Abstract
Diarrheal diseases are a great public health problem; they are among the most causes leading to morbidity and mortality of infants and children particularly in developing countries and even in developed countries. Rotavirus is the most common cause of severe gastroenteritis in infants and young children in both developed and developing countries. The purpose of this study was to determine the incidence rate of Rotavirus infection, its genotypes, and risk factors among children with diarrhea in Taiz, Yemen. 795 fecal samples were collected from children (less than 5 years old), suffering from diarrhea and attending the Yemeni-Swedish Hospital (YSH) in Taiz , Yemen, from November 2006 to February 2008. Rotavirus was detected by enzyme linkage immunosorbent assay (ELISA) on stool specimens of children. Genotypes of Rotavirus were characterized by reverse transcriptase-polymerase chain reaction (RT-PCR) and polyacrylamide gel electrophoresis (PAGE). The results showed that 358 (45.2%) were Rotavirus-positive and the most prevalent genotypes were G2P[4] (55%), followed by G1P[8] (15%). In addition, Rotavirus was found through the whole year; however, higher frequency during the summer season (53.4%) and lower frequency during the winter season (37.1%).
1. Introduction
Diarrheal diseases are a great public health problem that leads to morbidity and mortality of infants and children particularly in developing countries and even in developed countries [1]. In developing countries, Rotavirus is the most important cause of severe gastroenteritis among young children. Recent conservative estimates have indicated that 702,000 children die each year from Rotavirus disease and that up to 85% of these deaths occur in low-income countries [2, 3]. In many countries, the disease burden and epidemiology of Rotavirus are unknown because of the lack of adequate data or because there are no studies that have been conducted recently. In developed countries, Rotavirus remains a major clinical problem with 80% of children developing Rotavirus diarrhea in their first 3 years and the highest rates of illness occurring during the second year [4, 5]. In the United States of America (USA), approximately 20–60 deaths occur every year among children aged less than 5 years [6, 7] due to Rotavirus. Each year, Rotavirus infection results in the hospitalization of an estimated 70,000 children and 100 children died annually in the USA from complications of Rotavirus infection [7].
The aims of this study were to estimate the prevalence of Rotavirus infection, to detect the Rotavirus genotypes, and to determine the most cause factors of Rotavirus diarrhea among children under 5 years old in Taiz, Yemen.
2. Materials and Methods
2.1. Study Area
Taiz is one of the largest governorates in Yemen, with an area of 10.000 km2. It is the highest populated governorate in Yemen; its population reaches up to 2.5 million inhabitants, of which 49% are males and 51% are females, organized in 23 districts. The total population of children under the age of five years is 540,000 according to the last national census made in 2004 by the Central Statistical Organization, Sana'a [8]. In the last few years, the city has suffered from a shortage of water resources and, as a result, the government constructed several dams; one of them is near the northern part of the city. The sewage system creates two major water collections in the north part of the city, 2-3 Km2 away from the dam. The water from the sewage system is also used in agricultural activities.
2.2. Study Design
A cross-sectional observational study was conducted by recruiting case-series of children who have diarrhea within the age group of less than 5 years of age. The study was done in the Yemeni-Swedish Hospital (YSH) and the National Center for Public Health Laboratories (NCPHL) in Taiz from November 2006 to February 2008. YSH was the only public hospital which admits children and practices mother health care in Taiz city.
2.3. Sample Size
With respect to the investigation's third object of risk factors for Rotavirus, recruiting 795 samples provided the study with more than 80% of power to detect an odd ratio of 2 or more, assuming that the prevalence of Rotavirus infection was 20%–30%. Children were selected by a random sample, by selecting every fifth child who came with diarrhea during the study period. Many patients were coming from the neighboring governorates, because of the long history of good reputation for being the first specialized hospital in Yemen. Seventy-three samples were positive for Rotavirus; they were sent for genotyping by PCR in the Naval Medical Research Unit 3 (NAMRU-3), Cairo, Egypt.
2.4. Ethical Issue
Patients, mothers, or child's guardian received a simple explanation for the aim of the study as an ethical issue. If they agreed, the sample was collected and an interview was conducted. Confidentiality of the collected data was achieved by keeping data record in a locked room with limited access to the research team only.
2.5. Samples Collection
Within two days after hospitalization, at least 4–8 mg of stool was directly collected and stored in a sterile plastic container. Samples were kept at 2–8°C, for a maximum of 8 days, until they were transported to the laboratory where they were stored at −20°C prior to analysis. Clinical information was extracted from the mothers or child's guardian. Information included the child's sex, age at admission, symptoms, hydration status, height, weight, and length of hospital stay.
