Abstract
AIM: To evaluate the feasibility and short-term efficacy of laparoscopic spleen-preserving splenic hilar (No. 10) lymphadenectomy to treat advanced upper gastric cancer (AUGC).
METHODS: Between January and December 2012, 108 laparoscopic spleen-preserving No. 10 lymphadenectomy along with total gastrectomy with routine D2 lymphadenectomy were performed consecutively at our hospital to treat clinical T2-3 (cT2-3) upper gastric cancers. The preoperative clinical T stage was cT2 in 36 patients and cT3 in 72 patients. A prospectively designed database tracked the 108 patients, including the completeness of their medical records and the adequacy of follow-up. Patient clinicopathological characteristics, intraoperative and postoperative surgical outcomes, morbidity and mortality, lymph node (LN) dissection, and postoperative follow-up were analysed retrospectively.
RESULTS: Laparoscopic spleen-preserving No. 10 lymphadenectomy was successful in all 108 patients. The mean operation time was 169.3 ± 27.1 min, and the mean No. 10 lymphadenectomy time was 20.0 ± 5.7 min. The mean total blood loss was 46.2 ± 11.3 mL, and the mean blood loss from No. 10 lymphadenectomy was 14.3 ± 3.8 mL. The mean postoperative hospital stay was 11.9 ± 6.0 d. The intraoperative and postoperative morbidity rates were 3.7% and 12.0%, respectively; however, there was no postoperative mortality. A mean of 44.4 ± 17.6 LNs were retrieved from each specimen, including 3.0 ± 2.4 No. 10 LNs. Three patients (2.8%) with cT3 cancer had LN metastasis of the splenic hilus, including two patients with pathological T3 (pT3) and one patient with pathological T4a (pT4a) tumours, all located in the greater curvature. No splenic hilar LNs metastasis was evident in the patients with pT1 and pT2 tumours. At a median follow-up time of 18 mo (range, 12 to 23 mo), all patients were alive and none had experienced recurrent or metastatic disease.
CONCLUSION: Laparoscopic spleen-preserving No. 10 lymphadenectomy is feasible and effective to treat AUGC. Routine No. 10 lymphadenectomy may be unnecessary for AUGC without serosa invasion, unless T3 tumours are located in the greater curvature.
Keywords: Stomach neoplasms, Spleen-preservation, laparoscopy, Gastrectomy, Lymphadenectomy
Core tip: Several studies have shown that laparoscopic spleen-preserving No. 10 lymphadenectomy is feasible for patients with upper gastric cancer; however, the sample sizes in these studies were small. Thus, the value of the procedure must be further evaluated with large sample studies, and it is debatable whether routine No. 10 lymphadenectomy should be performed for advanced upper gastric cancer (AUGC) without serosa invasion. Therefore, we evaluated the feasibility and short-term efficacy of laparoscopic spleen-preserving No. 10 lymphadenectomy in 108 consecutive patients with AUGC (cT2-3). In addition, early follow-up results were also presented.
INTRODUCTION
Splenic hilar lymph nodes (No. 10 LNs) are LNs that are located in the splenic hilum, including those LNs adjacent to the splenic artery and distal to the pancreatic tail, on the roots of the short gastric arteries, and along the left gastroepiploic artery proximal to the first gastric branch[1]. Standard D2 LN dissection during total gastrectomy for advanced upper gastric cancer (AUGC) requires the removal of the No. 10 LNs[1]. Spleen-preserving No. 10 lymphadenectomy is technically feasible and safe for patients undergoing open surgery for upper gastric cancer[2], with lower postoperative morbidity and mortality rates than splenectomy[3-7]. Moreover, the radical effects and long-term survival rates were similar to those in patients who underwent splenectomy[8-12]. Total gastrectomy with spleen-preserving No. 10 lymphadenectomy is, therefore, increasingly used to treat patients with upper gastric cancer. Spleen-preserving D2 lymphadenectomy, however, requires an anatomical No. 10 lymphadenectomy, a procedure that is technically difficult because of the presence of intricate and complex vessels, and a narrow and deep space at the splenic hilum. Moreover, complete removal of No. 10 LNs is particularly difficult in obese patients and patients with splenic adhesions.
Laparoscopic gastrectomy with D2 lymphadenectomy is safe and feasible for patients with advanced gastric cancer[13-16]. Although several studies have shown that laparoscopic spleen-preserving No. 10 lymphadenectomy is feasible during total gastrectomy with D2 LN dissection for small numbers of patients with upper gastric cancer[17-19], few large-scale studies have assessed its success in patients with AUGC. Furthermore, it is debatable whether routine No. 10 lymphadenectomy should be performed during total gastrectomy for AUGC without serosa invasion. Therefore, in the current study, we evaluated the feasibility and short-term efficacy of laparoscopic spleen-preserving No. 10 lymphadenectomy in 108 consecutive patients with cT2-3 AUGC. In addition, early follow-up results were also presented.
