graft survival[20]. Thus, an early TC of 5-7 ng/mL is safe for preventing rejection, however, the effect of this concentration on long-term survival is unknown. In this study, we introduced conventional standard deviation (SD) into the evaluation of early TC exposure, and attempted to provide evidence that a TC of 5-7 ng/mL is beneficial for the long-term survival of LT patients. We further explored the ‘‘minimizing tacrolimus’’ strategy on long-term survival of the patients after LT.
MATERIALS AND METHODS
Ethics statement
Ethical approval was obtained from the Committee of Ethics in Biomedical Research of Zhejiang University and all participants provided written informed consent forms. The design of this research was hospital-based and was approved by the China Liver Transplant Registry.
Patient population
In the present prospective study, 319 patients who received LT between January 2009 and December 2011 at the First Affiliated Hospital of Zhejiang University School of Medicine (Hangzhou, China) were enrolled. The entire research was based on prospectively collected data. Subjects who received a liver from an ABO incompatible donor (n = 67), underwent combined transplantation (n = 1), and had blood TC monitored less than 3 times in 4 wk were excluded from the study. After exclusion, 235 patients were enrolled in the study; 38 of these patients were considered to have clinical rejection and a liver biopsy was performed. Routine blood TC was assessed before the first daily dose of TAC was administered. Daily TC monitoring was performed using the PRO-TracTM II Tacrolimus Elisa Kit (Diasorin, United States) following the manufacturer’s instructions. In addition, alanine aminotransferase, aspartate aminotransferase, albumin, white blood cell, bilirubin, urea and creatinine levels were examined and the baseline clinical characteristics of the patients, such as gender, age, ABO group, MELD score, and Plug score were recorded.
The study groups were provided with TAC as the primary immunosuppressant following LT, which was administered with corticosteroids and mycophenolate mofetil (MMF). TAC was initiated at a daily dose of 2-3 mg, according to renal function, in post-LT patients for the first month and was titrated down to 5-10 ng/mL for the next few months. 1000 mg methylprednisolone was initiated in the perioperative phase, tapered slowly and withdrawn within the first month after LT. MMF was administered at 500 mg twice daily after LT.
Similar intra-operative and post-transplant care was provided to all patients. Piperacillin-tazobactam (4.5 g) three times a day (tid) was prescribed for postoperative antimicrobial prophylaxis for at least a week. Fluconazole (200 mg) per day was administrated for antifungal prophylaxis for two weeks and intravenous ganciclovir (5 mg/kg) per day was provided for antiviral prophylaxis for 2 wk. Patients with HBV received lamivudine plus hepatitis B immunoglobulin for post-transplant prophylaxis.
The parameters measured were survival period after LT, blood TC, number of patients with graft rejection, renal insufficiency, infection, metabolic disorders and tumor recurrence. The median follow-up time was 872 d. Renal insufficiency was defined as serum creatinine ≥ 130 μmol/L, lasting at least 1 mo after LT.
Statistical analysis
The sample size estimation was based on previous logistic and Cox regression analyses of clinical trials[16]. Statistical analysis was performed using SPSS 17.0 (Chicago, IL, United States). Variables were displayed in frequency tables or expressed as means and standard deviations, or as medians and interquartile range. The χ2 test was used for comparison of frequencies and the optimal threshold value for TC (mean or standard deviation, SD) related to long-term survival was established by receiver operating characteristic curves or previous publications. Kaplan Meier curves and Cox regression analysis were used to determine the influence of early TC (mean or SD) on long-term outcomes. Each hypothesis tested was two tailed and significant if P < 0.05.
