Abstract
AIM: To demonstrate that administering heparanase inhibitor PI-88 at 160 mg/d is safe and promising in reducing hepatocellular carcinoma (HCC) recurrence for up to 3 year following curative resection.
METHODS: A total of 143 patients (83.1% of the 172 participants in the phase II study) participated in the follow-up study. Of these patients, 50 had received no treatment, 48 had received 160 mg/d PI-88, and 45 had received 250 mg/d PI-88 during the phase II trial. Safety parameters and the following efficacy endpoints were investigated: (1) time to recurrence; (2) disease-free survival; and (3) overall survival.
RESULTS: PI-88 at 160 mg/d delayed the onset and frequency of HCC recurrence, and provided a clinically significant survival advantage for up to 3 years after treatment compared with those of the control group: (1) the recurrence-free rate increased from 50% to 63%, and (2) time to recurrence at the 36th percentile was postponed by 78%. The efficacy of administering PI-88 at 250 mg/d was confounded by a high dropout rate (11 out of 54 patients). Additionally, subgroup analyses of patients with (1) multiple tumors or a single tumor ≥ 2 cm; and (2) hepatitis B or C revealed that administering PI-88 at 160 mg/d conferred the most significant survival advantage (56.8% improvement in disease-free survival, P = 0.045) for patients with both risk factors for recurrence.
CONCLUSION: Administering PI-88 at 160 mg/d is a safe and well-tolerated dosage that may confer significant clinical benefits for patients with HCC.
Keywords: Antiangiogenesis, Antimetastasis, Adjuvant therapy, Disease-free survival, Heparanase inhibitor, Hepatocellular carcinoma, PI-88, Tumor recurrence
Core tip: A phase II clinical trial demonstrated that heparanase inhibitor PI-88 at 160 mg/d is safe and promising in reducing hepatocellular carcinoma (HCC) recurrence for up to one year following curative resection. This observational follow-up study extended the follow-up period to 3 years. A total of 143 patients participated in the study. PI-88 at 160 mg/d delayed the onset and frequency of HCC recurrence, and provided a clinically significant survival advantage for up to 3 years after treatment. Subgroup analyses revealed that administering PI-88 at 160 mg/d conferred the most significant survival advantage for patients at high risk of recurrence.
INTRODUCTION
Hepatocellular carcinoma (HCC) is currently the fifth most common cancer and the third leading cause of cancer related deaths worldwide[1]. Traditionally, HCC has been more prevalent in Asia because of the prevalence of hepatitis B virus (HBV) infection. However, the incidence of HCC in the United States and Europe has risen in recent years because of increases in the number of hepatitis C virus (HCV) infections, consequently generating more interest in HCC research and treatment worldwide[2].
Surgical resection is a potentially curative therapy used to treat early-stage HCC; however, 50% to 80% of resection patients experience recurrence within 5 years[3,4]. Although numerous treatments, including oral and regional chemotherapy, interferon α and β, preoperative chemoembolization, and adoptive immunotherapy, have been investigated to reduce HCC recurrence, inconsistent and inconclusive results have prevented the adoption of these treatments in clinical practice[5-7]. Hence, there remains a dire clinical need for an adjuvant therapy to reduce the risk of postresection HCC recurrence[4,7,8].
There are 2 main types of postresection HCC recurrence. Intrahepatic metastatic recurrences develop from undetectable HCC dissemination prior to resection. De novo recurrences develop multicentrically and metachronously in the background liver, usually in patients with cirrhosis or chronic hepatitis[4,8]. Intrahepatic metastatic recurrence typically occurs within 2 years following resection, and de novo recurrence typically occurs 2 years following resection[6]. Although researchers who have conducted relevant molecular studies can differentiate between these types of recurrences to determine appropriate treatment strategies, these strategies are not widely used in clinical settings[4,8]. Currently, for convenience, recurrence in clinical settings is categorized as early or late, occurring within or after 2 years postresection, to approximate the likely mode of recurrence. Ideally, adjuvant therapies used to decrease postresection recurrence can inhibit both types of recurrence[4].
PI-88, a heparanase inhibitor, reduces HCC recurrence through 3 mechanisms. By inhibiting heparin sulfate (HS) degradation, PI-88 (1) preserves the integrity of the extracellular matrix (ECM) and (2) suppresses the release of angiogenic and fibroblastic growth factors (GFs) from the ECM. Moreover, the strong affinity of PI-88 to GFs enables PI-88 to (3) aggregate released GFs and block their activity. The antiangiogenic property of PI-88 stems from its ability to antagonize GF reception, and thereby restrict the necessary blood supply for both intrahepatic metastatic and de novo tumor proliferation. The antimetastatic property of PI-88 may stem from its ability to preserve ECM integrity, and thereby decrease basement invasion to further suppress intrahepatic metastatic recurrences[9-12]. Considering its ability to perform these dual functions, PI-88 can potentially suppress both types of HCC recurrences.
To investigate PI-88 as an adjuvant therapy for HCC recurrence, a randomized, multicenter Simon’s 2-stage design phase II trial was previously conducted to determine its safety, optimal dosage, and preliminary efficacy. The study results indicated that administering 160 mg/d for 36 wk postresection was a safe and optimal dosage that increased recurrence-free survival at 48 wk[13]. This observational follow-up study to the phase II trial was conducted to determine whether these effects lasted longer than 48 wk and improved overall survival.
