Abstract
AIM: To compare the prevalence and diversity of gastrointestinal (GI) symptoms in patients undergoing peritoneal dialysis (PD) and hemodialysis (HD).
METHODS: Two hundred and ninety-four end-stage renal disease patients participated in the study, including 182 HD and 112 PD patients. Dimension scores were calculated from a modified gastrointestinal symptom rating scale (GSRS) 18-item questionnaire, including items concerning eating dysfunction, and were used for measuring GI symptoms. Information on patient age, condition contributing to end-stage renal disease and the most recent dialysis adequacy assessment (serum Kt/V urea value) was obtained from the follow-up database and by interviewing patients and/or reviewing the medical records. Differences between the HD and PD groups were evaluated using Student’s t, Pearson’s χ2 or Fisher’s exact tests.
RESULTS: The overall prevalence of GI symptoms, defined by a GSRS > 1, in end-stage renal disease patients was 70.7% (208/294), which differed between HD and PD patients (76.4% vs 61.6%, P < 0.01). HD patients had a higher prevalence of constipation, abdominal pain and diarrhea compared to PD patients (36.3% vs 17.9%, 32.4% vs 5.4%, 17.6% vs 4.5%, respectively, P < 0.05). PD patients had a higher prevalence of reflux compared to HD patients (32.1% vs 24.2%, P < 0.05). Additionally, reflux and eating dysfunction were more severe in PD patients (GSRS: 1.71 ± 1.15 vs 1.30 ± 0.67, 1.57 ± 0.84 vs 1.39 ± 0.61, respectively, P < 0.05), whereas HD patients had greater abdominal pain, diarrhea and constipation (GSRS: 1.22 ± 0.39 vs 1.04 ± 0.19, 1.19 ± 0.53 vs 1.07 ± 0.35, 1.51 ± 0.83 vs 1.23 ± 0.58, respectively, P < 0.05). Finally, 14.8% (27/182) of HD patients presented with more than three GI symptoms, compared to 7.2% (8/112) of PD patients (P < 0.01).
CONCLUSION: HD and PD patients differ in prevalence, severity and diversity of GI symptoms.
Keywords: Gastrointestinal symptom, Hemodialysis, Peritoneal dialysis, End-stage renal disease, Constipation, Reflux, Eating dysfunction, Abdominal pain, Diarrhea, Indigestion
Core tip: End-stage renal disease patients undergoing dialysis frequently experience gastrointestinal symptoms. In agreement with previous studies, a majority of patients undergoing hemodialysis (HD) and peritoneal dialysis (PD) in the present study reported gastrointestinal symptoms. However, the results indicate that the prevalence and severity of various gastrointestinal symptoms differ between patients undergoing these two dialysis treatments. HD patients had a higher prevalence of and more severe constipation, abdominal pain and diarrhea, whereas PD patients experienced stronger and more frequent reflux and eating dysfunction. Furthermore, a significantly greater number of HD patients presented with more than three gastrointestinal symptoms.
INTRODUCTION
Gastrointestinal (GI) disorders are a common occurrence in the general population and significantly impair quality of life[1]. Furthermore, GI symptoms are common among patients with end-stage renal disease (ESRD)[2-5] and occur in 32%-85% of patients undergoing dialysis[6-8]. The incidence of GI symptoms can largely be attributed to the underlying conditions, such as increased level of uremic toxin, the effect of dialysis, lifestyle change, or the medications required for treatment[6,7]. Among patients undergoing regular hemodialysis (HD), 51.0%-70.7% experience some GI symptoms, which is significantly higher than that of controls[8,9] but lower than the reported 85% of peritoneal dialysis (PD) patients[8].
GI symptoms in PD patients most commonly include those of gastroesophageal reflux, dyspepsia and eating dysfunction[6,10]. Although PD patients are reportedly more likely than HD and pre-dialysis patients to suffer from these symptoms[6,7,11], few studies have directly or comprehensively evaluated these differences. Indeed, the increased prevalence of GI symptoms in PD patients remains controversial. Therefore, this study aimed to investigate the differences in the prevalence and diversity of GI symptoms between ESRD patients undergoing PD and those undergoing HD.