RESULTS
Study description
The pre-transplant diagnosis and the demographics of the patient population are listed in Table 1. In total, 235 subjects were enrolled, 59.6% of the subjects had hepatitis B-related disease (severe hepatitis or cirrhosis), 23.4% had hepatocarcinoma and 17% had other diseases (alcoholic cirrhosis in 15 cases, primary biliary cirrhosis in 8 cases, hepatolenticular degeneration in 3 cases, cholangiocarcinoma in 3 cases, diffuse biliary calculi in 3 cases, schistosoma-related cirrhosis in 3 cases, hepatitis C in 2 cases, glycogen storage disease in 2 cases, and hepatic veno-occlusive disease in 1 case). Of the 235 subjects, 47 patients (20%) died within the observation period and 124 patients (52.8%) experienced at least one TAC-related side effect (Table 2).
Table 1.
Clinical characteristics of 235 consecutive liver transplant patients
| Variables | Value | |
| Age (yr) | 47.11 ± 11.32 | |
| MELD score | 16 (range 6-50) | |
| Child score | 9 (range 5-15) | |
| Gender | Male | 203 (86.4) |
| Female | 32 (13.6) | |
| Etiology | HBV-related | 140 (59.6) |
| Hepatocarcinoma | 55 (23.4) | |
| Others | 40 (17) | |
| Blood tests | Bilirubin (μmol/L) (NR < 17.1) | 162.73 ± 205.39 |
| AST (IU/L) (NR < 50) | 240.46 ± 556.16 | |
| ALT (IU/L) (NR < 40) | 183.2 ± 325.93 | |
| Albumin (g/L) (NR 35-50) | 34.96 ± 5.47 | |
| WBC (× 109/L) (NR 3.5-11) | 5.96 ± 4.58 | |
| Urea (mmol/L) (NR 3-7.1) | 6.70 ± 5.29 | |
| Creatinine (μmol/L) (NR 60-97) | 94.37 ± 68.98 | |
| TC | 7.91 ± 2.28 | |
| ABO | A-A | 85 (36.2) |
| B-B | 51 (21.7) | |
| O-O | 87 (37) | |
| AB-AB | 12 (5.1) |
Data are expressed as absolute numbers (percentage) or mean ± SD. NR: Normal range; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; WBC: White blood cell; HBV: Hepatitis B virus.
Table 2.
Clinical Fk506-related side effects and cause of death
| All events (n) | First events only (n) | |
| Rejection | 38 | 20 |
| Infection | 55 | 26 |
| Renal insufficiency | 7 | 2 |
| Tumor recurrence | 36 | 18 |
| Metabolic disorder | 34 | 21 |
| Cause of death | ||
| MODS | 11 | |
| Tumor recurrence | 22 | |
| Graft loss | 3 | |
| Others | 11 |
MODS: Multiple organ dysfunction syndrome.
Relationship between mean TC and patient outcome
Patients were divided into four groups according to the mean TC intervals during the first four weeks after LT: 21 (8.9%) cases had a mean TC < 5 ng/mL (group 1), 57 (24.3%) cases had a mean TC of 5-7 ng/mL (group 2), 119 (50.6%) cases had a mean TC of 7-10 ng/mL (group 3) and 38 (16.2%) cases had a mean TC of 10-15 ng/mL (group 4). The survival curves of these groups are shown in Figure 1. TAC-related side effects observed during follow-up in each group are listed in Table 3. Pairwise comparisons between the groups were not statistically different (P > 0.05).
Figure 1.