MATERIALS AND METHODS
Patients
Between January and December 2012, 108 consecutive patients with cT2-3 AUGC underwent laparoscopic spleen-preserving No. 10 lymphadenectomy, along with total gastrectomy and routine D2 lymphadenectomy in the Department of Gastric Surgery, Fujian Medical University Union Hospital. Beginning in May 2007, the surgeon (Huang CM) in this study had performed more than 500 laparoscopy-assisted gastrectomies with D2 LN dissection in gastric cancer patients before attempting this procedure. Since then, a prospectively designed database has tracked all laparoscopy-assisted gastrectomies for gastric cancer. Moreover, the surgeon performed laparoscopic spleen-preserving No. 10 lymphadenectomy for advanced proximal gastric cancer using a left-sided approach[20]. This method was mastered after a learning curve of 40 patients[21]. The group of 108 consecutive patients was used for our retrospective analysis because of the completeness of their medical records and the adequacy of their follow-up. Upper gastric cancer was diagnosed by analysis of endoscopic biopsy specimens. Preoperative imaging studies were routinely performed following endoscopic examination, computed tomography (CT) scanning, abdominal ultrasonography (US) and endoscopic US. CT scans and multi-slice spiral CT angiography (MSCTA) were performed to assess preoperatively the splenic vascular anatomy (Figures 1 and 2). Advanced (cT2-T3) upper gastric cancer diagnosed by preoperative CT scanning and endoscopic US were enrolled in this study. Patients with clinical T1 (cT1) or clinical T4 (cT4) tumours, distant metastasis, or preoperative enlargement or integration of LNs were excluded. No patients had received preoperative chemoradiation therapy. Each preoperative patient was informed of the surgical procedure, including its advantages and risks. All patients provided written informed consent for the procedure before surgery, as well as for the publication of this report and any accompanying images.
Figure 1.
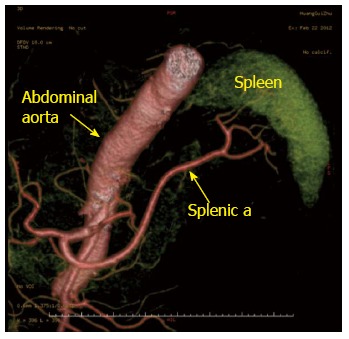
Preoperative computed tomography angiography showing the drainage of the splenic arteries. Abdominal aorta (arrow); splenic a (arrow). a: Artery.
Figure 2.
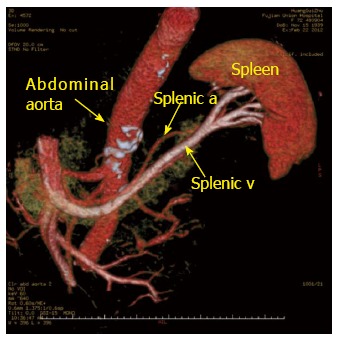
Preoperative computed tomography angiography showing the drainage of the splenic veins. Abdominal aorta (arrow); splenic a (arrow); splenic v (arrow). a: Artery; v: Vein.
In the current study, the No. 10 lymphadenectomy began with the surgeon using an ultrasonic scalpel to separate and reveal the end of the splenic arteries within the retropancreatic space at the superior border of the pancreatic tail, to divide the last short gastric artery. The No. 10 lymphadenectomy time referred to the time of this procedure. The blood loss during surgery was measured by estimating the volume of blood in the suction container and weighing the gauze with blood. Dissected LNs were classified according to the 3rd English edition of the Japanese classification of gastric carcinoma[1]. The clinical and pathological stagings were in accordance with the American Joint Committee on Cancer (AJCC) seventh edition of Gastric Cancer TNM Staging[22]. Follow-up was performed by trained investigators every 3 mo. Routine follow-up comprised a physical examination, laboratory tests, chest radiography, abdominopelvic ultrasonography or CT scans. Survival time was calculated from the time of surgical intervention until the last date of contact (December 31, 2013).
Surgical procedures
Patient positioning: The patient was placed in the reverse Trendelenburg position with the head elevated approximately 15° to 20°, and tilted with the left side up approximately 20° to 30°. A 10-mm trocar for the laparoscope was inserted below the umbilicus; a 12-mm trocar was inserted in the left upper quadrant as a major hand port; a 5-mm trocar was inserted in the left lower quadrant as an accessory port; a second 5-mm trocar for exposure was inserted in the left upper quadrant; and a third 5-mm trocar for exposure was inserted in the right lower quadrant. The surgeon stood on the left side of the patient; the assistant surgeon was on the right side; and the camera operator was situated between the patient’s legs.