2.6. Laboratory Analysis
2.6.1. Detection of Rotavirus by Enzyme Linked Immunosorbent Assay (ELISA)
Rotavirus status was ascertained by ELISA (IDEIA Kit-Dako Ltd., Cambridgeshire, UK). This kit was employed by a polyclonal antibody prepared against the common antigen presented on Rotavirus VP6 (a major group specific protein). These antibodies were used in a solid phase sandwich type ELISA using a microplate containing 96 wells [9].
2.6.2. Detection of Rotavirus Genotypes by Polymerase Chain Reaction (PCR)
Rotavirus genotypes were detected by reverse transcription-polymerase chain reaction (RT-PCR) on 45.2% of Rotavirus-positive samples. Two amplifications of RT-PCR were carried out for identification of Rotavirus gene 9 (G-genotyping) and gene 4 (P-genotyping). For determination of the G types, the viral RNA was extracted according to the instructions of QIAmp viral RNA Mini kit (Qiagen, USA) and specific primers were used for the VP7 genes of G serotypes and other specific primers for the VP4 genes of P serotypes. The extracted RNA was kept at −20°C until use [10, 11].
2.6.3. Agarose Gel Electrophoresis
All PCR products were also examined by gel electrophoresis in 2% agarose gel and the Rotavirus genotypes were determined by the molecular weight of the amplicons. On the other hand, gel separating nucleic acid requires staining in order to be visualized; the stain contained ethidium bromide and then was viewed under ultraviolet illumination [10, 11].
2.7. Statistical Methods
Interview and laboratory data were analyzed using Epi Info. The descriptive analysis and the chi-square test were used to make comparisons among categorical variables. For all statistical analyses, a P value of less than 0.05 was considered statistically significant.
3. Results
3.1. Characteristics and Clinical History of Patients
3.1.1. Age and Sex
Fecal samples were collected from 795 children (less than 5 years of age) diagnosed with diarrhea during the period from November 2006 to February 2008. All children had diarrhea for a period of 1-2 days before hospitalization. Rotavirus infection was detected in 45.0% using ELISA technique. The general characteristics of patients were shown in Table 1. The age range of patients was from 1 to 59 months and the median age of subjects was 9 months with range of 6–14 months, where 50% of cases occurred between 6 and 14 months. Rotavirus diarrhea was represented in the males as 61.5% while in the females it was represented as 38.5%. However, this difference was not statistically significant (P = 0.8). The higher frequency of Rotavirus diarrhea was in infants between 7 and 11 months (32.1%) and the lower frequency was in infants between 1 and 6 months (29.6%) (Table 2). Also, the incidence rate among the studied patients was 66.3% new cases of Rotavirus in age group per 100.000 inhabitants.
Table 1.
General characteristics of patients (n = 795).
| Personal data | Number | Ratio |
|---|---|---|
| Sex | ||
| Male | 485 | 61.1 |
| Female | 310 | 38.9 |
| Age (months) | ||
| 1–6 | 310 | 38.9 |
| 7–11 | 276 | 34.7 |
| 12–17 | 155 | 19.5 |
| 18–23 | 55 | 6.9% |
| 24–60 | 80 | 10.1 |
| Frequency of defecation/day (time) | ||
| 1–4 | 165 | 20.8 |
| 5-6 | 190 | 23.9 |
| 7–9 | 162 | 20.4 |
| 10+ | 278 | 34.9 |
| Duration of diarrhea (day) | ||
| 1-2 | 334 | 42.0 |
| 3-4 | 343 | 43.2 |
| 5+ | 118 | 14.8 |
| Hospitalization | ||
| Inpatient | 421 | 53.0 |
| Outpatient | 374 | 47.0 |
Table 2.
Ratio of Rotavirus infection and characteristic of patients in Taiz, Yemen.
| Personal data | Positive | Negative | χ 2 | P value | ||
|---|---|---|---|---|---|---|
| Number | Ratio | Number | Ratio | |||
| Sex | ||||||
| Male | 220 | 61.5 | 265 | 60.6 | ||
| Female | 138 | 38.5 | 172 | 39.4 | ||
| Age (months) | ||||||
| 1–6 | 106 | 29.6 | 123 | 28.1 | ||
| 7–11 | 115 | 32.1 | 161 | 36.8 | ||
| 12–17 | 70 | 19.6 | 85 | 19.5 | 3.74 | 0.29 |
| 18–23 | 43 | 12.0 | 12 | 2.8 | ||
| 24–60 | 24 | 6.7 | 56 | 12.8 | ||
| Frequency of defecation/day (time) | ||||||
| 1–4 | 72 | 20.1 | 93 | 21.3 | ||
| 5-6 | 77 | 21.5 | 113 | 25.9 | ||
| 7–9 | 79 | 22.1 | 83 | 18.9 | 2.93 | 0.4 |
| 10+ | 130 | 36.3 | 148 | 33.9 | ||
| Duration of diarrhea (day) | ||||||
| 1-2 | 161 | 45.0 | 173 | 39.6 | ||
| 3-4 | 159 | 44.4 | 184 | 42.1 | 9.45 | 0.009∗ |
| 5+ | 38 | 10.6 | 80 | 18.3 | ||
| Hospitalization | ||||||
| Inpatient | 196 | 54.7 | 225 | 51.5 | 0.84 | 0.39 |
| Outpatient | 162 | 45.3 | 212 | 48.5 | ||
∗Significant.