MATERIALS AND METHODS
Participants
Active PD and HD patients were recruited from the Blood Purification Center at the Changhai Hospital (Shanghai, China), including both inpatients and outpatients who had been receiving dialysis for at least three months. Patients with dementia, severe infectious illness, hepatocholecystopathy, peritonitis in the last three months, unstable blood pressure or glucose levels, or unwillingness to participate in the study were excluded. Informed consent was obtained from all patients in the study, which was approved by the Ethics Committee of Changhai Hospital.
Rating of gastrointestinal symptoms
Participants were asked to complete a modified gastrointestinal symptom rating scale (GSRS) questionnaire to evaluate the presence and severity of general GI symptoms during the previous 2 wk. The GSRS is a 15-item questionnaire with a 7-grade Likert scale (1 = none, 2 = minor, 3 = mild, 4 = moderate, 5 = moderately severe, 6 = severe, and 7 = very severe discomfort) that was originally constructed as an interview-based rating scale to evaluate a wide range of GI symptoms[12] and later modified to become a self-administered questionnaire[13]. The items are grouped into five dimensions, including abdominal pain (three items), reflux (two items), indigestion (four items), diarrhea (three items), and constipation (three items) syndromes. In addition, an eating dysfunction dimension was included, concerning early satiety, difficulties in eating normal portions and postprandial pain, which was developed in a manner analogous to the GSRS[14]. A dimension score was calculated as the mean value of the items belonging to the specific syndrome with a minimum value of 1 and a maximum value of 7.
Patient information
Information concerning age, disease leading to ESRD, diabetic status, and duration of dialysis was obtained by interviewing patients and/or reviewing the medical records. The most recent serum Kt/V urea, an index of dialysis adequacy, was obtained from the follow-up database. Kt/V was calculated using the Daugirdas formula[15].
Statistical analysis
Analyses were performed with SPSS for Windows, version 19.0 (IBM, Armonk, NY, United States). Student’s t-tests were used to compare continuous variables between HD and PD patients when appropriate, and Pearson’s χ2 or Fisher’s exact tests were used for categorical variables. Data are presented as the mean and standard deviation for continuous variables that are normally distributed, and as percentages for categorical variables. A P value < 0.05 was considered statistically significant.
RESULTS
Patient characteristics
In total, two hundred and ninety-four ESRD patients participated in the study and completed the questionnaires, including 182 HD patients and 112 PD patients. Patients undergoing HD and PD did not differ with regard to age, sex, the presence of diabetes mellitus, or the mean dialysis duration (Table 1). The most common cause of ESRD was chronic glomerular nephritis, followed by hypertensive nephropathy and diabetic nephropathy. Dialysis efficacies did not differ between the groups, with 66.7% (62/93) of PD patients able to keep their total (renal + peritoneal) Kt/V above the recommended 1.7[16], and 73.7% (132/179) of HD patients able to achieve a delivered Kt/V value of more than 1.2[17].
Table 1.
Clinical features of the study population n (%)
| Clinical feature | HD (n = 182) | PD(n = 112) | P value |
| Age (yr) | 58.67 ± 14.39 | 59.67 ± 14.19 | 0.56 |
| Female | 75 (41.2) | 51 (45.5) | 0.47 |
| Diabetes mellitus | 37 (20.3) | 27 (24.1) | 0.47 |
| Disease leading to chronic renal failure | |||
| Chronic glomerular nephritis | 85 (46.7) | 40 (35.7) | |
| Hypertensive nephropathy | 38 (20.9) | 34 (30.4) | |
| Diabetic nephropathy | 27 (14.8) | 22 (19.6) | |
| Polycystic kidney disease | 9 (4.9) | 7 (6.2) | |
| Gout | 5 (2.7) | 3 (2.7) | |
| Others | 18 (9.0) | 6 (5.4) | |
| Duration of dialysis, mean months | 55.54 ± 38.47 | 48.90 ± 31.01 | 0.11 |
| Kt/V target reached1 | 132 (73.7) | 62 (66.7) | 0.26 |
Restricted to the 179 HD and 93 PD patients for whom complete information on Kt/V was available. HD: Hemodialysis; PD: Peritoneal dialysis.