Other lymphadenectomy: The gastrocolic ligament was divided using an ultrasonic scalpel along the border of the transverse colon. The right gastroepiploic vein and the right gastroepiploic artery were vascularised and divided to dissect the No. 6 LNs. The stomach was lifted toward the head to expose the gastropancreatic fold. The LNs along the proximal splenic artery (No. 11p) at the upper border of pancreatic body were removed. The dissection was then continued rightward. The fatty connective tissue, including the LNs along the celiac trunk (No. 9), the left gastric artery (No. 7), and the common hepatic artery (No. 8a) were removed en-block with the left gastric vein and the left gastric artery being vascularised and divided. The LNs around the right gastric artery (No. 5) and along the surface of the proper hepatic artery (No. 12a) were then dissected and removed. Subsequently, the liver was held up to divide the hepatogastricum ligament along the lower border of the liver and the LNs around lesser curvature (No. 3) were removed. Finally, the phrenoesophageal membrane and both vagus nerves were divided and the LNs around the abdominal oesophagus (No. 1 and 2) were dissected.
No. 10 lymphadenectomy: The patient was subsequently tilted, with the left side up approximately 20° to 30° and subjected to a 20° upward head tilt. The surgeon then moved to stand between the patient’s legs, and the assistant and camera operator were both on the patient’s right side. Before surgery, the assistant placed the greater omentum behind the stomach to keep the visual field clear, and pulled and tensed the gastrosplenic ligament. The surgeon gently pressed the tail of the pancreas toward the lower left, exposing the splenic hilum. The surgeon separated the membrane of the body and tail of the pancreas to reach the posterior space at the superior border of the pancreas, and opened the vascular envelope at the end of the splenic arteries. The surgeon dissected away the lymphatic fatty tissue on the surface of the inferior splenic lobar artery from the lower pole of the spleen, vascularised the left gastroepiploic artery issuing from the inferior splenic lobar artery, and then cut the left gastroepiploic artery (No. 4sb) from the origin. The assistant then placed the free omentum between the liver and the stomach and continually pulled the posterior wall of the fundus and body of the stomach to the upper right. The surgeon gently pressed the pancreas to fully reveal the retropancreatic space and the space inside the splenorenal ligament. The surgeon then tracked the termini of the splenic vessels along the completely vascularised the lower lobar vessels of the spleen within the space inside the splenorenal ligament. Next, the surgeon carefully dissected the fatty lymphatic tissue around the splenic vessels (No. 11d) along the latent anatomic spaces on the surface of the splenic vessels. At this time, the assistant gently pulled up the lymphatic fatty tissue at the surface of the inferior splenic lobar artery. Starting from the root of the left gastroepiploic artery, the surgeon, using the non-functional face of the ultrasonic scalpel, closed the surface of the inferior splenic lobar artery. The surgeon used the ultrasonic scalpel to carefully dissect the lymphatic fatty tissue and to vascularise the inferior splenic lobar artery. After the latter became visible, the short gastric arteries issuing from the inferior splenic lobar artery were skeletonised and divided at their roots, resulting in complete vascularisation of the inferior splenic lobar artery. The fatty tissues and gastric tissues were pulled up by the assistant, and the surgeon dissected the lymphatic fatty tissue on the surface of the superior splenic lobar artery, starting from the root of the artery towards the upper pole of the spleen, as described for vascularisation of the inferior splenic lobar artery. One branch of the short gastric artery issuing from the superior splenic lobar artery was skeletonised and divided at its root. This procedure resulted in LN dissections at the front of the splenic vessels (Figure 3). The assistant then pulled the root of the inferior splenic lobar artery towards the upper right, revealing the lymphatic fatty tissue behind the splenic hilum. The latter was pulled up by the surgeon towards the lower left to maintain tension. The lymphatic fatty tissue behind the splenic hilum was then dissected (No. 10) (Figure 4). A piece of gauze was placed behind the splenic hilum to indicate that the vessels had been vascularised and the LNs had been completely dissected.
Figure 3.
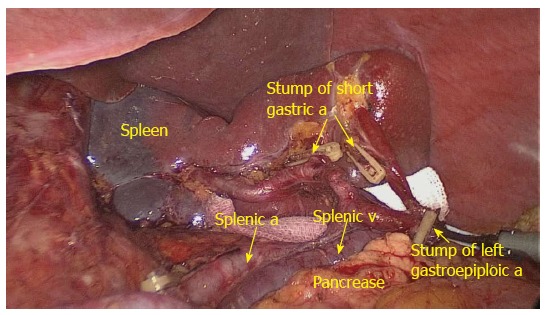
No. 10 lymph nodes lymphadenectomy at the front of the splenic vessels (anterior view). Dividing left gastroepiploic a (arrow); dividing short gastric a (arrow); splenic a (arrow); splenic v (arrow); a: Artery; v: Vein.
Figure 4.