3.1.2. Symptoms of Rotavirus Diarrhea
In addition, the clinical findings were diagnosed and recorded. Fever, vomiting, and diarrhea were the main symptoms (50.5%), while diarrhea alone was recorded in 10.7% (Table 3).
Table 3.
Symptoms of Rotavirus infection among children in Taiz, Yemen (n = 391).
| Symptoms | Rotavirus | Total | Odd ratio | P value | ||||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | |||||||
| Number | % | Number | % | Number | % | |||
| Diarrhea alone | 21 | 10.7 | 23 | 11.8 | 44 | 11.3 | 0.89 | 0.75 |
| Vomiting alone | 2 | 1.0 | 3 | 1.5 | 5 | 1.3 | 1.5 | 0.65 |
| Diarrhea and vomiting | 71 | 36.2 | 83 | 42.6 | 154 | 39.4 | 1.3 | 0.20 |
| Diarrhea, vomiting, and fever | 99 | 50.5 | 85 | 43.6 | 184 | 47.1 | 0.76 | 0.17 |
| Upper respiratory tract infection | 3 | 1.5 | 1 | 0.5 | 4 | 1.0 | 0.33 | 0.31 |
|
| ||||||||
| Total | 196 | 100.0 | 195 | 100.0 | 391 | 100.0 | ||
3.1.3. Rehydration Therapy and Misprescription Intake Antibiotics
On the other hand, a total of 421 (53.0%) of 795 patients were admitted as inpatients for intravenous rehydration therapy; 196 patients of them had Rotavirus diarrhea, while 374 (47.0%) were seen in the outpatient ward receiving oral rehydration solution and 162 had Rotavirus diarrhea; this difference was not statistically significant (P = 0.39) (Table 2). Also, the results showed that about 60.5% of the patients infected with Rotavirus were given antibiotics empirically as a sort of treatment inside hospital by doctors (P = 0.001) and there was misprescription intake of antibiotics from the health worker (P = 0.025) (Table 4).
Table 4.
Clinical history and Rotavirus diarrhea among children in Taiz, Yemen.
| Type of house (n = 195) |
Rotavirus | P value | Odd ratio | 95% confidence interval |
|---|---|---|---|---|
| Number | ||||
| Clinical history (n = 731) | ||||
| Health worker treatment | 107 | 1.89 | 0.62 | 1.23–2.76 |
| Costive medicines | 22 | 1.19 | 0.03∗ | 0.62–2.25 |
| Antibiotics | 100 | 1.83 | 0.003∗ | 1.22–2.76 |
| Oral rehydration | 107 | 0.92 | 0.59 | 0.68–1.26 |
| Public hospital treatment | 163 | 0.78 | 0.93 | 0.54–1.59 |
| Costive medicines | 19 | 1.29 | 0.473 | 0.64–2.63 |
| Antibiotics | 107 | 2.2 | 0.0001∗ | 1.45–3.26 |
| Oral rehydration solution | 106 | 1.54 | 0.034 | 1.03–2.29 |
∗Significant.
3.2. Seasonality of Rotavirus Diarrhea
As for seasonal distribution, Rotavirus was found through the whole year. However, summer and winter seasons were significantly associated with Rotavirus infection; however, higher frequency during the summer season (140 cases, 53.4%, and P = 0.001) and lower frequency during the winter season (83 cases, 37.1%, and P = 0.005) (Table 5). In addition, Rotavirus exhibited peaks in May and June months (Figure 1).
Table 5.
Seasonality of Rotavirus diarrhea among children in Taiz, Yemen.
| Season | Rotavirus | Total | χ 2 | P value | ||||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | |||||||
| Number | % | Number | % | Number | % | |||
| Winter | 83 | 37.1 | 141 | 62.9 | 224 | 10.44 | 0.02 | 0.88 |
| Spring | 100 | 44.6 | 124 | 55.4 | 224 | 12.57 | 0.17 | 0.67 |
| Summer | 140 | 53.4 | 122 | 56.6 | 262 | 17.61 | 0.03 | 0.86 |
| Autumn | 35 | 41.2 | 50 | 58.8 | 85 | 04.40 | 0.17 | 0.68 |
|
| ||||||||
| Total | 358 | 45 | 437 | 55 | 795 | 45 | ||
Figure 1.