In the previous phase II study, the primary endpoint was the recurrence-free survival rate; in this follow-up study, its more conventional equivalent, disease-free survival (DFS), was assessed. The efficacy endpoints analyzed in this follow-up study included (1) time-to-recurrence (TTR); (2) DFS; and (3) overall survival (OS). DFS and OS were assessed because both are reliable, clinically relevant endpoints[8]. This follow-up study also included subgroup analyses and further investigation to prepare for the design of a double-blind, randomized phase III confirmatory study.
MATERIALS AND METHODS
Summary of phase II study methods
From June 2004 to December 2006, a randomized phase II trial designed according to Simon’s 2-stage design was conducted in 6 medical centers in Taiwan, to determine the safety and optimal dosage of PI-88 in the adjuvant setting[14]. In total, 215 patients were screened, and 172 patients were randomized into 3 groups: 58 patients received no treatment, 57 patients received 160 mg/d PI-88, and 57 patients received 250 mg/d PI-88. PI-88 was provided in lyophilized powder form and produced by Progen Pharmaceuticals Limited (Brisbane, Queensland, Australia). As indicated in Figure 1, patients in Groups B and C received 9 cycles of the respective PI-88 treatments. Each cycle lasted 4 wk: weeks 1 through 3 each consisted of 4 consecutive days of treatment followed by 3 d without treatment; week 4 was a no-treatment week. After 9 treatment cycles (36 wk), no treatment was administered in the subsequent 12 wk before the final assessment in week 48. At the end of the study, data for assessing the safety and efficacy endpoints, including the tumor recurrence-free rate, TTR, and 1-year survival rate, were collected[13].
Figure 1.
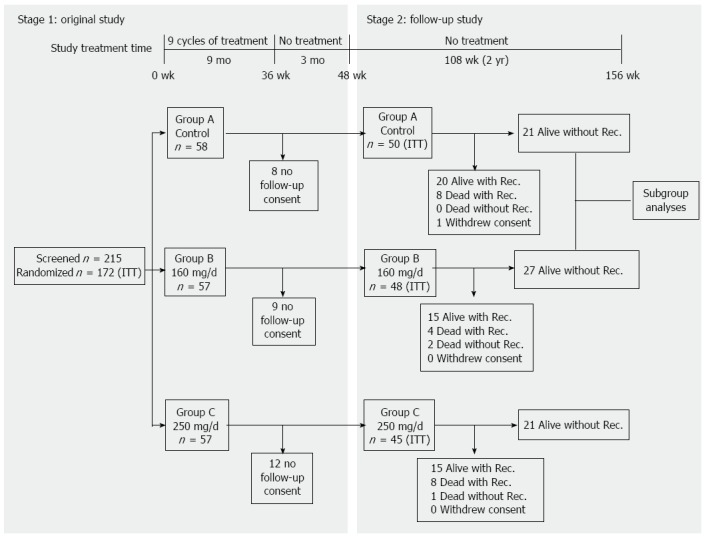
Graphic representation of phase II and follow-up study design, timeline and cohort relationships. ITT: Intent-to-treat; Rec.: Recovery.
Follow-up study design
During the observational follow-up study, recurrence and survival data were collected for 2 years to examine the long-term efficacy of PI-88. All of the participants in the phase II study were invited to participate in the follow-up study. In the follow-up study, the period between week 48, when the first patient in the phase II trial completed his or her last visit during that week, and week 156, when the last patient completed his or her last 2-year follow-up visit, in January 2009 was investigated (Figure 1).
In both the clinical trial and the follow-up study, all of the patients received follow-up care according to Taiwanese standard-of-care guidelines: vital signs were checked, alfa fetoprotein and liver enzyme tests were conducted, and abdominal ultrasonograph and abdominal computed tomography (CT) scans were taken at outpatient clinics every 3 mo. CT scans and ultrasonographs were alternately performed every 1.5 mo. Additional CT scans were performed when HCC recurrence was suspected. In patients with HCC recurrence, treatment strategy was determined by the responsible investigator, basing on practice guidelines of individual institute. Treatment-related adverse events that occurred in the phase II trial were also monitored throughout the follow-up study.
Ethical considerations
The follow-up protocol was approved by the institutional review board at each center and conformed to the ethical guidelines of the Declaration of Helsinki and local laws. Written informed consent was provided by the patient directly or by family members of patients who had passed away prior to the start of the follow-up study.
Patient demographics and baseline condition
As shown in Figure 1, 143 patients, or 83.1% of those in the phase II study, participated in the follow-up study. Of these patients, 50 patients received no treatment (Group A), 48 patients received 160 mg/d PI-88 (Group B), and 45 patients received 250 mg/d PI-88 (Group C). A logistic regression model was used to explore disparities in the baseline characteristics (1) among Groups A, B, and C in the follow-up study; and (2) between the phase II and follow-up study cohorts. The investigated baseline factors included tumor features, Cancer of the Liver Italian Program score, Eastern Cooperative Oncology Group performance score, Child-Pugh status, viral hepatitis activity, and vascular invasion (Table 1).
Table 1.