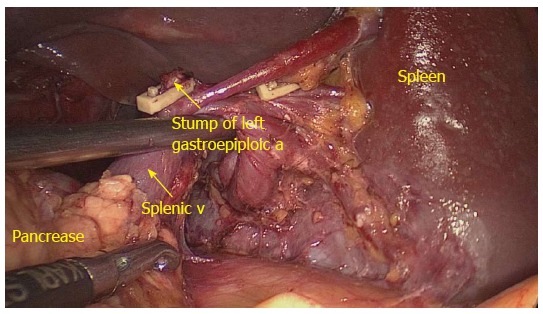
No. 10 lymph nodes lymphadenectomy behind the splenic vessels (posterior view). Dividing left gastroepiploic a (arrow); splenic vein (arrow); a: Artery; v: Vein.
Digestive tract reconstruction: The duodenum was transected 2 cm below the pylorus with a 60-mm laparoscopic cartridge linear stapling device through the major hand port. Finally, a longitudinal laparotomy was performed using a 6-8 cm skin incision at the epigastrium, and the specimen was extracted from the peritoneal cavity. The transaction of the oesophagus and Roux-en-Y oesophagojejunostomy was carried out using a circular stapler. A side-to-side jejunojejunostomy was performed by hand suture.
RESULTS
Patient clinicopathological characteristics
The 108 patients included 87 males (80.6%) and 21 females (19.4%) with a mean age of 62.5 ± 9.2 years (range, 24-82 years) and mean body mass index (BMI) of 22.1 ± 2.9 kg/m2 (range: 14.5-34.5 kg/m2). The preoperative clinical T stage was cT2 in 36 patients (33.3%) and cT3 in 72 patients (66.7%). The postoperative pathological TNM stages included pT1 (n = 12), pT2 (n = 14), pT3 (n = 73), and pT4a (n = 9); pN0 (n = 31), pN1 (n = 17), pN2 (n = 23), and pN3 (n = 37); IA (n = 9), IB (n = 8), IIA (n = 19), IIB (n = 14), IIIA (n = 23), IIIB (n = 29) and IIIC (n = 6) (Table 1).
Table 1.
Baseline demographic and clinicopathological characteristics of patients
| Characteristic | Value | |
| Gender | Male/female | 87/21 |
| Age (yr) | 62 ± 9 | |
| Tumour size (cm) | 5.0 ± 2.6 | |
| BMI (kg/m2) | 22.1 (14.5-34.5) | |
| Tumour location | Lesser curvature/greater curvature/anterior wall/posterior wall/circumferential involvement | 30/21/15/19/23 |
| Pathological type | Differentiated/undifferentiated type | 43/65 |
| cT stage | T2/T3 | 36/72 |
| pT stage | T1/T2/T3/T4a | 12/14/73/9 |
| pN stage | N0/N1/N2/N3 | 31/17/23/37 |
| TNM stage | IA/IB/IIA/IIB/IIIA/IIIB/IIIC | 9/8/19/14/23/29/6 |
Data are expressed as mean ± SD. BMI: Body mass index; cT stage: Clinical tumour stage; pT stage: Pathological tumour stage.
Intraoperative and postoperative surgical outcomes
For all 108 patients, the mean operation time was 169.3 ± 27.1 min, and the mean No. 10 lymphadenectomy time was 20.0 ± 5.7 min. The mean estimated blood loss was 46.2 ± 11.3 mL, and the mean estimated blood loss for No. 10 lymphadenectomy was 14.3 ± 3.8 mL. The mean times to first flatus, fluid diet, and soft diet were 3.4 ± 1.1, 4.7 ± 1.6 and 8.3 ± 4.2 d, respectively, and the mean postoperative hospital stay was 11.9 ± 6.0 d (Table 2).
Table 2.
Intraoperative and postoperative surgical outcomes
| Item | Value |
| Operation time (min) | 169.3 ± 27.1 |
| Blood loss (mL) | 46.2 ± 11.3 |
| No. 10 lymphadenectomy (min) | 20.0 ± 5.7 |
| No. 10 lymphadenectomy blood loss (mL) | 14.3 ± 3.8 |
| Time to first flatus (POD) | 3.4 ± 1.1 |
| Time to fluid diet (POD) | 4.7 ± 1.6 |
| Time to soft diet (POD) | 8.3 ± 4.2 |
| Hospital stay (POD) | 11.9 ± 6.0 |
Data are expressed as mean ± SD. No. 10 lymphadenectomy: Splenic hilar lymphadenectomy; POD: Postoperative days.
Morbidity and mortality
Four patients experienced intraoperative complications, giving an intraoperative morbidity rate of 3.7%. One patient experienced each of the following complications: injury to the transverse colon, injury to the splenic envelope, bleeding from the gastric coronary vein and bleeding from the gastric short arteries. All complications were treated during successfully laparoscopic surgery. No patient required conversion to laparotomy, and no patient required splenectomy because of intraoperative injury to the splenic blood vessels or the spleen itself. Postoperative complications occurred in 13 patients, giving a morbidity rate of 12.0%. These complications included abdominal infection in two patients, pulmonary infection in eight patients, inflammatory intestinal obstruction in one patient, chylous fistula in one patient, and anastomotic leakage in one patient. These postoperative complications were all successfully treated with conservative methods, and none of these patients required a second operation (Table 3). No patient experienced an operative splenic infarction, haemorrhage of the splenic blood vessels, or complications of spleen itself. The 30-d mortality rate for the total patient population was 0%.