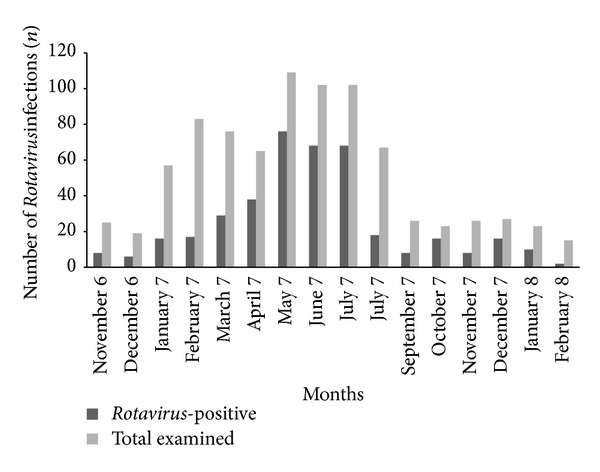
Monthly seasonality of Rotavirus diarrhea among children in Taiz, Yemen.
3.3. Effect of Feeding on Rotavirus Diarrhea Outcome
In addition, the effect of breast-feeding versus mixed- and artificial-feeding on the outcome of the Rotavirus infection was studied in infants and young children; the results showed that the rate of the Rotavirus gastroenteritis was 67.5% of the breast-feeding and 32.5% of the artificial-feeding (Table 6).
Table 6.
Effect of feeding on outcome of Rotavirus diarrhea among children in Taiz, Yemen (n = 385).
| Feeding if <2 years | Rotavirus | Odd ratio | P value | 95% confidence interval | |
|---|---|---|---|---|---|
| Number | % | ||||
| Exclusively breast fed | 34 | 17.8 | 0.57 | 0.026∗ | 0.35–0.94 |
| Mixed with artificial formulas | 35 | 18.3 | 0.78 | 0.38 | 0.46–1.34 |
| Mixed with cow/goat milk | 20 | 10.5 | 0.81 | 0.56 | 0.41–1.62 |
| Mixed with weaning food | 40 | 20.9 | 0.83 | 0.47 | 0.50–1.38 |
| Mixed with artificial formulas/weaning food | 15 | 7.9 | 1.05 | 0.89 | 0.51–2.19 |
| Exclusively artificial formulas | 15 | 7.9 | 0.77 | 0.52 | 0.35–1.69 |
| Artificial formulas/weaning food | 25 | 13.1 | 0.75 | 0.38 | 0.39–1.43 |
| Mixed with cow/goat milk | 7 | 3.7 | 1.28 | 0.63 | 0.46–3.50 |
|
| |||||
| Total | 191 | 100.0 | |||
∗Significant.
3.4. Effect of Accommodation and Pattern Education on Rotavirus Diarrhea Outcome
The relationship between the Rotavirus infection and the type of house and education level of mothers was not found, while the Rotavirus infection and father educational level was statistically significant (P = 0.01). The source of water itself seems to have no effect in the transmission of Rotavirus. 39.0% of studied patients had animals at home; of those patients 41.3% had Rotavirus infection, but there was no statistical significance (P = 0.09). There was statistical significance (P = 0.026) between the presence of the latrine and its absence with infected Rotavirus patients (Table 7). Also, regarding the area of residence, only 7.0% of Rotavirus infections were from the semiurban area; 36.3% were from urban area, whereas 56.7% were from rural area. However, this difference was not statistically significant (P = 0.48) (Table 8).
Table 7.
Effect of accommodation on outcome of Rotavirus diarrhea among children in Taiz, Yemen.
| Risk factors | Rotavirus | P value | Odd ratio | 95% confidence interval | |
|---|---|---|---|---|---|
| Number | % | ||||
| Type of house (n = 390) | |||||
| Flat | 20 | 10.3 | 0.83 | 0.93 | 0.49–1.78 |
| House | 175 | 89.7 | |||
| Hut, shack | 0 | 00.0 | 0.08 | 1.02 | 0.94–1.97 |
| Total | 195 | 100 | |||
|
| |||||
| Education level (n = 466) | |||||
| Father (illiterate) | 183 | 85.5 | 1.8 | 0.01∗ | 1.14–2.977 |
| Total | 214 | 100.0 | |||
| Mother (illiterate) | 123 | 57.5 | 1.01 | 0.92 | 0.705–1.473 |
| Total | 214 | 100.0 | |||
|
| |||||
| Latrine (n = 195) | |||||
| Latrine is available | 191 | 98.0 | 3.4 | 0.026∗ | 1.09–10.65 |
| Nonlatrine | 4 | 2.0 | 1.1 | 0.62 | 0.62–2.28 |
| Total | 195 | 100.0 | |||
|
| |||||
| Source of water (n = 466) | |||||
| Tap water | 75 | 35.1 | 0.4 | 0.84 | |
| Well | 89 | 41.6 | 0.9 | 0.98 | |
| Stream/spring | 11 | 5.1 | 0.46 | 1.3 | 0.61–2.91 |
| Water trunk | 21 | 9.8 | 0.97 | 1.0 | 0.55–1.87 |
| Others (treated water) | 18 | 8.4 | 0.33 | 1.3 | 0.73–2.53 |
| Total | 214 | 100.0 | |||
|
| |||||
| Animal at home (n = 472) | |||||
| Yes | 76 | 34.9 | 0.09 | 0.7 | 0.49–1.05 |
| Total | 218 | 100.0 | |||
∗Significant.