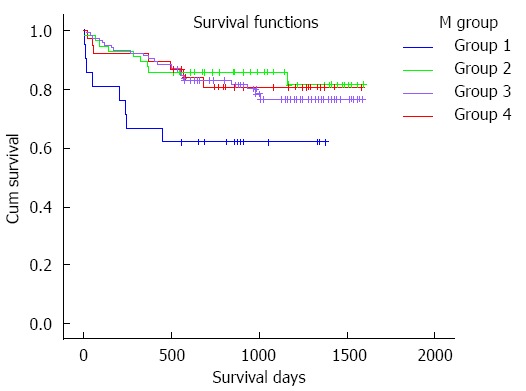
Patient survival curve according to the mean tacrolimus concentration. The survival time of group 1 is obviously shorter than other groups (group 1 vs group 2, 911.3 ± 131.6 d vs 1381.1 ± 66.1 d, P = 0.018; group 1 vs group 3, 911.3 ± 131.6 d vs 1327.3 ± 47.8 d, P = 0.024; group 1 vs group 4, 911.3 ± 131.6 d vs 1343.2 ± 83.1 d, P = 0.067) while the rates in groups 2, 3 and 4 are almost the same (group 2 vs group 3, 1381.1 ± 66.1 d vs 1327.3 ± 47.8 d, P = 0.501; group 2 vs group 4, 1381.1 ± 66.1 d vs 1343.2 ± 83.1 d, P = 0.774; group 3 vs group 4, 1327.3 ± 47.8 d vs 1343.2 ± 83.1 d, P = 0.801). Group 1: Mean tacrolimus concentration is 0-5 ng/mL, Group 2: 5-7 ng/mL, Group 3: 7-10 ng/mL, and Group 4: 10-15 ng/mL.
Table 3.
Classification of groups according to mean tacrolimus concentration, death rate and related side effects in respective categories
| Group | 1 | 2 | 3 | 4 |
| TC interval (ng/mL) | 0-5 | 5-7 | 7-10 | 10-15 |
| Total number | 21 | 57 | 119 | 38 |
| Number of death | 8 | 9 | 23 | 7 |
| Cause of death | ||||
| MODS | 4 | 4 | 2 | 1 |
| Tumor recurrence | 2 | 4 | 13 | 3 |
| Graft loss | 1 | 1 | 0 | 1 |
| Others | 1 | 0 | 8 | 2 |
| Rejection | 2 | 14 | 16 | 6 |
| Infection | 6 | 14 | 24 | 11 |
| Renal insufficiency | 2 | 2 | 2 | 1 |
| Tumor recurrence | 5 | 7 | 19 | 5 |
| Metabolic disorder | 6 | 8 | 15 | 5 |
MODS: Multiple organ dysfunction syndrome; TC: Tacrolimus concentration.
Relationship between the SD of TC and patient outcome
The patients were divided into three groups according to the SD of TC intervals during the first four weeks after LT: 21 (8.9%) cases had a SD < 2 (group 1), 118 (50.2%) cases had a SD of 2-4 (group 2) and 96 (40.9%) cases had a SD > 4 (group 3). The survival curve is shown in Figure 2. TAC-related side effects during the follow-up period are listed in Table 4. Pairwise comparisons between the groups were not statistically different (P > 0.05).
Figure 2.
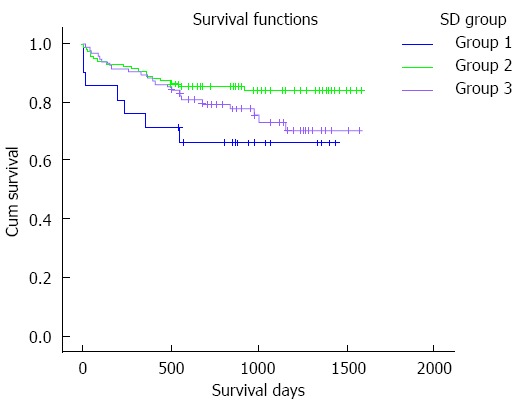
Patient survival curve according to the standard deviation of tacrolimus concentration. The survival time of group 2 is obviously longer than that of other groups (group 1 vs group 2, 1029.6 ± 131.3 d vs 1388.8 ± 45.4 d, P = 0.032; group 3 vs group 2, 1274.9 ± 57.0 d vs 1388.8 ± 45.4 d, P = 0.044) while in groups 1 and 3, the rates are not statistically different (1029.6 ± 131.3 d vs 1274.9 ± 57.0 d, P = 0.286). Note: Group 1: Standard deviation (SD) of tacrolimus concentration was 0-2 ng/mL, Group 2: 2-4 ng/mL, and Group 3: > 4 ng/mL.
Table 4.