Prevalence of GI symptoms in the HD and PD groups
In total, the overall prevalence of GI symptoms (GSRS > 1) in ESRD patients was 70.7% (208/294). A significantly larger number of patients in the HD group had a GSRS > 1 (139/182; 76.4%), compared to 61.6% (69/112) of patients in the PD group (P < 0.01) (Figure 1). In the HD group, more patients suffered from constipation, abdominal pain and diarrhea (36.3%, 32.4% and 17.6%, respectively), compared with those in the PD group (17.9%, 5.4% and 4.5%, respectively; P < 0.01 for all) (Figure 2). Although both groups show similar prevalences of indigestion and eating dysfunction, PD patients had a higher prevalence of reflux symptoms than HD patients (32.1% vs 24.2%, P < 0.05).
Figure 1.
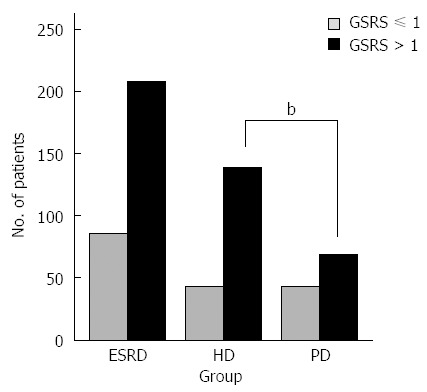
Gastrointestinal symptom rating scale scores. Numbers of end-stage renal disease (ESRD), hemodialysis (HD) and peritoneal dialysis (PD) patients with Gastrointestinal Symptom Rating Scale (GSRS) scores ≤ or > 1. bP < 0.01, HD vs PD.
Figure 2.

Prevalence of gastrointestinal symptoms. The prevalence of various gastrointestinal symptoms in hemodialysis (HD) and peritoneal dialysis (PD) patients. aP < 0.05, HD vs PD.
GSRS scores in the HD and PD groups
GSRS scores were significantly different between HD and PD patients in all dimensions except for indigestion (P < 0.05) (Table 2). Rating scores for abdominal pain, diarrhea and constipation were higher in HD patients, whereas PD patients reported more severe reflux and eating dysfunction.
Table 2.
Comparison of gastrointestinal symptom rating scale scores between the two groups
| Dimension | HD (n = 182) | PD (n = 112) | P value |
| Abdominal pain | 1.22 ± 0.39 | 1.04 ± 0.19 | 0.000 |
| Reflux | 1.30 ± 0.67 | 1.71 ± 1.15 | 0.001 |
| Indigestion | 1.23 ± 0.47 | 1.32 ± 0.56 | 0.153 |
| Diarrhea | 1.19 ± 0.53 | 1.07 ± 0.35 | 0.019 |
| Constipation | 1.51 ± 0.83 | 1.23 ± 0.58 | 0.001 |
| Eating dysfunction | 1.39 ± 0.61 | 1.57 ± 0.84 | 0.049 |
HD: Hemodialysis; PD: Peritoneal dialysis.
Diversity of GI symptoms in the HD and PD groups
The majority of the studied population had at least one reported GI symptom. Although most patients reported one to three symptoms, 27 HD patients reported experiencing four or more symptoms, compared to only eight of the PD patients (Table 3). There was a significantly different distribution of the number of GI symptoms between these two groups (P < 0.01).
Table 3.
Number of gastrointestinal symptoms according to the gastrointestinal symptom rating scale
| Group |
No. of symptoms |
P value1 | ||
| 0 | 1-3 | 4-5 | ||
| HD | 43 | 112 | 27 | 0.009 |
| PD | 43 | 61 | 8 | |
Pearson’s χ2 analysis. HD: Hemodialysis; PD: Peritoneal dialysis.