Table 8.
Distribution of Rotavirus diarrhea among children according to the area of residence in Taiz, Yemen (n = 795).
| Area of residence | Rotavirus | Total | χ 2 | P value | ||||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | |||||||
| Number | % | Number | % | No. | % | |||
| Rural | 203 | 56.7 | 255 | 58.4 | 458 | 34.5 | 0.22 | 0.64 |
| Urban | 130 | 36.3 | 144 | 33.0 | 274 | 57.6 | 1.47 | 0.48 |
| Semiurban | 25 | 7.0 | 38 | 8.7 | 63 | 7.9 | 0.79 | 0.37 |
|
| ||||||||
| Total | 358 | 100.0 | 437 | 100.0 | 795 | 100.0 | ||
3.5. Genotypes of Rotavirus Infection
G and P genotypes of Rotavirus were detected in only 73 out of 358 (20%) by RT-PCR assay. G2P[4] (55.0%) was the predominant genotype, followed by G1P[8] (15.0%), also 4% of G1G2P[8], 4% G1G2P[4] P[8], and 7% of G2 untypeable and 15% of other genotypes (Figure 2). On the other hand, the seasonal variation of the most prevalent Rotavirus genotypes (G1P[8] and G2P[4]) was shown in Table 9.
Figure 2.
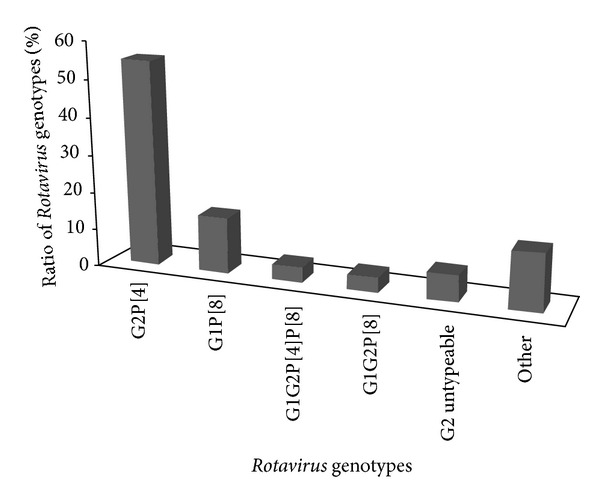
Common genotypes of Rotavirus diarrhea among children in Taiz, Yemen.
Table 9.
Seasonality of Rotavirus genotypes among children in Taiz, Yemen (n = 44).
| Season | Rotavirus genotypes | Total | χ 2 | P value | ||||
|---|---|---|---|---|---|---|---|---|
| G1P[8] | % | G2P[4] | % | Number | % | |||
| Winter | 3 | 6.81 | 11 | 25 | 14 | 31.8 | 0.02 | 0.88 |
| Spring | 2 | 4.54 | 9 | 20.45 | 11 | 25.0 | 0.17 | 0.67 |
| Summer | 2 | 4.54 | 6 | 13.63 | 8 | 18.2 | 0.03 | 0.86 |
| Autumn | 3 | 6.81 | 8 | 18.18 | 11 | 25.0 | 0.17 | 0.68 |
|
| ||||||||
| Total | 10 | 22.7 | 34 | 77.3 | 44 | 100.0 | ||
4. Discussion
Rotavirus is a major cause of severe diarrhea of infants and young children. The present study showed that Rotavirus was the most common cause of diarrhea (45.2%) in infants and young children less than five years old in Taiz. Comparing to other studies carried out in different countries in the world, the present study results agreed with a study done by El Assouli et al. in Jeddah, Saudi Arabia, which stated that Rotavirus infection was 46% [12], while the higher rate of Rotavirus infection was recorded by Alicia et al. in Spain (55%) [13].