Intent-to-treat patient demographics and baseline characteristic in the follow-up study n (%)
| Group A untreated (n = 50)1 | Group B 160 mg/d (n = 48) | Group C 250 mg/d (n = 45) | Overall (n = 143) | P-value2 | |
| Age (yr) | |||||
| mean ± SD | 55.9 ± 12.2 | 52.3 ± 12.6 | 54.3 ± 11.9 | 54.2 ± 12.2 | 0.3565 |
| Age group (yr) | |||||
| Age < 65 | 36 (72.0) | 38 (79.2) | 37 (82.2) | 111 (77.6) | 0.4667 |
| Age ≥ 65 | 14 (28.0) | 10 (20.8) | 8 (17.8) | 32 (22.4) | |
| Sex | |||||
| Female | 13 (26.0) | 10 (20.8) | 9 (20.0) | 32 (22.4) | 0.7445 |
| Male | 37 (74.0) | 38 (79.2) | 36 (80.0) | 111 (77.6) | |
| Alcohol use | |||||
| Never or rarely | 43 (86) | 35 (72.9) | 36 (80.0) | 114 (79.7) | 0.7547 |
| Monthly | 1 (2.0) | 1 (2.1) | 1 (2.2) | 3 (2.1) | |
| Weekly | 2 (4.0) | 6 (12.5) | 3 (6.7) | 11 (7.7) | |
| Daily | 4 (8.0) | 6 (12.5) | 5 (11.1) | 15 (10.5) | |
| Clip stage | |||||
| 0 | 30 (60.0) | 25 (52.1) | 26 (57.8) | 81 (56.6) | 0.6169 |
| 1 | 13 (26.0) | 12 (25.0) | 10 (22.2) | 35 (24.5) | |
| 2 | 4 (8.0) | 6 (12.5) | 7 (15.6) | 17 (11.9) | |
| 3 | 1 (2.0) | 5 (10.4) | 2 (4.4) | 8 (5.6) | |
| 4 | 2 (4.0) | 0 | 0 | 2 (1.4) | |
| Ecog performance status score | |||||
| 0 | 42 (84.0) | 40 (83.3) | 40 (88.9) | 122 (85.3) | 0.6496 |
| 1 | 7 (14.0) | 8 (16.7) | 5 (11.1) | 20 (14.0) | |
| 2 | 1 (2.0) | 0 | 0 | 1 (0.7) | |
| Child-Pugh score | |||||
| 5/6 | 48 (96.0) | 46 (95.8) | 43 (95.6) | 137 (95.8) | 0.6702 |
| 7 | 1 (2.0) | 2 (4.2) | 2 (4.4) | 5 (3.5) | |
| 8 | 1 (2.0) | 0 | 0 | 1 (0.7) | |
| New York Heart Association classification of functional capacity class activity | |||||
| Class I | 48 (96.0) | 47 (97.9) | 44 (97.8) | 139 (97.2) | 0.8144 |
| Class II | 2 (4.0) | 1 (2.1) | 1 (2.2) | 4 (2.8) | |
| Differentiation of tumor | |||||
| Well differentiated | 2 (4.0) | 7 (14.6) | 4 (8.9) | 13 (9.1) | 0.2487 |
| Moderately differentiated | 34 (68.0) | 23 (47.9) | 26 (57.8) | 83 (58.0) | |
| Poorly differentiated or anaplasia | 14 (28.0) | 18 (37.5) | 15 (33.3) | 47 (32.9) | |
| Liver cirrhosis | |||||
| Absence | 19 (38.0) | 20 (41.7) | 12 (26.7) | 51 (35.7) | 0.6023 |
| Presence | 28 (56.0) | 24 (50.0) | 29 (64.4) | 81 (56.6) | |
| Not assessed | 3 (6.0) | 4 (8.3) | 4 (8.9) | 11 (7.7) | |
| Hepatitis activity | |||||
| Absence | 7 (14.0) | 4 (8.3) | 5 (11.1) | 16 (11.2) | 0.8234 |
| Presence | 34 (68.0) | 35 (72.9) | 29 (64.4) | 98 (68.5) | |
| Not assessed | 9 (18.0) | 9 (18.8) | 11 (24.4) | 29 (20.3) | |
| Vein invasion (microscopic) | |||||
| Absence | 42 (84.0) | 36 (75.0) | 36 (80.0) | 114 (79.7) | 0.7375 |
| Presence | 8 (16.0) | 11 (22.9) | 8 (17.8) | 27 (18.9) | |
| Not assessed | 0 (0) | 1 (2.1) | 1 (2.2) | 2 (1.4) | |
| Macro vascular invasion | |||||
| Absence | 47 (94.0) | 42 (87.5) | 42 (93.3) | 131 (91.6) | 0.4493 |
| Presence | 3 (6.0) | 6 (12.5) | 3 (6.7) | 12 (8.4) | |
Includes 1 patient who withdrew consent during follow-up study;
P-value on Age is by using analyses of variance, on CLIP stage by using Cochran-Mantel-Haenszel modified ridit scores for mean scores difference, on others by using χ2 test. Differences in patient demographics and baseline characteristics were not statistically significant.
Statistical analysis
Of the participants in the phase II trial, 24 patients did not participate in the follow-up study. These individuals are not represented in the follow-up study data; they were counted at the end of the phase II study (week 48) to achieve conservative endpoint estimations for all of the 3-year study data.
Efficacy endpoints of interest in this study included (1) TTR; (2) DFS; and (3) OS. TTR, DFS, and OS were respectively defined as the time until each of the following events occurred: recurrence only, recurrence or death (unrelated to HCC recurrence), and death only; patients who withdrew consent were included until their drop-out times. The DFS event time for patients whose deaths were recurrence-related was defined as the time of recurrence.