Classification of groups according to standard deviation of Tacrolimus concentration, death rate and related side effects in respective categories
| Group | 1 | 2 | 3 |
| SD interval | 0-2 | 2-4 | > 4 |
| Total number | 21 | 118 | 96 |
| Number of death | 7 | 18 | 22 |
| Cause of death | |||
| MODS | 4 | 4 | 3 |
| Tumor recurrence | 2 | 7 | 13 |
| Graft loss | 0 | 2 | 1 |
| Others | 1 | 5 | 5 |
| Rejection | 1 | 21 | 16 |
| Infection | 5 | 25 | 25 |
| Renal insufficiency | 0 | 6 | 1 |
| Tumor recurrence | 3 | 14 | 19 |
| Metabolic disorder | 2 | 20 | 12 |
MODS: Multiple organ dysfunction syndrome; SD: Standard deviation.
Cox regression analysis
Predictive factors, such as gender, age, primary diagnosis, Plug score, MELD score, mean TC, and TC SD were analyzed first by a univariate, then by a multivariate Cox regression model. In the univariate Cox regression model, gender (female, P = 0.041), diagnosis (malignancy, P = 0.03), Plug score (> 10, P = 0.042), mean TC (< 5 ng/mL, P = 0.013) and TC SD (< 2 or > 4, P = 0.049) demonstrated statistical significance, while in the multivariate model, gender (female, P = 0.022), diagnosis (malignancy, P = 0.03), mean TC (< 5 ng/mL, P = 0.016) and TC SD (< 2 or > 4, P = 0.024) showed statistical significance in predicting overall survival after LT (Table 5). Therefore, male patients, those with a benign primary diagnosis, mean TC > 5 ng/mL and a TC SD of 2-4 showed a survival benefit.
Table 5.
Variables in multivariate Cox regression equation of long-term survival
| B | SE | Wald | df | Sig. | Exp(B) | Lower | Upper | |
| Gender | -0.809 | 0.352 | 5.284 | 1 | 0.022 | 0.445 | 0.224 | 0.888 |
| Age | 0.252 | 0.612 | 0.170 | 1 | 0.680 | 1.287 | 0.388 | 4.273 |
| Plug score | 0.640 | 0.364 | 3.093 | 1 | 0.079 | 1.897 | 0.929 | 3.870 |
| Meld score | -0.281 | 0.266 | 1.113 | 1 | 0.291 | 0.755 | 0.448 | 1.272 |
| Diagnosis | -0.351 | 0.162 | 4.691 | 1 | 0.030 | 0.704 | 0.512 | 0.967 |
| SD of TC | 0.345 | 0.153 | 5.063 | 1 | 0.024 | 1.412 | 1.045 | 1.907 |
| Mean TC | -0.960 | 0.397 | 5.830 | 1 | 0.016 | 0.383 | 0.176 | 0.835 |
SD: Standard deviation; TC: Tacrolimus concentration.
Evaluation of survival rate in selected patients
Patients were divided into two groups according to the selected mean TC and SD intervals during the first four weeks after LT: 107 (50.2%) patients had a mean TC > 5 ng/mL and SD of 2-4 (group 0), and 106 (49.8%) patients had a mean TC > 5 ng/mL and SD < 2 or SD > 4 (group 1). The survival curve is shown in Figure 3. The 3-year overall survival of group 0 was over 80% and was approximately 70% in group 1.
Figure 3.
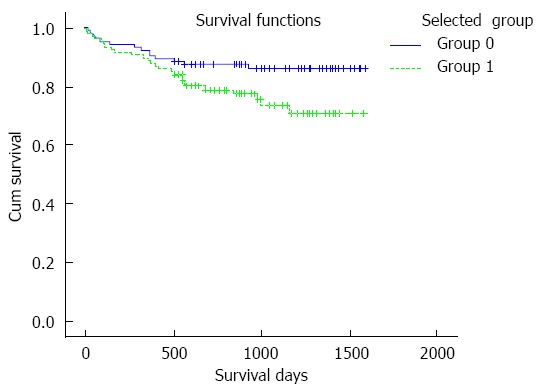
Patient survival curve according to the selected mean tacrolimus concentration and standard deviation. The survival time in group 0 is obviously longer than in group 1 (group 0 vs group 1, 1418.8 ± 44.5 d vs 1275.3 ± 53.7 d, P = 0.043). Note: Group 0: mean tacrolimus concentration (TC) > 5 ng/mL and standard deviation 2-4, Group 1: mean TC > 5 ng/mL and SD < 2 or > 4.