On the other hand, the lower percentages of Rotavirus infection were reported in many countries. The percentage of Rotavirus infection in infants and young children was 40% in Kuwait [14], 37% in both Turkey [15] and Erbil, Iraqi Kurdistan [16], 33% in Jordan [17], and 31% in Oman [18].
The rate of Rotavirus infection obtained in this study was more than those obtained in developed countries. For example, Rotavirus was found to be responsible for 10% of diarrhea in USA [19] and about 23.4% in infant diarrheal cases in the northwestern Ecuador [20]. The difference between the results may be due to the age of patients, duration of the study, and the methods used in detecting Rotavirus. In this study, two of the major global human Rotavirus genotypes (G2P[4] 55.0% and G1P[8] 15.0%) were detected and these genotypes were recorded in other studies in the neighboring Saudi Arabia [21]. In addition, in Yemen, Rotavirus infection was detected in 27% with genotypes that included G1P[8] (55%), G9P[8] (21%), and G2P[4] (12%) with G12 comprising 3% of strain types [22].
The infants who were less than one year old had the highest frequency of Rotavirus infection. Many other studies showed similar relationship between Rotavirus and age like the previous study in Yemen for children less than three years old who had diarrhea significantly higher than the young children [23], while this result agreed with other studies done in Egypt [24], Iran [25, 26], and Kuwait [14], where the higher frequency of Rotavirus was among infants less than 12 months. Also, the peak incidence of Rotavirus diarrhea in developing countries occurs between 6 and 11 months of age [27]. On the other hand, the difference between male and female in this study was not significant (P = 0.8), this result agreed with a previous study done in Hanoi [28].
During the period of the present study Rotavirus diarrhea occurred throughout the year, but the greatest number of Rotavirus diarrheas was identified in May and June; this result agreed with a study done in Jordan, where Rotavirus was more frequent during the summer months, June to August [29]. In addition, Rotavirus infection was reported in the cold, dry season in Tunisia [30]. This is also similar to the pattern seen in some African countries where Rotavirus infection had been recorded, such as Morocco [31, 32], Algeria [33], and Egypt [34], where epidemiological studies showed the same seasonal occurrence. This agreed with the result in this study which found that its peak was in the summer. Rotavirus infection has been called a winter disease in the temperate zones, whereas in tropical setting Rotavirus occurs through the whole year and this is in agreement with the current study.
The high prevalence of Rotavirus diarrhea among inpatients (54.7%) was not statistically significant in comparison with the prevalence of Rotavirus diarrhea in outpatients (45.3%) (P = 0.36) and this explains the role of Rotavirus in hospitalization of infants and children less than five years of age and the severity of diarrhea due to Rotavirus in practical medicine. Many other studies showed the burden of hospitalization due to Rotavirus infection. Rotavirus accounted for 30%–50% of diarrheal hospitalization in less than 5-year infants and children in England [35], 40% in diarrheal consultations in Argentina [36] and in New York, Rotavirus infection was reported in more than 30% of diarrhea cases in children less than 5 years of age [37].
The frequent association of fever and vomiting and the significant association of vomiting with Rotavirus diarrhea are not conclusive in the clinical diagnosis of Rotavirus infection, because such symptoms are constitutional and present with other causes of diarrhea. Nonetheless, the frequent vomiting with diarrhea leads to the risk of dehydration in Rotavirus infection more than other diarrheal infections and therefore increases the need for hospitalization.
The association between breast-feeding and Rotavirus infection may explain the poor personal hygiene practice. This link indicates their method of transmission, that is, the fecal-oral route. While breast-feeding protects against all-cause diarrhea in infants, no evidence shows that breast-feeding confers specific protection against viral gastrointestinal infection [38]. Several studies demonstrated that breast-feeding did not prevent acquisition of Rotavirus [39, 40]. Breast-feeding had no effect on reducing the severity of the symptoms of Rotavirus infection in this study. This was supported by other studies in Baghdad [41] and Yemen [23]. However, a beneficial effect of breast-feeding against Rotavirus infection was reported in other studies [42] which found that breast-feeding reduces the amount of vomiting in infants with Rotavirus infection [43] but the degrees of protection offered against Rotavirus infection vary in different populations [44].
There is no statistical significance between domestic animals and Rotavirus infection (P = 0.09). This result was supported by Al-Khasra et al. [23]. Socioeconomic status including level of education, type of house, source of water, and latrine was also studied as risk factor for transmission of Rotavirus. The latrine inside the home was significantly associated with Rotavirus infection but the other factors had no association. In a community-based survey carried out in two residential areas of different sanitary and socioeconomic conditions in Basra, Iraq, it was suggested that there is possibility of favoring Rotavirus transmission by poor personal hygiene, poor sanitation, and low educational level [45]. However, other studies did not prove the role of socioeconomic factors or sanitation except for the crowdedness factor [45].