The Kaplan-Meier estimator was used to determine TTR, DFS, and OS probabilities. TTR Hazard ratios were calculated using a Log rank test [logS(t, treated)/logS(t, untreated)]. Fisher’s exact test was used to determine the statistical significance of the differences in the TTR, DFS, and OS probabilities among the 3 groups.
Subgroup analyses
Subgroup analyses were conducted to determine the efficacy of PI-88 administered at 160 mg/d in reducing HCC recurrence in patients with tumors and host factors that influence recurrence. Patients with multiple tumors, or a single tumor ≥ 2 cm, were included in the intermediate-risk subgroup. Patients with both (1) multiple tumors or a single tumor ≥ 2 cm; and (2) chronic hepatitis B or C infection were included in the high-risk subgroup[5]. DFS, the rate of DFS improvement, and statistical significance were calculated for both of the subgroups with respect to the controls.
To investigate the effects of patient population heterogeneity as suggested by Forner and Roayaie[15], statistical analyses comparing the recurrence trends between cohorts, including and excluding Child-Pugh class B patients, were conducted.
RESULTS
Summary of phase II study results
In addition to being safe and well-tolerated throughout the 9 treatment cycles, PI-88 administered at 160 mg/d demonstrated the following efficacy improvements compared with those of the control group: (1) the recurrence-free rate increased from 50% to 63% and (2) TTR at the 36th percentile was postponed by 78%. The efficacy of administering PI-88 at 250 mg/d was confounded by a high dropout rate (11 out of 54 patients); the higher dosage was not determined to confer additional clinical advantages. Overall, the results supported the possible efficacy of administering PI-88 at 160 mg/d in decreasing and delaying recurrence, and prolonging disease-free survival in the short term[13,15].
Patient demographics
Statistical analyses revealed that the patient baseline demographics and tumor statuses among the 3 groups in the follow-up cohort, and between the respective follow-up and phase II study groups, were not significant.
Treatment of recurrent HCC
Overall, during the 156 wk follow-up period, 61 (36.3%) of the 168 patients received treatment for recurrent HCC. The details of treatment strategies among different groups of patients are shown in Table 2.
Table 2.
Other treatment or medication for recurrent hepatocellular carcinoma during the 156 wk follow-up period n (%)
| Anti-HCC therapy | Group A untreated (n = 58) | Group B 160 mg/d (n = 56) | Group C 250 mg/d (n = 54) | Total (n = 168) |
| At least one shown below | 22 (37.9) | 17 (30.4) | 22 (40.7) | 61 (36.3) |
| Chemotherapy | 3 (5.2) | 4 (7.1) | 4 (7.4) | 11 (6.5) |
| Percutaneous ethanol injection therapy | 2 (3.4) | 3 (5.4) | 3 (5.6) | 8 (4.8) |
| Radiofrequency ablation | 2 (3.4) | 3 (5.4) | 3 (5.6) | 8 (4.8) |
| Radiotherapy | 3 (5.2) | 1 (1.8) | 1 (1.9) | 5 (3.0) |
| Surgical resection | 5 (8.6) | 5 (8.9) | 7 (13.0) | 17 (10.1) |
| Transcatheter arterial chemoembolization | 18 (31.0) | 11 (19.6) | 15 (27.8) | 44 (26.2) |
| Thalidomide | 2 (3.4) | 1 (1.8) | 1 (1.9) | 4 (2.4) |
| Liver transplantation | 0 (0.0) | 1 (1.8) | 1 (1.9) | 2 (1.2) |
| Sorafenib | 1 (1.7) | 1 (1.8) | 1 (1.9) | 3 (1.8) |
| New clinical trial | 3 (5.1) | 1 (1.8) | 6 (11.3) | 10 (6.0) |
HCC: Hepatocellular carcinoma.
Safety profiles
As shown in Table 3, only thrombocytopenia and elevated serum aminotransferase levels persisted from the phase II study to the follow-up study. Common treatment-related adverse effects (AEs), such as neutropenia, injection site pain and hemorrhage, and the prolongation of activated partial thromboplastin time, were not observed in the follow-up study up to week 156[13]. Higher incidences of elevated serum aminotransferase levels in the 250 mg/d group were detected before week 60 (3 mo of follow-up). However, in the absence of antiHBV or anti-HCV treatment, most liver enzyme abnormalities returned to normal by the end of the first year of the follow-up study (week 102).
Table 3.
Adverse events (with > 5% incidence) possibly related to treatment observed at the end of the phase II study and in the follow-up study n (%)
| Timeline | Week-48 | Week-60 | Week-102 | Week-156 |
| End of phase II study | 3 mo into follow-up study | 1 yr into follow-up study | End of follow-up study | |
| MedDRA system | ||||
| Blood and lymphatic system disorders: Thrombocytopenia | ||||
| Group B: 160 mg/d | 2 (4.2) | 2 (4.2) | 2 (4.2) | 0 (0.0) |
| Group C: 250 mg/d | 3 (6.7) | 3 (6.7) | 3 (6.7) | 3 (6.7) |
| P-value1 | 0.671 | 0.671 | 0.671 | 0.109 |
| Investigations; elevated ALT/elevated AST | ||||
| Group B: 160 mg/d | 2 (4.2)/3 (6.3) | 2 (4.2)/3 (6.3) | 2 (4.2)/1 (2.1) | 1 (2.1)/0 (0.0) |
| Group C: 250 mg/d | 7 (15.6)/7 (15.6) | 7 (15.6)/7 (15.6) | 2 (4.4)/1 (2.2) | 1 (2.2)/1 (2.2) |
| P-value1 | 0.0843/0.1893 | 0.0843/0.1893 | 1.0000/1.0000 | 1.0000/0.4839 |
Fisher’s exact test. Treatment-related adverse events that were observed only in the phase II study are excluded. All adverse events in the 160 mg/d treatment group reverted to baseline levels by the end of the follow-up study. Adverse events were more frequently observed in the 250 mg/d treatment group. ALT: Alanine aminotransferase; AST: Aspartate aminotransferase.