Table 3.
Intraoperative and postoperative complications
| Item | Value | Incidence |
| Intraoperative complications (n) | 4 | 3.7% |
| Transverse colon injury | 1 | |
| Spleen injury | 1 | |
| Left gastric vein bleeding | 1 | |
| Gastric short arteries bleeding | 1 | |
| Postoperative complications (n) | 13 | 12.0% |
| Pulmonary infection | 8 | |
| Abdominal infection | 2 | |
| Anastomotic leakage | 1 | |
| Intestinal obstruction | 1 | |
| Chylous fistula | 1 |
LN dissection
The total number of LNs in all 108 patients was 4797, with a mean of 44.4 ± 17.6 LNs retrieved from each specimen. The total number of No. 10 LNs in all patients was 327, with a mean of 3.0 ± 2.4 No. 10 LNs retrieved per patient. Three patients (2.8%) had LN metastasis of the splenic hilus, including two patients with pT3 tumours and one patient with pT4a tumours, all located in the greater curvature (Table 4). There was no No. 10 LN metastasis in the patients with pT1 and pT2 tumours.
Table 4.
Lymph nodes dissection results
| Item | Value |
| Total No. of retrieved LNs | 4797 |
| Mean No. of retrieved LNs | 44.4 ± 17.6 |
| Total No. of retrieved No. 10 LNs | 327 |
| Mean No. of retrieved No. 10 LNs | 3.0 ± 2.4 |
| Total No. of No. 10 LNs metastasis | 3 |
| No. of No. 10 LNs metastasis in pT3 | 2 |
| No. of No. 10 LNs metastasis in pT4a | 1 |
| No. 10 LNs metastasis rate | 2.8% |
| No. 10 LNs metastasis rate in pT3 | 2.7% |
| No. 10 LNs metastasis rate in pT4a | 11.1% |
Data are expressed as mean ± SD. LN: Lymph node; No. 10 LN: Splenic hilar lymph node; pT3: Pathological T3 stage; pT4a: Pathological T4a stage.
Postoperative follow-up
The 108 patients were followed up for a median 18 mo (range: 12-23 mo). No patient died or experienced tumour recurrence or metastasis during the follow-up period.
DISCUSSION
D2 lymphadenectomy, including the removal of No. 10 LNs, has become the standard surgical procedure for patients with curable AUGC[1,6]. In recent years, with advances in surgical concepts, improvements in the anatomical techniques and the progress of organ retention, spleen-preserving No. 10 LN dissection has been used increasingly for AUGC patients[2,7,9,12]. However, this procedure is technically difficult, not only because of the intricate and complex blood vessels, but also because of the deep and limited operative space in the splenic hilum. On the one hand, in open surgery, the complete removal the No. 10 LNs often requires the mobilisation of the spleen from the abdominal cavity, which obviously increases patient trauma, elongates operation time, and is especially difficult for obese patients and patients with splenic adhesions. On the other hand, maintaining the spleen within the abdominal cavity and performing spleen-preserving No. 10 LN dissection directly would not completely remove all LNs, because the exposure would be insufficient. Similar to open surgery, spleen-preserving No. 10 LN dissection is also one of the most difficult procedures in laparoscopic surgery. Previously, a few studies have reported the feasibility of laparoscopic spleen-preserving splenic hilar LN dissection for AUGC[17-19] patients; however, the sample sizes in these studies were small; thus, the value of the procedure needed to be further evaluated by studies with large samples. According to the 3rd English edition of Japanese classification of gastric carcinoma[1], splenic hilar lymphadenectomy is unnecessary for cT1 tumours and laparoscopic surgery applied to cT4 tumours has been controversial. In the current study, therefore, we studied the feasibility and short-term efficacy of laparoscopic spleen-preserving No. 10 lymphadenectomy in 108 consecutive patients with stage cT2-T3 upper gastric cancer. Our data showed that the average time needed for No. 10 LN dissection was approximately 20 min, with less bleeding and shorter postoperative hospital stays, suggesting that laparoscopic spleen-preserving No. 10 lymphadenectomy is technically feasible.