DISCUSSION
The present study indicates that GI symptoms are common in dialysis patients, with an overall prevalence of 70.7%, similar to a previous report on chronic kidney disease patients[18]. However, these symptoms are more prevalent in HD than in PD patients. Previous studies have reported inconsistent prevalences of GI symptoms, ranging from 32% to 79% in HD patients[4,9,19,20] and 42% to 62% in PD patients[7,10,21], and 73.6% of patients with continuous ambulatory peritoneal dialysis (CAPD) had abnormal upper gastrointestinal endoscopic findings[22]. However, our results indicate a higher prevalence among HD patients, despite similar sample sizes. Such discrepancies may be attributed to the differences in questionnaires used.
The results of this study suggest that the severity of GI symptoms was quite different between HD and PD patients. A greater number of HD patients complained of abdominal pain, diarrhea and constipation, in agreement with previous observations[2,20,23]. The abdominal pain may be associated with peptic ulcers, as Chachati et al[24] observed a high incidence of upper GI pathology, including ulcers, during the first two years of HD, which declined with HD duration. A similar finding was reported in another study where peptic ulcers were shown to be less likely to recur in patients receiving HD for prolonged therapy durations[25]. It is possible that HD patients are more susceptible to ischemic colitis due to hypotensive episodes, which are likely to occur during the initial stages of dialysis[21]. The prevalence of diarrhea in the HD patients of the current study was similar to results from a study by Cano et al[2] who used a Rome II questionnaire to evaluate GI symptoms. However, another previous study reported a much lower incidence, though that study found a gender difference, with women reporting more detrimental symptoms[23]. However, constipation was the most common and severe presenting GI symptom among HD patients, similar to findings from Yasuda et al[26] showing that constipation was more than three times more prevalent in HD compared to CAPD patients. In the clinic, diet and fluid restrictions, lack of physical exercise and the need for certain medications, phosphate binders in particular, by HD patients may contribute to constipation[27].
Similar to the results of previous studies[6-8,10,11], PD patients in our study experienced more pronounced reflux and eating dysfunction. These symptoms may be exacerbated by the filling of the abdominal cavity with dialysate fluid during PD, which increases the intra-abdominal pressure[28] and frequency of acid reflux episodes[29] as well as lowers the esophageal sphincter pressure. PD has also been reported as an independent pathophysiological factor for esophageal acid exposure[30]. Additionally, delayed gastric emptying in PD patients[13,31,32] and the glucose dialysate may play a metabolic role in gastric emptying[33]. An international cross-sectional study found that 33% of 224 CAPD patients were not well-nourished and 8% had severe malnutrition, of which one of the leading causes was eating dysfunction[34] that may have resulted from the associated food aversion, early satiety and changes in taste and smell[20]. Eating dysfunction could also be partly due to local or systemic circulatory insufficiency, hypergastrinemia and higher levels of ammonia and inflammation[35].
To our knowledge, this is the first study directly comparing the diversity of GI symptoms in HD and PD patients. GI symptoms were common in dialysis patients, but the prominent symptoms unexpectedly differed between patients undergoing different dialysis therapies. Although the majority of patients complained of few and minor symptoms, approximately 10% suffered from more than three GI symptoms, which occurred in a greater percentage of HD than PD patients. The underlying mechanism is not clear, but hemodynamic changes, delayed gastric emptying, loss of residual renal function, and inadequate dialysis, such as protein-bound uremic toxin that cannot be effectively cleared by HD, may all contribute.
In conclusion, the present study demonstrated a high prevalence of GI symptoms in HD and PD patients, with constipation, abdominal pain and diarrhea more frequent and severe in HD patients, and reflux more prominent in PD patients. However, the results are not in complete agreement with previous studies, thus necessitating further evaluation in a larger population of dialysis patients.
ACKNOWLEDGMENTS
We would like to thank Xie-Qin You and Xing Lu for their assistance with grammar and statistical analysis, respectively.
COMMENTS
Background
Gastrointestinal (GI) symptoms are commonly reported among patients with end-stage renal disease and correlate to nutritional status, life quality, and even mortality. Until now, little attention has been paid to these GI symptoms, especially in hemodialysis (HD) patients.