Compliance: We defined compliance in those subjects who received ≥ 80% of the required doses (12 doses/cycle × 9 cycles × 80%). Rate of compliance in Groups B and C is shown in Table 4, categorized by drop-out status. In general, compliance was lower in Group C than that in Group B. For those subjects remaining in the phase II study, the rate of compliance was also lower in Group C compared with Group B although not statistically significant.
Table 4.
Rate of compliance1 categoried by drop-out status n (%)
| Drop-out status | Group B 160 mg/d (n = 56) | Group C 250 mg/d (n = 54) | P-value2 |
| Drop-outs without recurrence | 5 | 11 | 0.214 |
| < 80% compliance | 3 (60.0) | 10 (90.9) | |
| ≥ 80% compliance | 2 (40.0) | 1 (9.1) | |
| Non-withdrawal subjects | 51 | 43 | 0.171 |
| < 80% compliance | 11 (21.6) | 15 (34.9) | |
| ≥ 80% compliance | 40 (78.4) | 28 (65.1) |
1≥ 80% compliance denotes received ≥ 80% of required doses (12 doses/cycle × 9 cycles × 80);
Fisher’s exact test.
Efficacy endpoint analyses
TTR: TTR at the 48th percentile for Groups A, B, and C occurred in weeks 95.1, 143.9, and 94.4, respectively (Figure 2A). Compared with Group A, the TTR of Group B increased by 51.3%, which represents a hazard ratio of 0.688. Although this hazard ratio was not statistically significant, the TTR curve difference between Groups A and B was more pronounced than between Groups A and C throughout the course of the 3-year study. Table 3 also shows that Group B demonstrated substantial, although not statistically significant, rates of TTR improvement compared with those of Group A at weeks 48 (35.1%) and 156 (21.8%).
Figure 2.
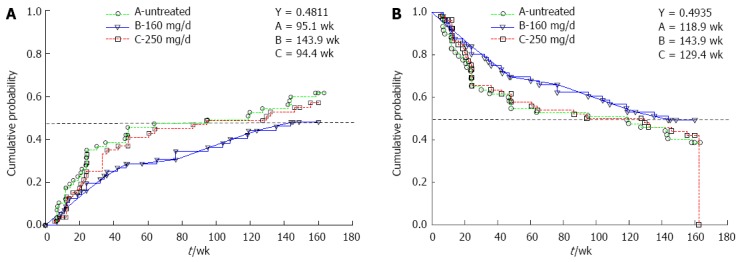
3-year probability for Groups A, B and C. A: Time-to-recurrence; B: Disease-free survival.
DFS: As shown in Figure 2B, the DFS trends for Group B were more distinct compared with those of Groups A and C throughout the 3-year study. Although the DFS probabilities at the end of the 3-year study of both Groups A (38.5%) and B (49.4%) were lower than the respective probabilities at week 48 (54.1% and 68.4%), the magnitude of the rate of DFS improvement observed at week 48 (26.4%) was maintained up to the end of the follow-up study (28.1%).
OS: As shown in Table 5, the OS probabilities of Groups A and B were comparable at the end of the phase II and follow-up studies. Although the OS of Group A was higher than that of Group B at week 48, this trend was reversed at week 156.
Table 5.
Summary of time-to-recurrence, disease-free survival probability, and overall survival results from the follow-up study n (%)
| Probability | Phase II study | 3-yr study | ||
|
Week-48 |
Week-156 |
|||
| Group A untreated | Group B 160 mg/d | Group A untreated | Group B 160 mg/d | |
| TTR probability1 | 45.9% | 29.8% | 61.5% | 48.1% |
| Difference | -16.1% | -13.4% | ||
| 95%CI | -33.6-1.5 | -31.5-4.7 | ||
| Rate of improvement2 | 35.1% | 21.8% | ||
| P value3 | 0.086 | 0.187 | ||
| DFS probability4 | 54.1% | 68.4% | 38.5% | 49.4% |
| Difference | 14.3% | 10.8% | ||
| 95%CI | -3.4-32.0 | -7.3-29.0 | ||
| Rate of improvement | 26.4% | 28.1% | ||
| P value | 0.129 | 0.257 | ||
| OS probability | 90.9% | 88.6% | 81.0% | 82.8% |
| Difference | -2.3% | 1.7% | ||
| 95%CI | -13.4-8.8 | -12.4-15.9 | ||
| Rate of improvement | -2.5% | 2.2% | ||
| P value | 0.760 | 1.000 | ||
1,4Values from Figure 2 respectively;
Percent change in endpoint probability of treated group from untreated control;
Fisher’s exact test. Although not statistically significant, time-to-recurrence (TTR) and disease-free survival (DFS) probabilities and rates of improvements in the 160 mg/d group indicate substantial clinical advantages. Similarities in the two rates of DFS improvement indicate that the clinical benefits of 36 wk of treatment with 160 mg/d PI-88 persisted for up to 3 years. Despite the clinical survival benefits indicated by DFS, overall survival (OS) benefits were inconclusive.