Previous studies reported that the intraoperative complication rate of laparoscopic gastric surgery was 2.6%-4.4%[23,24]. Consistent with these findings, we observed intraoperative complications in four of 108 patients (3.7%). None of our patients required conversion to laparotomy, and no patient required splenectomy because of injury to the spleen or splenic blood vessels. Postoperative complications were reported in 8.7%-25.0% of patients who underwent open spleen-preserving No. 10 lymphadenectomy for upper gastric cancer[2,9,11,12], and a recent study reported postoperative complications in two of 15 (13.3%) patients with upper gastric cancer who underwent laparoscopic spleen-preserving No. 10 lymphadenectomy[17]. In the current study, we found that 13 of 108 patients (12.0%) experienced postoperative complications, but no patient died within 30-d of follow-up, suggesting that laparoscopic spleen-preserving No. 10 LNs dissection is safe and does not increase postoperative morbidity and mortality rates. In our experience, the keys to successful No. 10 LN dissection are a skilled laparoscopic technique, familiarity with the minimally invasive vascular anatomy of the splenic hilum area, and a cooperative surgical team. Moreover, the laparoscope, with its unique perspective, lighting and amplification, can more clearly visualise the splenic vasculature, nerves, fascia and other structures, thereby reducing damage to the splenic vessels and spleen, and assisting the surgeon in performing spleen-preserving No. 10 lymphadenectomy without splenic mobilisation.
The number of dissected LNs is an important assessment of the outcome of LN dissection. The average number of No. 10 LNs dissected per patient has been reported as three LNs during open radical surgery for upper gastric cancer involving splenectomy[25] and 1.7 LNs during open radical surgery with spleen-preserving No. 10 lymphadenectomy[26]. During laparoscopic spleen-preserving No. 10 lymphadenectomy, the average numbers of No. 10 LNs dissected per patient were 2.7[17] and 2.6[19], indicating that a similar number of No. 10 LNs were dissected during laparoscopic and open surgery. In the current study, the average number of No. 10 LNs dissected was 3.0, which was similar to the other reports. No. 10 LNs are prone to metastasis in AUGC[25], and the metastasis rate to the No. 10 LNs reportedly ranges from 5.1% to 20.9%[9-11,27,28]. Moreover, the No. 10 LNs metastasis rate is related to the tumour location, depth of invasion, other total LNs metastasis status and size of the primary tumour[9-11,27,28]. In the current study, we observed metastases in these LNs in only three of 108 patients (2.8%), including two patients with pT3 and one patient with pT4a tumours, all located in the greater curvature; however, there was no No. 10 LNs metastasis in patients with pT1 and pT2 tumours. Therefore, this present study suggested that routine No. 10 lymphadenectomy may be unnecessary for AUGC without serosa invasion, unless T3 tumours are located in the greater curvature.
Patient survival after radical gastrectomy is important to evaluate its efficacy. The short and long term survival rates were greater in patients undergoing open spleen-preserving No. 10 lymphadenectomy for the treatment of upper stomach cancer[4-6]. In Hyung’s study, none of the 15 patients who underwent a laparoscopic procedure died or experienced tumour recurrence after a median follow-up period of 21 mo[17]. We found that after a median follow-up time of 18 mo, none of our patients experienced recurrence or metastasis. Longer follow-up periods, however, are required to determine the long-term efficacy of this procedure.
In conclusion, laparoscopic spleen-preserving No. 10 lymphadenectomy is feasible and effective for patients with AUGC. However, routine No. 10 lymphadenectomy may be unnecessary for AUGC without serosa invasion, unless T3 tumours are located in the greater curvature. In addition, multi-centre, prospective, randomised controlled studies involving greater numbers of patients and longer follow-up times, are needed to confirm its long-term efficacy.
COMMENTS
Background
Standard D2 lymph node (LN) dissection during total gastrectomy for advanced upper gastric cancer (AUGC) requires the removal of the No. 10 LNs, according to the 3rd English edition of Japanese classification of gastric carcinoma. Total gastrectomy with spleen-preserving No. 10 lymphadenectomy is increasingly used in open surgery to treat patients with upper gastric cancer. Several studies have shown that laparoscopic spleen-preserving No. 10 lymphadenectomy is feasible for patients with upper gastric cancer; however, the sample sizes in these studies were small, and the value of the procedure must be further evaluated by studies with large sample sizes. Furthermore, it remains controversial whether routine No. 10 lymphadenectomy should be performed for AUGC without serosa invasion.
Research frontiers
Laparoscopic spleen-preserving No. 10 lymphadenectomy has been difficult to accomplish because of the possibilities of injury to splenic vessels and the parenchyma of the spleen or pancreas.
Innovations and breakthroughs
The results of the current study demonstrate that the average time needed for laparoscopic spleen-preserving No. 10 lymphadenectomy was approximately 20 min, and included less bleeding and shorter postoperative hospital stays. Moreover, the intraoperative and postoperative morbidity rates were 3.7% and 12.0%, respectively, and there was no postoperative mortality. At the same time, a mean of 3.0 ± 2.4 No. 10 LNs were retrieved per patient. Three patients (2.8%) had LN metastasis of the splenic hilus, including two patients with pT3 and one patient with pT4a tumours, all located in the greater curvature. At a median follow-up of 18 mo (range, 12 to 23 mo), no patient died or experienced tumour recurrence or metastasis during the follow-up period.