DISCUSSION
TAC, a calcineurin inhibitor, first developed by Starzl[21], is the foundation of conventional immunosuppression in LT recipients, and is preferable to cyclosporine as demonstrated in numerous randomized studies[3,22]. As mentioned previously, TAC has unwanted side effects which are a significant issue in clinical practice. To reduce TAC-related side effects, considerable efforts should be made to offer patients the ‘‘minimizing tacrolimus’’ strategy, and early exposure to TC between 5 and 10 ng/mL which has already been confirmed to prevent acute rejection. Within our cohort of patients given TAC-based regimens, mean TC > 5 ng/mL during the initial four weeks following LT had almost the same survival curve as that for 10-15 ng/mL (the current recommended target range) without increasing graft rejection, which is consistent with previous data[19]. In addition, SD is yet another productive and important index in judging TC level. High SD in serial TC levels is related to an increased likelihood of late rejection and graft loss in pediatric organ transplant recipients[23]. Within our clinical setting, SD of 2-4 was shown to be the optimal SD for long-term survival, SD > 4 (in accordance with other reports) and SD < 2 (may due to the small number of patients (n = 21) or insensitivity to TAC) were both associated with a low patient survival rate. Additionally, Cox regression analysis showed that male gender, Plug score (< 10), primary diagnosis of benign disease, mean TC > 5 ng/mL and TC SD of 2-4 are independent factors in the long-term survival of patients, further confirming our results. Chronic TAC exposure may lead to numerous adverse reactions including infection, renal dysfunction, tumor recurrence, rejection, and metabolic disorders[17], and low TC was reported to be linked to rejection[24-26]. In our study, low early exposure to TAC was not associated with these adverse reactions or rejection, and this was mainly due to the brief exposure time which was not continuous or protracted; the entire population attained exactly the same target concentration of 5-10 ng/mL during maintenance treatment for 4-6 wk following LT.
This is the first time that the indices, mean TC and SD, to determine the effect of early TAC exposure on long-term survival have been incorporated, and it was found that the inclusion of mean TC > 5 ng/mL and SD of 2-4 in the optimal early exposure of TAC after LT led to an overall 3-year survival of over 80%. In our study the ‘‘minimizing tacrolimus’’ strategy showed the same rate of survival as the advised level of 10-15 ng/mL and did not increase the incidence of adverse reactions or graft rejection. Thus, this ‘‘minimizing tacrolimus’’ strategy is safe and practical for LT patients. However, it is clear that the ‘‘minimizing tacrolimus’’ strategy should be included with suitable scientific analysis of every patient, integrating clinical examination, biochemical evaluation and pathological analysis, in addition to the pharmacodynamics and pharmacokinetics of TAC.
In conclusion, there are no increased risks when the TC level is reduced compared with the currently recommended level (10-15 ng/mL) during the early phase after LT. We combined the mean and SD to evaluate TAC exposure and suggest that mean TC of 5-10 ng/mL and SD of 2-4 in the initial four weeks following LT are optimal for long-term survival. This ‘‘minimizing tacrolimus’’ strategy protects against unwanted effects, particularly graft rejection. However, further data are needed to support this strategy in order to confirm the results of this study.