Subgroup analyses
Table 6 illustrates the effects of PI-88 on patients with tumors and host factors that influence recurrence. Two trends were observed: First, a correlation between DFS improvement and the number of risk factors promoting recurrence was determined. Numerically, this was demonstrated by the increase in DFS improvement in the untreated subgroups from 26.4% to 56.8% at week 48 and from 28.1% to 56.8% at week 156. Similarly, the most significant DFS improvement rate was observed in the high-risk subgroup. Second, the phase II cohort exhibited a slightly higher rate of DFS improvement compared with that of the respective 3-year cohorts. Overall, only the high-risk cohort achieved a statistically significant DFS improvement (56.8%) at the end of the phase II study (P = 0.045).
Table 6.
Subgroup analyses comparing disease-free survival probabilities of the 160 mg/d group to their respective controls in the phase II and follow-up studies
| Subgroup analyses | Phase II study | 3-yr study | ||
|
Week 48 |
Week 156 |
|||
| Group A untreated | Group B 160 mg/d | Group A untreated | Group B 160 mg/d | |
| Study cohort | ||||
| DFS probability | 54.1% | 68.4% | 38.5% | 49.4% |
| Difference | 14.3% | 10.8% | ||
| 95%CI | -3.4-32.0 | -7.3-29.0 | ||
| Rate of improvement1 | 26.4% | 28.1% | ||
| P value2 | 0.129 | 0.257 | ||
| Intermediate-risk group (multiple or single tumor ≥ 2 cm) | ||||
| DFS probability | 45.1% | 63.8% | 33.0% | 48.4% |
| Difference | 18.7% | 15.4% | ||
| 95%CI | -0.8-38.2 | -3.9-34.7 | ||
| Rate of improvement | 41.5% | 46.6% | ||
| P value | 0.104 | 0.150 | ||
| High-risk group (multiple or single tumor ≥ 2 cm and positive HBV/HCV infection)3 | ||||
| DFS probability | 41.4% | 64.9% | 29.6% | 46.4% |
| Difference | 23.5% | 16.8% | ||
| 95%CI | 2.0-45.0 | -4.4-38.1 | ||
| Rate of improvement | 56.8% | 56.8% | ||
| P value | 0.045 | 0.163 | ||
1,2 Table 3 footnote 2 and 3;
Values from Figure 3. The clinical benefits of 160 mg/d PI-88 were more pronounced in intermediate and high-risk groups. Statistically significant survival benefits were observed in the high-risk group at the end of the phase II study. TTR: Time-to-recurrence; DFS: Disease-free survival; OS: Overall survival; HBV: Hepatitis B virus; HCV: Hepatitis C virus.
The results of the Child-Pugh class A and B subgroup analysis were inconclusive because neither a clinical nor statistical difference was demonstrated (data not shown).
DISCUSSION
The results of this follow-up study corroborated the findings of the phase II study: PI-88 administered at 160 mg/d is well-tolerated and may be effective in reducing HCC recurrence. Furthermore, previously observed clinical benefits during the initial 36 wk of active treatment persisted for up to 3 years with minimal AEs. Because numerous premature withdrawals caused by treatment-related toxicities occurred, PI-88 administered at 250 mg/d (Group C) did not confer significant clinical benefits. The following discussion focuses on the 160 mg/d dosage.
Overall, these results suggest that PI-88 administered at 160 mg/d may confer clinical benefits on HCC patients whose tumors have been surgically removed with curative intent. The 36-wk 160 mg/d PI-88 treatment delayed median TTR to over 3 years, and decreased the 3-year TTR probability by 21.8%. In addition to delaying the onset and decreasing the frequency of recurrence, PI-88 treatment also conferred survival advantages; the DFS rate of the 160 mg/d cohort, 49.4%, was the highest rate observed among the cohorts and demonstrated a 28.1% improvement compared with that of the control group. Similar DFS improvements observed at the end of the phase II and follow-up studies (26.4% and 28.1%) suggest that the survival benefits of short term PI-88 treatment were maintained for 2 additional years without implementing additional treatment. The OS results, as expected in a small population study for adjuvant cancer therapy, were inconclusive. Although the clinical benefits did not reach statistical significance, active treatment was limited to only 36 wk in the original phase II study, and the prolonged use of 160 mg/d PI-88 may result in more pronounced benefits; further trials are necessary to verify this.
Patient stratification observed using subgroup analyses further supported PI-88 efficacy. Because the presence of multiple tumors or a single tumor ≥ 2 cm was correlated with high degrees of intrahepatic tumor spread and vascular invasion[5], patients with multiple tumors or a single tumor ≥ 2 cm, together with chronic HBV and HCV infection, are more susceptible to recurrence after surgery. The decreasing DFS probabilities in the untreated cohort exemplify this trend (Table 4). Moreover, the constant DFS probabilities and increasing DFS improvements in the treated intermediate-risk and high-risk groups reinforce the efficacy of PI-88 in reducing recurrence, and also suggest that its effects may be more pronounced in patients with factors that promote HCC recurrence.
The high-risk subgroup exhibited a DFS improvement of 56.8% at the end of the phase II study (P = 0.045), and this result was statistically significant. Although this rate of DFS improvement was maintained up to the end of this follow-up study, the magnitude of this improvement was not statistically significant (P = 0.163). This suggests that the change in DFS improvement is not a reflection of PI-88 treatment efficacy; instead, it is possibly a reflection of the decrease in sample size, which may have prevented clinically significant benefits from reaching statistical significance. Thus, a larger patient population is required in the phase III study to verify the statistical significance of the clinical efficacy of PI-88.