Applications
The study results suggest that laparoscopic spleen-preserving No. 10 lymphadenectomy is feasible and effective for AUGC. Routine No. 10 lymphadenectomy may be unnecessary for AUGC without serosa invasion, unless T3 tumours are located in the greater curvature. The results should encourage more surgeons to perform laparoscopic total gastrectomy with No. 10 lymphadenectomy and will aid the acceptance of this procedure as a surgical option for AUGC patients.
Terminology
For spleen-preserving No. 10 lymphadenectomy, surgeons do not need to remove the spleen during the No. 10 lymphadenectomy when performing total gastrectomy with D2 LN dissection. Body mass index was used as an objective index to indicate massive obesity. The cut-off value was chosen according to the World Health Organisation guidelines for the Western Pacific region.
Peer review
This is a good work in which the authors evaluate the feasibility and short-term efficacy of laparoscopic spleen-preserving No. 10 lymphadenectomy for AUGC. Congratulations to the authors for the excellence of their work. All the contents in this study are appropriately presented. This manuscript is well written and documented. Additionally, this manuscript adds new knowledge to the literature.
Footnotes
Supported by the National Key Clinical Specialty Discipline Construction Program of China, No. [2012]649
P- Reviewer: Abd Ellatif ME, Coskun A, Ferreira Caboclo JL S- Editor: Ma N L- Editor: Stewart G E- Editor: Zhang DN
References
- 1.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 2.Schwarz RE. Spleen-preserving splenic hilar lymphadenectomy at the time of gastrectomy for cancer: technical feasibility and early results. J Surg Oncol. 2002;79:73–76. doi: 10.1002/jso.10036. [DOI] [PubMed] [Google Scholar]
- 3.Weitz J, Jaques DP, Brennan M, Karpeh M. Association of splenectomy with postoperative complications in patients with proximal gastric and gastroesophageal junction cancer. Ann Surg Oncol. 2004;11:682–689. doi: 10.1245/ASO.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 4.Hartgrink HH, van de Velde CJ, Putter H, Bonenkamp JJ, Klein Kranenbarg E, Songun I, Welvaart K, van Krieken JH, Meijer S, Plukker JT, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol. 2004;22:2069–2077. doi: 10.1200/JCO.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Bonenkamp JJ, Songun I, Hermans J, Sasako M, Welvaart K, Plukker JT, van Elk P, Obertop H, Gouma DJ, Taat CW. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet. 1995;345:745–748. doi: 10.1016/s0140-6736(95)90637-1. [DOI] [PubMed] [Google Scholar]
- 6.Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–449. doi: 10.1016/S1470-2045(10)70070-X. [DOI] [PubMed] [Google Scholar]
- 7.Csendes A, Burdiles P, Rojas J, Braghetto I, Diaz JC, Maluenda F. A prospective randomized study comparing D2 total gastrectomy versus D2 total gastrectomy plus splenectomy in 187 patients with gastric carcinoma. Surgery. 2002;131:401–407. doi: 10.1067/msy.2002.121891. [DOI] [PubMed] [Google Scholar]
- 8.Lee KY, Noh SH, Hyung WJ, Lee JH, Lah KH, Choi SH, Min JS. Impact of splenectomy for lymph node dissection on long-term surgical outcome in gastric cancer. Ann Surg Oncol. 2001;8:402–406. doi: 10.1007/s10434-001-0402-0. [DOI] [PubMed] [Google Scholar]
- 9.Yu W, Choi GS, Chung HY. Randomized clinical trial of splenectomy versus splenic preservation in patients with proximal gastric cancer. Br J Surg. 2006;93:559–563. doi: 10.1002/bjs.5353. [DOI] [PubMed] [Google Scholar]
- 10.Aoyagi K, Kouhuji K, Miyagi M, Imaizumi T, Kizaki J, Shirouzu K. Prognosis of metastatic splenic hilum lymph node in patients with gastric cancer after total gastrectomy and splenectomy. World J Hepatol. 2010;2:81–86. doi: 10.4254/wjh.v2.i2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nashimoto A, Yabusaki H, Matsuki A. The significance of splenectomy for advanced proximal gastric cancer. Int J Surg Oncol. 2012;2012:301530. doi: 10.1155/2012/301530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh SJ, Hyung WJ, Li C, Song J, Kang W, Rha SY, Chung HC, Choi SH, Noh SH. The effect of spleen-preserving lymphadenectomy on surgical outcomes of locally advanced proximal gastric cancer. J Surg Oncol. 2009;99:275–280. doi: 10.1002/jso.21229. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Ahn SH, Park do J, Kim HH, Lee HJ, Yang HK. Laparoscopic total gastrectomy with D2 lymphadenectomy for advanced gastric cancer. World J Surg. 2012;36:2394–2399. doi: 10.1007/s00268-012-1669-y. [DOI] [PubMed] [Google Scholar]