Rotavirus was isolated from the nasopharyngeal secretion of two children out of thirty with upper respiratory tract infections [46]. In a recent study, Rotavirus was isolated from the oropharyngeal aspirates of 25 out of 89 infants with respiratory tract infections and 33 control patients without the disease [47]. This is in agreement with the current study, which shows that Rotavirus was detected in three patients out of four with upper respiratory tract infection (75.0%). This supports the other route of transmission of Rotavirus, which is respiratory droplets. Antibiotics were prescribed for 43.9% of all patients at the time of sample collection and for 45.4% of inpatients; 60.5% of patients with Rotavirus were given antibiotics empirically. Another study in Yemen indicated that antibiotics were used in 57.7% of children infected with Rotavirus [23]. This indicates the huge reliance on antibiotics treatment. This reflects the inappropriate diagnosis and lack of facilities of testing. Antibiotics should not be given routinely to children with diarrhea because they are ineffective and may lead to serious side effects [48].
In conclusion, this study revealed that Rotavirus is a major cause of diarrhea in infants and children in Taiz, Yemen. The incidence rate among the studied patients was found to be 66.3 new cases of Rotavirus in the studied age group per 100.000 inhabitants. The most predominant genotypes were G2P[4] (55.0%), followed by G1P[8] (15.0%); Rotavirus was found through the whole year with less-defined seasonal variation. However, the greatest number of the cases was identified in May and June of the year. The peak incidence of Rotavirus diarrhea in infants and young children in Taiz occurs between 7 and 11 months of age.
Acknowledgments
The authors would like to thank the director and staff of the Yemeni-Swedish Hospital of Taiz, National Center for Laboratories of Public Health (NCLPH) of Taiz, Tihama Foundation for Drug Studies and Research (TFDSR), Hodeidah, and Yemen and the United State of Naval Medical Research Unit 3 (US NAMRU-3), Cairo, Egypt, and Dr. Abdullah Al-Tayar and Jamal Bathar, for their fruitful assistance. This work was supported by grant from the Eastern Mediterranean Regional Office of the World Health organization (EMRO-WHO). Also, the authors would like to thank Mr. Ibrahim Jibreel, lecturer at the Deptaertment of Translation, Faculty of Human and Social Sciences, University of Sciences and Technology, Hodeidah Branch, for his fruitful assistance, and Mr. Hamza Khayrat, M.D. holder in English language, Department of English Language, Faculty of Education, Hodeidah University.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Dr. Abdulmalik Al-Badani (Ph.D. in pediatrics) contributed to clinical investigation and treatment of children; Ms. Leena Al-Areqi collected the sample, analyzed the data, and wrote the paper; Mr. Abdulatif Majily did field work implementation and data interpretation; Dr. Saleh AL-Sallami and Dr. Anwar AL-Madhagi supervised the progress of the project and protocol development; Mohammed Amood AL-Kamarany contributed to data interpretation, writing, and revision of paper. Abdulmalik Al-Badani and Leena Al-Areqi have equally contributed to this paper. The authors have participated equally in contribution of this work and they have given final approval to the version to be submitted for publication.
References
- 1.Snyder JD, Merson MH. The magnitude of the global problem of acute diarrhoeal disease: a review of active surveillance data. Bulletin of the World Health Organization. 1982;60(4):605–613. [PMC free article] [PubMed] [Google Scholar]
- 2.Cunliffe NA, Nakagomi O. A critical time for rotavirus vaccines: a review. Expert Review of Vaccines. 2005;4(4):521–532. doi: 10.1586/14760584.4.4.521. [DOI] [PubMed] [Google Scholar]
- 3.Parashar UD, Gibson CJ, Bresee JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerging Infectious Diseases. 2006;12(2):304–306. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gurwith M, Wenman W, Hinde D, Feltham S, Greenberg H. A prospective study of rotavirus infection in infants and young children. Journal of Infectious Diseases. 1981;144(3):218–224. doi: 10.1093/infdis/144.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez WJ, Kim HW, Brandt CD, et al. Longitudinal study of rotavirus infection and gastroenteritis in families served by a pediatric medical practice: clinical and epidemiologic observations. Pediatric Infectious Disease Journal. 1987;6(2):170–176. doi: 10.1097/00006454-198702000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Kilgore PE, Holman RC, Clarke MJ, Glass RI. Trends of diarrheal disease— associated mortality in US children, 1968 through 1991. Journal of the American Medical Association. 1995;274(14):1143–1148. [PubMed] [Google Scholar]
- 7.Center for Disease Control and Prevention (CDC ) The National Healthy Mothers Healthy Babies Coalition, Are You Rotavirus Ready? 2006. [Google Scholar]
- 8.National Census-Central Statistical Organization. Sana’a, Census, 2004.