As per expert suggestion[8,15], the TTR and OS endpoints were also assessed in this follow-up study. The TTR endpoint effectively revealed the efficacy of PI-88 in delaying recurrence. Although certain regulatory agencies prefer using OS as a measure of efficacy, properly powering OS as an early-stage cancer adjuvant therapy may be difficult for multiple reasons. One reason is that other therapeutic modalities, such as alternative medicine, may confound the efficacy and survival advantages of the treatment under study. Another reason is that, because resection is potentially curative, the extended observation time may have allowed deaths unrelated to the treatment or HCC recurrence to confound the study data. Finally, compliance to PI-88 and access to other therapy or medicine for the treatment of recurrent HCC may also confound the OS. In this observation study, treatment strategy for the recurrent tumor was determined individually by the investigator. Selection bias may significantly influence the overall outcomes of HCC recurrence, which however could not be controlled. Thus, the inconsistent OS probabilities observed in this study may be attributed to these reasons or to the limited sample size.
To investigate the implications of patient heterogeneity, as suggested by Forner and Roayaie[15], subgroup analysis excluding the 4 (2 patients each from Groups A and B) borderline Child-Pugh B patients was performed. Unfortunately, because of the small sample size, the results were inconclusive (Figure 3).
Figure 3.
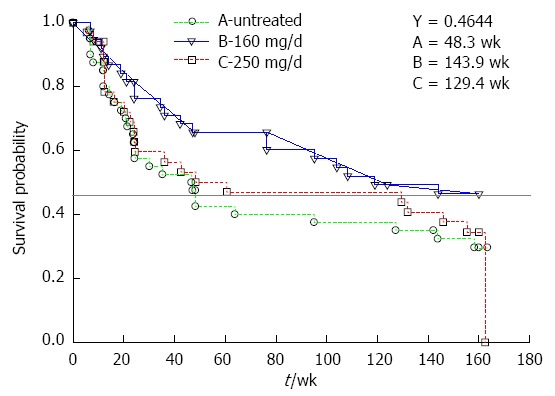
Subgroup analysis of 3-year disease-free survival probability for high-risk subgroups in Groups A, B and C.
The results of this study were consistent with the known mechanisms of PI-88. As a heparanase inhibitor, PI-88 (1) antagonizes interactions between angiogenic GFs and their receptors; (2) inhibits the release of HS-bound angiogenic and fibroblastic GFs; and (3) inhibits the heparanase degradation of ECM[9]. Collectively, these mechanisms produce the antiangiogenic and antimetastatic effects of PI-88, enabling it to simultaneously reduce intrahepatic metastatic and de novo HCC recurrences. However, because the causes of de novo recurrences are multifactorial, administering combinational therapy using agents with various mechanisms, such as antiviral or other molecular target agents, may confer even greater clinical benefits[16-18].
In addition to limitations in sample size, additional factors may have precluded the attainment of more statistically significant outcomes in this study. Because active cancer treatments are typically administered until either progression or recurrence occurs, prolonged active PI-88 treatment may have conferred even more favorable survival benefits than those observed. Moreover, because resection is potentially curative, extrapolating the length of time required to achieve statistically significant outcomes is difficult, especially because resection and death rates are declining because of technological advances[19,20].
Overall, the findings of this follow-up study are consistent with the findings of the original study, and provide insights that can be used to aid the design of a more conclusive phase III trial for the approval of adjuvant PI-88. High tumor recurrence rates and the lack of a standard of care following resection have created a dire need for adjuvant HCC agents; based on the results of the current study, PI-88 is a promising candidate for fulfilling that need.
ACKNOWLEDGMENTS
We extend our thanks to the researchers who participated in this PI-88 HCC phase II study: Lin YJ, Shan YS, and Chou TC of National Cheng Kung University Hospital, Tainan, Taiwan; and Tsai CC and Wang BW of Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan. We are indebted to James Wang and Angela Tseng for their statistical assistance. We would also like to thank our study coordinators Chen CY, Lee R, and Lai R for their contributions to this follow-up trial.
COMMENTS
Background
Hepatocellular carcinoma (HCC) recurrence is common after curative resection. There remains a dire clinical need for an adjuvant therapy to reduce the risk of postresection HCC recurrence. A phase II clinical trial demonstrated that heparanase inhibitor PI-88 at 160 mg/d is safe and promising in reducing HCC recurrence for up to one year following curative resection.
Research frontiers
Considering the ability of PI-88 to perform these dual functions (antiangiogenesis and antimetastasis), PI-88 can potentially suppress HCC recurrences. This observation study aims to investigate the longterm benefit of PI-88 as an adjuvant therapy for HCC recurrence.
Innovations and breakthroughs
This observational follow-up study extended the follow-up period to 3 years. PI-88 at 160 mg/d delayed the onset and frequency of HCC recurrence, and provided a clinically significant survival advantage for up to 3 years after treatment. Subgroup analyses revealed that administering PI-88 at 160 mg/d conferred the most significant survival advantage for patients at high risk of recurrence.
Applications
The findings of this follow-up study provide insights that can be used to aid the design of a more conclusive phase III trial for the approval of adjuvant PI-88.