COMMENTS
Background
Liver transplantation (LT) is a life-saving technique for patients with end-stage liver disease. Tacrolimus (TAC) is the cornerstone immunosuppressant used to prevent graft rejection in many LT centers. However, frequent TAC-related toxicities are observed and these are a major concern in LT patients. A high blood TAC concentration, especially during the early phase after LT, is associated with adverse effects such as hepatocellular carcinoma, early renal impairment and even death. Thus, the ‘‘minimizing tacrolimus’’ strategy is very important for better long-term outcome, especially in the early phase.
Research frontiers
The primary outcomes of liver transplantation are poor, however, improvements in surgical techniques and in donor liver protection, specifically new developments in immunosuppressive drugs, have led to enhanced graft survival rates. Tacrolimus is one of the immunosuppressant drugs used to prevent graft rejection in many LT centers. Due to TAC-related toxicity, numerous studies have demonstrated that lower early TAC concentration (TC) level also known as the ‘‘minimizing tacrolimus’’ strategy avoids these over immunosuppression side effects.
Innovations and breakthroughs
The parameter measured during tacrolimus exposure in previous studies has mainly been mean concentration. In this study, the authors combined mean TC and standard deviation (SD) to determine the effect of early TAC exposure on long-term survival and found that a mean TC > 5 ng/mL and SD of 2-4 were optimal levels for early TAC exposure in LT patients, which is lower than the currently recommended level of 10-15 ng/mL.
Applications
This study showed that there are no increased risks when the TC level is reduced below the currently recommended level (10-15 ng/mL). The authors recommend that mean TC of 5-10 ng/mL and SD of 2-4 during the initial four weeks following LT are optimal for long-term survival, and this ‘‘minimizing tacrolimus’’ strategy prevents unwanted effects and graft rejection. Therefore, early TAC exposure could be reduced to 5-10 ng/mL in clinical practice and the outcome of patients may be predicted according to the early mean TC and SD.
Terminology
“Minimizing tacrolimus” means lowering the tacrolimus dosage to avoid related toxicity and prevent graft rejection.
Peer review
This study introduced a novel method to evaluate the low level of TC by mean and SD. The authors report the outcomes of a prospective study of tacrolimus mean levels and SD in patients undergoing liver transplantation. The data of the complication rates reported at different tacrolimus levels is useful, as it is the identification of an optimal level of tacrolimus for maintenance.
Footnotes
Supported by National S and T Major Program, No. 2012ZX10002004; National Natural Science Foundation of China, No. 81373160 and No. 81302074
P- Reviewer: Boucek C, Wang GY S- Editor: Gou SX L- Editor: O’Neill M E- Editor: Wang CH
References
- 1.Varo Pérez E, Castroagudín JF. The future of liver transplantation. Transplant Proc. 2010;42:613–616. doi: 10.1016/j.transproceed.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 2.A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation. The U.S. Multicenter FK506 Liver Study Group. N Engl J Med. 1994;331:1110–1115. doi: 10.1056/NEJM199410273311702. [DOI] [PubMed] [Google Scholar]