Research frontiers
The gastrointestinal symptom rating scale (GSRS) has been used widely for evaluating the presence of GI symptoms in the population. The authors in this study used the GSRS to evaluate more than two hundred dialysis patients, and compared the prevalence, severity and diversity of GI symptoms between patients receiving HD or peritoneal dialysis (PD).
Innovations and breakthroughs
Previous studies have presented cross-sectional descriptions of GI symptoms in PD and HD patients. The prevalence, severity and diversity of GI symptoms are directly compared in more than two hundred PD and HD patients in the present study. The results show that HD patients have a higher prevalence and severity of constipation, abdominal pain and diarrhea, whereas PD patients have a higher prevalence and severity of reflux and more severe eating dysfunction. Moreover, a greater number of HD patients presented with more than three GI symptoms.
Applications
The results of this study suggest that GI symptoms differ in patients with PD and HD, and clinicians should therefore have differential focus and treatments for patients with GI symptoms undergoing dialysis.
Terminology
Kt/V urea is an index used to quantify HD and PD treatment adequacy: K refers to the dialyzer clearance of urea, t is the dialysis time, and V is the volume of distribution of urea, approximately equal to the patient’s total body water. The US National Kidney Foundation designates the Kt/V target as ≥ 1.2 for HD and ≥ 1.7/wk for PD patients.
Peer review
This is a good research study in which authors compare the GI symptoms in patients undergoing PD and HD. The results are interesting and suggest that GI symptoms differ between PD and HD patients, and clinicians should therefore evaluate and differentially treat GI symptoms in dialysis patients.
Footnotes
P- Reviewer: Shu KH, Zouiten-Mekki L S- Editor: Nan J L- Editor: Wang TQ E- Editor: Zhang DN
References
- 1.Jones R. Primary care research and clinical practice: gastroenterology. Postgrad Med J. 2008;84:454–458. doi: 10.1136/pgmj.2008.068361. [DOI] [PubMed] [Google Scholar]
- 2.Cano AE, Neil AK, Kang JY, Barnabas A, Eastwood JB, Nelson SR, Hartley I, Maxwell D. Gastrointestinal symptoms in patients with end-stage renal disease undergoing treatment by hemodialysis or peritoneal dialysis. Am J Gastroenterol. 2007;102:1990–1997. doi: 10.1111/j.1572-0241.2007.01321.x. [DOI] [PubMed] [Google Scholar]
- 3.Murtagh FE, Addington-Hall J, Higginson IJ. The prevalence of symptoms in end-stage renal disease: a systematic review. Adv Chronic Kidney Dis. 2007;14:82–99. doi: 10.1053/j.ackd.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Hammer J, Oesterreicher C, Hammer K, Koch U, Traindl O, Kovarik J. Chronic gastrointestinal symptoms in hemodialysis patients. Wien Klin Wochenschr. 1998;110:287–291. [PubMed] [Google Scholar]
- 5.Fallone CA, Mayrand S. Gastroesophageal reflux and hyperacidity in chronic renal failure. Perit Dial Int. 2001;21 Suppl 3:S295–S299. [PubMed] [Google Scholar]
- 6.Strid H, Simrén M, Johansson AC, Svedlund J, Samuelsson O, Björnsson ES. The prevalence of gastrointestinal symptoms in patients with chronic renal failure is increased and associated with impaired psychological general well-being. Nephrol Dial Transplant. 2002;17:1434–1439. doi: 10.1093/ndt/17.8.1434. [DOI] [PubMed] [Google Scholar]