Terminology
As a heparanase inhibitor, PI-88 (1) antagonizes interactions between angiogenic growth factors and their receptors, (2) inhibits the release of heparin sulfate-bound angiogenic and fibroblastic growth factors, and (3) inhibits the heparanase degradation of extracellular matrix. Collectively, these mechanisms produce the antiangiogenic and antimetastatic effects of PI-88, enabling it to simultaneously reduce intrahepatic metastatic and de novo HCC recurrences.
Peer review
The authors present an important follow-up study of outcomes following PI-88 treatment as adjuvant therapy for hepatocellular carcinoma. This study was well done and well-presented.
Footnotes
Supported by NIH Clinical Trial Registration, No. NCT00247728 (this trial was cosponsored by Progen Industries Limited, Brisbane, Australia and Medigen Biotechnology Corporation, Taipei, Taiwan) to Chen PJ, Lai KL and Chang SSC; Taiwan Liver Disease Consortium, the National Research Program for Biopharmaceuticals, and the National Science Council, Taiwan, NSC100-2325-B-002-052; NSC102-2325-B-002-079
P- Reviewer: Lin J, Sangro B, Yanev SG S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
References
- 1.Faloppi L, Scartozzi M, Maccaroni E, Di Pietro Paolo M, Berardi R, Del Prete M, Cascinu S. Evolving strategies for the treatment of hepatocellular carcinoma: from clinical-guided to molecularly-tailored therapeutic options. Cancer Treat Rev. 2011;37:169–177. doi: 10.1016/j.ctrv.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 3.Ye SL, Takayama T, Geschwind J, Marrero JA, Bronowicki JP. Current approaches to the treatment of early hepatocellular carcinoma. Oncologist. 2010;15 Suppl 4:34–41. doi: 10.1634/theoncologist.2010-S4-34. [DOI] [PubMed] [Google Scholar]
- 4.Poon RT. Prevention of recurrence after resection of hepatocellular carcinoma: a daunting challenge. Hepatology. 2011;54:757–759. doi: 10.1002/hep.24569. [DOI] [PubMed] [Google Scholar]
- 5.Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10–24. doi: 10.1097/00000658-200007000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishii H, Yamamoto J, Ikari T. Adjuvant treatments for resectable hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2008;15:459–462. doi: 10.1007/s00534-008-1359-1. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz JD, Schwartz M, Mandeli J, Sung M. Neoadjuvant and adjuvant therapy for resectable hepatocellular carcinoma: review of the randomised clinical trials. Lancet Oncol. 2002;3:593–603. doi: 10.1016/s1470-2045(02)00873-2. [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 9.Kudchadkar R, Gonzalez R, Lewis KD. PI-88: a novel inhibitor of angiogenesis. Expert Opin Investig Drugs. 2008;17:1769–1776. doi: 10.1517/13543784.17.11.1769. [DOI] [PubMed] [Google Scholar]
- 10.Joyce JA, Freeman C, Meyer-Morse N, Parish CR, Hanahan D. A functional heparan sulfate mimetic implicates both heparanase and heparan sulfate in tumor angiogenesis and invasion in a mouse model of multistage cancer. Oncogene. 2005;24:4037–4051. doi: 10.1038/sj.onc.1208602. [DOI] [PubMed] [Google Scholar]
- 11.Cochran S, Li C, Fairweather JK, Kett WC, Coombe DR, Ferro V. Probing the interactions of phosphosulfomannans with angiogenic growth factors by surface plasmon resonance. J Med Chem. 2003;46:4601–4608. doi: 10.1021/jm030180y. [DOI] [PubMed] [Google Scholar]
- 12.Parish CR, Freeman C, Brown KJ, Francis DJ, Cowden WB. Identification of sulfated oligosaccharide-based inhibitors of tumor growth and metastasis using novel in vitro assays for angiogenesis and heparanase activity. Cancer Res. 1999;59:3433–3441. [PubMed] [Google Scholar]
- 13.Liu CJ, Lee PH, Lin DY, Wu CC, Jeng LB, Lin PW, Mok KT, Lee WC, Yeh HZ, Ho MC, et al. Heparanase inhibitor PI-88 as adjuvant therapy for hepatocellular carcinoma after curative resection: a randomized phase II trial for safety and optimal dosage. J Hepatol. 2009;50:958–968. doi: 10.1016/j.jhep.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 14.Thall PF, Simon R, Ellenberg SS. Two-stage selection and testing designs for comparative clinical trials. Biometrika. 1988;75:303–310. [Google Scholar]
- 15.Forner A, Roayaie S. Clinical research in hepatocellular carcinoma: study design and endpoints. J Hepatol. 2009;50:850–853. doi: 10.1016/j.jhep.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 16.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 17.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 18.Gedaly R, Angulo P, Hundley J, Daily MF, Chen C, Evers BM. PKI-587 and sorafenib targeting PI3K/AKT/mTOR and Ras/Raf/MAPK pathways synergistically inhibit HCC cell proliferation. J Surg Res. 2012;176:542–548. doi: 10.1016/j.jss.2011.10.045. [DOI] [PubMed] [Google Scholar]
- 19.Fan ST, Mau Lo C, Poon RT, Yeung C, Leung Liu C, Yuen WK, Ming Lam C, Ng KK, Ching Chan S. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20-year experience. Ann Surg. 2011;253:745–758. doi: 10.1097/SLA.0b013e3182111195. [DOI] [PubMed] [Google Scholar]
- 20.Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229:322–330. doi: 10.1097/00000658-199903000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]