- 3.Haddad EM, McAlister VC, Renouf E, Malthaner R, Kjaer MS, Gluud LL. Cyclosporin versus tacrolimus for liver transplanted patients. Cochrane Database Syst Rev. 2006;(4):CD005161. doi: 10.1002/14651858.CD005161.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Venkataramanan R, Shaw LM, Sarkozi L, Mullins R, Pirsch J, MacFarlane G, Scheller D, Ersfeld D, Frick M, Fitzsimmons WE, et al. Clinical utility of monitoring tacrolimus blood concentrations in liver transplant patients. J Clin Pharmacol. 2001;41:542–551. doi: 10.1177/00912700122010429. [DOI] [PubMed] [Google Scholar]
- 5.Zahir H, McCaughan G, Gleeson M, Nand RA, McLachlan AJ. Factors affecting variability in distribution of tacrolimus in liver transplant recipients. Br J Clin Pharmacol. 2004;57:298–309. doi: 10.1046/j.1365-2125.2003.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiesner RH, Fung JJ. Present state of immunosuppressive therapy in liver transplant recipients. Liver Transpl. 2011;17 Suppl 3:S1–S9. doi: 10.1002/lt.22410. [DOI] [PubMed] [Google Scholar]
- 7.Koesukwiwat U, Jayanta S, Leepipatpiboon N. Validation of a liquid chromatography-mass spectrometry multi-residue method for the simultaneous determination of sulfonamides, tetracyclines, and pyrimethamine in milk. J Chromatogr A. 2007;1140:147–156. doi: 10.1016/j.chroma.2006.11.099. [DOI] [PubMed] [Google Scholar]
- 8.Ansermot N, Fathi M, Veuthey JL, Desmeules J, Hochstrasser D, Rudaz S. Quantification of cyclosporine A in peripheral blood mononuclear cells by liquid chromatography-electrospray mass spectrometry using a column-switching approach. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;857:92–99. doi: 10.1016/j.jchromb.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Crettol S, Venetz JP, Fontana M, Aubert JD, Ansermot N, Fathi M, Pascual M, Eap CB. Influence of ABCB1 genetic polymorphisms on cyclosporine intracellular concentration in transplant recipients. Pharmacogenet Genomics. 2008;18:307–315. doi: 10.1097/FPC.0b013e3282f7046f. [DOI] [PubMed] [Google Scholar]
- 10.Boillot O, Seket B, Dumortier J, Pittau G, Boucaud C, Bouffard Y, Scoazec JY. Thymoglobulin induction in liver transplant recipients with a tacrolimus, mycophenolate mofetil, and steroid immunosuppressive regimen: a five-year randomized prospective study. Liver Transpl. 2009;15:1426–1434. doi: 10.1002/lt.21905. [DOI] [PubMed] [Google Scholar]
- 11.Calmus Y, Kamar N, Gugenheim J, Duvoux C, Ducerf C, Wolf P, Samuel D, Vanlemmens C, Neau-Cransac M, Salamé E, et al. Assessing renal function with daclizumab induction and delayed tacrolimus introduction in liver transplant recipients. Transplantation. 2010;89:1504–1510. doi: 10.1097/TP.0b013e3181db8cf0. [DOI] [PubMed] [Google Scholar]
- 12.Trunečka P, Boillot O, Seehofer D, Pinna AD, Fischer L, Ericzon BG, Troisi RI, Baccarani U, Ortiz de Urbina J, Wall W. Once-daily prolonged-release tacrolimus (ADVAGRAF) versus twice-daily tacrolimus (PROGRAF) in liver transplantation. Am J Transplant. 2010;10:2313–2323. doi: 10.1111/j.1600-6143.2010.03255.x. [DOI] [PubMed] [Google Scholar]
- 13.Boudjema K, Camus C, Saliba F, Calmus Y, Salamé E, Pageaux G, Ducerf C, Duvoux C, Mouchel C, Renault A, et al. Reduced-dose tacrolimus with mycophenolate mofetil vs. standard-dose tacrolimus in liver transplantation: a randomized study. Am J Transplant. 2011;11:965–976. doi: 10.1111/j.1600-6143.2011.03486.x. [DOI] [PubMed] [Google Scholar]