- 7.Dong R, Guo ZY. Gastrointestinal symptoms in patients undergoing peritoneal dialysis: multivariate analysis of correlated factors. World J Gastroenterol. 2010;16:2812–2817. doi: 10.3748/wjg.v16.i22.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salamon K, Woods J, Paul E, Huggins C. Peritoneal dialysis patients have higher prevalence of gastrointestinal symptoms than hemodialysis patients. J Ren Nutr. 2013;23:114–118. doi: 10.1053/j.jrn.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Strid H, Fjell A, Simrén M, Björnsson ES. Impact of dialysis on gastroesophageal reflux, dyspepsia, and proton pump inhibitor treatment in patients with chronic renal failure. Eur J Gastroenterol Hepatol. 2009;21:137–142. doi: 10.1097/MEG.0b013e3283200047. [DOI] [PubMed] [Google Scholar]
- 10.Anderson JE, Yim KB, Crowell MD. Prevalence of gastroesophageal reflux disease in peritoneal dialysis and hemodialysis patients. Adv Perit Dial. 1999;15:75–78. [PubMed] [Google Scholar]
- 11.Chong VH, Tan J. Prevalence of gastrointestinal and psychosomatic symptoms among Asian patients undergoing regular hemodialysis. Nephrology (Carlton) 2013;18:97–103. doi: 10.1111/nep.12000. [DOI] [PubMed] [Google Scholar]
- 12.Svedlund J, Sjödin I, Dotevall G. GSRS--a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33:129–134. doi: 10.1007/BF01535722. [DOI] [PubMed] [Google Scholar]
- 13.Dimenäs E, Glise H, Hallerbäck B, Hernqvist H, Svedlund J, Wiklund I. Quality of life in patients with upper gastrointestinal symptoms. An improved evaluation of treatment regimens? Scand J Gastroenterol. 1993;28:681–687. doi: 10.3109/00365529309098272. [DOI] [PubMed] [Google Scholar]
- 14.Svedlund J, Sullivan M, Liedman B, Lundell L. Long term consequences of gastrectomy for patient’s quality of life: the impact of reconstructive techniques. Am J Gastroenterol. 1999;94:438–445. doi: 10.1111/j.1572-0241.1999.874_c.x. [DOI] [PubMed] [Google Scholar]
- 15.Daugirdas JT. Second generation logarithmic estimates of single-pool variable volume Kt/V: an analysis of error. J Am Soc Nephrol. 1993;4:1205–1213. doi: 10.1681/ASN.V451205. [DOI] [PubMed] [Google Scholar]
- 16.Lo WK, Bargman JM, Burkart J, Krediet RT, Pollock C, Kawanishi H, Blake PG. Guideline on targets for solute and fluid removal in adult patients on chronic peritoneal dialysis. Perit Dial Int. 2006;26:520–522. [PubMed] [Google Scholar]
- 17.Charra B, Calemard E, Ruffet M, Chazot C, Terrat JC, Vanel T, Laurent G. Survival as an index of adequacy of dialysis. Kidney Int. 1992;41:1286–1291. doi: 10.1038/ki.1992.191. [DOI] [PubMed] [Google Scholar]
- 18.Thomas R, Panackal C, John M, Joshi H, Mathai S, Kattickaran J, Iqbal M. Gastrointestinal complications in patients with chronic kidney disease--a 5-year retrospective study from a tertiary referral center. Ren Fail. 2013;35:49–55. doi: 10.3109/0886022X.2012.731998. [DOI] [PubMed] [Google Scholar]
- 19.Nespor SL, Holley JL. Patients on hemodialysis rely on nephrologists and dialysis units for maintenance health care. ASAIO J. 1992;38:M279–M281. doi: 10.1097/00002480-199207000-00037. [DOI] [PubMed] [Google Scholar]
- 20.Bossola M, Luciani G, Rosa F, Tazza L. Appetite and gastrointestinal symptoms in chronic hemodialysis patients. J Ren Nutr. 2011;21:448–454. doi: 10.1053/j.jrn.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Lee SW, Song JH, Kim GA, Yang HJ, Lee KJ, Kim MJ. Effect of dialysis modalities on gastric myoelectrical activity in end-stage renal disease patients. Am J Kidney Dis. 2000;36:566–573. doi: 10.1053/ajkd.2000.16195. [DOI] [PubMed] [Google Scholar]