- 14.Shinohara T, Satoh S, Kanaya S, Ishida Y, Taniguchi K, Isogaki J, Inaba K, Yanaga K, Uyama I. Laparoscopic versus open D2 gastrectomy for advanced gastric cancer: a retrospective cohort study. Surg Endosc. 2013;27:286–294. doi: 10.1007/s00464-012-2442-x. [DOI] [PubMed] [Google Scholar]
- 15.Hottenrott C. The long-term efficacy of laparoscopic surgery in early and advanced gastric cancer. Surg Endosc. 2012;26:3695–3696. doi: 10.1007/s00464-012-2275-7. [DOI] [PubMed] [Google Scholar]
- 16.Scatizzi M, Kröning KC, Lenzi E, Moraldi L, Cantafio S, Feroci F. Laparoscopic versus open distal gastrectomy for locally advanced gastric cancer: a case-control study. Updates Surg. 2011;63:17–23. doi: 10.1007/s13304-011-0043-1. [DOI] [PubMed] [Google Scholar]
- 17.Hyung WJ, Lim JS, Song J, Choi SH, Noh SH. Laparoscopic spleen-preserving splenic hilar lymph node dissection during total gastrectomy for gastric cancer. J Am Coll Surg. 2008;207:e6–11. doi: 10.1016/j.jamcollsurg.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 18.Hur H, Jeon HM, Kim W. Laparoscopic pancreas- and spleen-preserving D2 lymph node dissection in advanced (cT2) upper-third gastric cancer. J Surg Oncol. 2008;97:169–172. doi: 10.1002/jso.20927. [DOI] [PubMed] [Google Scholar]
- 19.Okabe H, Obama K, Kan T, Tanaka E, Itami A, Sakai Y. Medial approach for laparoscopic total gastrectomy with splenic lymph node dissection. J Am Coll Surg. 2010;211:e1–e6. doi: 10.1016/j.jamcollsurg.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Jia-Bin W, Chang-Ming H, Chao-Hui Z, Ping L, Jian-Wei X, Jian-Xian L. Laparoscopic spleen-preserving No. 10 lymph node dissection for advanced proximal gastric cancer in left approach: a new operation procedure. World J Surg Oncol. 2012;10:241. doi: 10.1186/1477-7819-10-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu J, Huang CM, Zheng CH, Li P, Xie JW, Wang JB, Lin JX. Learning curve of laparoscopy spleen-preserving splenic hilar lymph node dissection for advanced upper gastric cancer. Hepatogastroenterology. 2013;60:296–300. doi: 10.5754/hge12641. [DOI] [PubMed] [Google Scholar]
- 22.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 23.Azagra JS, Ibañez-Aguirre JF, Goergen M, Ceuterick M, Bordas-Rivas JM, Almendral-López ML, Moreno-Elola A, Takieddine M, Guérin E. Long-term results of laparoscopic extended surgery in advanced gastric cancer: a series of 101 patients. Hepatogastroenterology. 2006;53:304–308. [PubMed] [Google Scholar]
- 24.Ryu KW, Kim YW, Lee JH, Nam BH, Kook MC, Choi IJ, Bae JM. Surgical complications and the risk factors of laparoscopy-assisted distal gastrectomy in early gastric cancer. Ann Surg Oncol. 2008;15:1625–1631. doi: 10.1245/s10434-008-9845-x. [DOI] [PubMed] [Google Scholar]
- 25.Mönig SP, Collet PH, Baldus SE, Schmackpfeffer K, Schröder W, Thiele J, Dienes HP, Hölscher AH. Splenectomy in proximal gastric cancer: frequency of lymph node metastasis to the splenic hilus. J Surg Oncol. 2001;76:89–92. doi: 10.1002/1096-9098(200102)76:2<89::aid-jso1016>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 26.Fatouros M, Roukos DH, Lorenz M, Arampatzis I, Hottentrott C, Encke A, Kappas AM. Impact of spleen preservation in patients with gastric cancer. Anticancer Res. 2005;25:3023–3030. [PubMed] [Google Scholar]
- 27.Ikeguchi M, Kaibara N. Lymph node metastasis at the splenic hilum in proximal gastric cancer. Am Surg. 2004;70:645–648. [PubMed] [Google Scholar]
- 28.Kunisaki C, Makino H, Suwa H, Sato T, Oshima T, Nagano Y, Fujii S, Akiyama H, Nomura M, Otsuka Y, et al. Impact of splenectomy in patients with gastric adenocarcinoma of the cardia. J Gastrointest Surg. 2007;11:1039–1044. doi: 10.1007/s11605-007-0186-z. [DOI] [PubMed] [Google Scholar]