- 14.Rodríguez-Perálvarez M, Germani G, Papastergiou V, Tsochatzis E, Thalassinos E, Luong TV, Rolando N, Dhillon AP, Patch D, O’Beirne J, et al. Early tacrolimus exposure after liver transplantation: relationship with moderate/severe acute rejection and long-term outcome. J Hepatol. 2013;58:262–270. doi: 10.1016/j.jhep.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez-Perálvarez M, Tsochatzis E, Naveas MC, Pieri G, García-Caparrós C, O’Beirne J, Poyato-González A, Ferrín-Sánchez G, Montero-Álvarez JL, Patch D, et al. Reduced exposure to calcineurin inhibitors early after liver transplantation prevents recurrence of hepatocellular carcinoma. J Hepatol. 2013;59:1193–1199. doi: 10.1016/j.jhep.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Kershner RP, Fitzsimmons WE. Relationship of FK506 whole blood concentrations and efficacy and toxicity after liver and kidney transplantation. Transplantation. 1996;62:920–926. doi: 10.1097/00007890-199610150-00009. [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez-Perálvarez M, Germani G, Darius T, Lerut J, Tsochatzis E, Burroughs AK. Tacrolimus trough levels, rejection and renal impairment in liver transplantation: a systematic review and meta-analysis. Am J Transplant. 2012;12:2797–2814. doi: 10.1111/j.1600-6143.2012.04140.x. [DOI] [PubMed] [Google Scholar]
- 18.Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 19.Nashan B, Saliba F, Durand F, Barcéna R, Herrero JI, Mentha G, Neuhaus P, Bowles M, Patch D, Bernardos A, et al. Pharmacokinetics, efficacy, and safety of mycophenolate mofetil in combination with standard-dose or reduced-dose tacrolimus in liver transplant recipients. Liver Transpl. 2009;15:136–147. doi: 10.1002/lt.21657. [DOI] [PubMed] [Google Scholar]
- 20.Karie-Guigues S, Janus N, Saliba F, Dumortier J, Duvoux C, Calmus Y, Lorho R, Deray G, Launay-Vacher V, Pageaux GP. Long-term renal function in liver transplant recipients and impact of immunosuppressive regimens (calcineurin inhibitors alone or in combination with mycophenolate mofetil): the TRY study. Liver Transpl. 2009;15:1083–1091. doi: 10.1002/lt.21803. [DOI] [PubMed] [Google Scholar]
- 21.Starzl TE, Todo S, Fung J, Demetris AJ, Venkataramman R, Jain A. FK 506 for liver, kidney, and pancreas transplantation. Lancet. 1989;2:1000–1004. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Grady JG, Hardy P, Burroughs AK, Elbourne D. Randomized controlled trial of tacrolimus versus microemulsified cyclosporin (TMC) in liver transplantation: poststudy surveillance to 3 years. Am J Transplant. 2007;7:137–141. doi: 10.1111/j.1600-6143.2006.01576.x. [DOI] [PubMed] [Google Scholar]
- 23.Pollock-Barziv SM, Finkelstein Y, Manlhiot C, Dipchand AI, Hebert D, Ng VL, Solomon M, McCrindle BW, Grant D. Variability in tacrolimus blood levels increases the risk of late rejection and graft loss after solid organ transplantation in older children. Pediatr Transplant. 2010;14:968–975. doi: 10.1111/j.1399-3046.2010.01409.x. [DOI] [PubMed] [Google Scholar]
- 24.Capron A, Lerut J, Latinne D, Rahier J, Haufroid V, Wallemacq P. Correlation of tacrolimus levels in peripheral blood mononuclear cells with histological staging of rejection after liver transplantation: preliminary results of a prospective study. Transpl Int. 2012;25:41–47. doi: 10.1111/j.1432-2277.2011.01365.x. [DOI] [PubMed] [Google Scholar]
- 25.Barbier L, Garcia S, Cros J, Borentain P, Botta-Fridlund D, Paradis V, Le Treut YP, Hardwigsen J. Assessment of chronic rejection in liver graft recipients receiving immunosuppression with low-dose calcineurin inhibitors. J Hepatol. 2013;59:1223–1230. doi: 10.1016/j.jhep.2013.07.032. [DOI] [PubMed] [Google Scholar]
- 26.Uesugi M, Hosokawa M, Shinke H, Hashimoto E, Takahashi T, Kawai T, Matsubara K, Ogawa K, Fujimoto Y, Okamoto S, et al. Influence of cytochrome P450 (CYP) 3A4*1G polymorphism on the pharmacokinetics of tacrolimus, probability of acute cellular rejection, and mRNA expression level of CYP3A5 rather than CYP3A4 in living-donor liver transplant patients. Biol Pharm Bull. 2013;36:1814–1821. doi: 10.1248/bpb.b13-00509. [DOI] [PubMed] [Google Scholar]