- 22.Urganci N, Ozcelik G, Kalyoncu D, Geylani Gulec S, Akinci N. Serum gastrin levels and gastroduodenal lesions in children with chronic renal failure on continuous ambulatory peritoneal dialysis: a single-center experience. Eur J Gastroenterol Hepatol. 2012;24:924–928. doi: 10.1097/MEG.0b013e3283543ee7. [DOI] [PubMed] [Google Scholar]
- 23.Silva LF, Lopes GB, Matos CM, Brito KQ, Amoedo MK, Azevedo MF, Sá Araújo MJ, Martins MS, Lopes AA. Gastrointestinal symptoms and nutritional status in women and men on maintenance hemodialysis. J Ren Nutr. 2012;22:327–335. doi: 10.1053/j.jrn.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Chachati A, Godon JP. Effect of haemodialysis on upper gastrointestinal tract pathology in patients with chronic renal failure. Nephrol Dial Transplant. 1987;1:233–237. [PubMed] [Google Scholar]
- 25.Tseng GY, Lin HJ, Fang CT, Yang HB, Tseng GC, Wang PC, Hung TL, Deng YC, Cheng YT, Huang CH. Recurrence of peptic ulcer in uraemic and non-uraemic patients after Helicobacter pylori eradication: a 2-year study. Aliment Pharmacol Ther. 2007;26:925–933. doi: 10.1111/j.1365-2036.2007.03438.x. [DOI] [PubMed] [Google Scholar]
- 26.Yasuda G, Shibata K, Takizawa T, Ikeda Y, Tokita Y, Umemura S, Tochikubo O. Prevalence of constipation in continuous ambulatory peritoneal dialysis patients and comparison with hemodialysis patients. Am J Kidney Dis. 2002;39:1292–1299. doi: 10.1053/ajkd.2002.33407. [DOI] [PubMed] [Google Scholar]
- 27.Lee A. Constipation in patients on peritoneal dialysis: a literature review. Ren Soc Aust J. 2011;7:122–129. [Google Scholar]
- 28.Shay S, Schreiber M, Richter J. Compliance curves during peritoneal dialysate infusion are like a distensible tube and are similar at multiple UGI sites. Am J Gastroenterol. 1999;94:1034–1041. doi: 10.1111/j.1572-0241.1999.01010.x. [DOI] [PubMed] [Google Scholar]
- 29.Kim MJ, Kwon KH, Lee SW. Gastroesophageal reflux disease in CAPD patients. Adv Perit Dial. 1998;14:98–101. [PubMed] [Google Scholar]
- 30.Cekin AH, Boyacioglu S, Gursoy M, Bilezikci B, Gur G, Akin ED, Ozdemir N, Yilmaz U. Gastroesophageal reflux disease in chronic renal failure patients with upper GI symptoms: multivariate analysis of pathogenetic factors. Am J Gastroenterol. 2002;97:1352–1356. doi: 10.1111/j.1572-0241.2002.05772.x. [DOI] [PubMed] [Google Scholar]
- 31.Schoonjans R, Van VB, Vandamme W, Van HN, Verdievel H, Vanholder R, Lameire N, De VM. Dyspepsia and gastroparesis in chronic renal failure: the role of Helicobacter pylori. Clin Nephrol. 2002;57:201–207. doi: 10.5414/cnp57201. [DOI] [PubMed] [Google Scholar]
- 32.Van V, Schoonjans RS, Struijk DG, Verbanck JJ, Vanholder RC, Van B, Lefebvre RA, De V, Lameire NH. Influence of dialysate on gastric emptying time in peritoneal dialysis patients. Perit Dial Int. 2002;22:32–38. [PubMed] [Google Scholar]
- 33.Strid H, Simrén M, Björnsson ES. Overuse of acid suppressant drugs in patients with chronic renal failure. Nephrol Dial Transplant. 2003;18:570–575. doi: 10.1093/ndt/18.3.570. [DOI] [PubMed] [Google Scholar]
- 34.Jones MR. Etiology of severe malnutrition: results of an international cross-sectional study in continuous ambulatory peritoneal dialysis patients. Am J Kidney Dis. 1994;23:412–420. doi: 10.1016/s0272-6386(12)81004-3. [DOI] [PubMed] [Google Scholar]
- 35.Bacci MR, Chehter EZ. Dyspepsia among patients with chronic kidney disease: a cross sectional study. Int Arch Med. 2013;6:43. doi: 10.1186/1755-7682-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]