- 9.Taniguchi K, Urasawa T, Morita Y, Greenberg HB, Urasawa S. Direct serotyping of human rotavirus in stools by an enzyme-linked immunosorbent assay using serotype 1-, 2-, 3-, and 4- specific monoclonal antibodies to VP7. Journal of Infectious Diseases. 1987;155(6):1159–1166. doi: 10.1093/infdis/155.6.1159. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Manual of Rotavirus Detection and Characterization Methods. WHO; 2009. [Google Scholar]
- 11.Logan C, O’Leary JJ, O’Sullivan N. Real-time reverse transcription-PCR for detection of rotavirus and adenovirus as causative agents of acute viral gastroenteritis in children. Journal of Clinical Microbiology. 2006;44(9):3189–3195. doi: 10.1128/JCM.00915-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Assouli SM, Banjar ZM, Mohammed KA, Zamakhchari FT. Rotavirus infection in children in Saudi Arabia. The American Journal of Tropical Medicine and Hygiene. 1992;46(9):272–277. doi: 10.4269/ajtmh.1992.46.272. [DOI] [PubMed] [Google Scholar]
- 13.Alicia SF, Vanessa M, Slivia M, et al. Human Rotavirus G9 and G3 as major cause of disease in hospitalized children, spain. Emerging Infectious Diseases. 2006;12(10):304–306. doi: 10.3201/eid1210.060384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sethi SK, Al-Nakib W, Khuffash FA, Majeed HA. Acute diarrhoea and rotavirus infections in young children in Kuwait. Annals of Tropical Paediatrics. 1984;4(2):117–121. doi: 10.1080/02724936.1984.11748321. [DOI] [PubMed] [Google Scholar]
- 15.Karadag A, Acikgoz ZC, Avci Z, et al. Childhood diarrhoea in Ankara, Turkey: epidemiological and clinical features of rotavirus-positive versus rotavirus-negative cases. Scandinavian Journal of Infectious Diseases. 2005;37(4):269–275. doi: 10.1080/00365540410020983. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed HM, Coulter JBS, Nakagomi O, et al. Molecular characterization of rotavirus gastroenteritis strains, Iraqi Kurdistan. Emerging Infectious Diseases. 2006;12(5):824–826. doi: 10.3201/eid1205.051422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Youssef M, Shurman A, Bougnoux M, Rawashdeh M, Bretagne S, Strockbine N. Bacterial, viral and parasitic enteric pathogens associated with acute diarrhea in hospitalized children from northern Jordan. FEMS Immunology and Medical Microbiology. 2000;28(3):257–263. doi: 10.1111/j.1574-695X.2000.tb01485.x. [DOI] [PubMed] [Google Scholar]
- 18.Aithala G, Al Dhahry SHS, Saha A, Elbualy MS. Epidemiological and clinical features of Rotavirus gastroenteritis in Oman. Journal of Tropical Pediatrics. 1996;42(1):54–57. doi: 10.1093/tropej/42.1.54. [DOI] [PubMed] [Google Scholar]
- 19.Parashar DU, Gibson CJ, Bresee JS, Glass RI. Rotavirus and severe childhood diarrhea centers for disease control and prevention (CDC) Atlanta, Georgia, USA. 2006;12(2):304–306. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Endara P, Trueba G, Solberg OD, et al. Symptomatic and subclinical infection with rotavirus P[8]G9, rural Ecuador. Emerging Infectious Diseases. 2007;13(4):574–580. doi: 10.3201/eid1304.061285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kheyami AM, Nakagomi T, Nakagomi O, Dove W, Hart CA, Cunliffe NA. Molecular epidemiology of rotavirus diarrhea among children in Saudi Arabia: first detection of G9 and G12 strains. Journal of Clinical Microbiology. 2008;46(4):1185–1191. doi: 10.1128/JCM.02244-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirby A, Al-Eryani A, Al-Sonboli N, et al. Rotavirus and norovirus infections in children in Sana'a, Yemen. Tropical Medicine and International Health. 2011;16(6):680–684. doi: 10.1111/j.1365-3156.2011.02756.x. [DOI] [PubMed] [Google Scholar]
- 23.Al-Khasra KR, Nasher MA, Jabber AA. The detection of rotavirus among infants and young children suffering from diarrhea in Sana'a. Yemen Medical Journal. 2002;4(1):60–63. [Google Scholar]
- 24.Radwan SF, Gabr MK, El-Maraghi S, El-Saifi AF. Serotyping of group a rotaviruses in Egyptian neonates and infants less than 1 year old with acute diarrhea. Journal of Clinical Microbiology. 1997;35(11):2996–2998. doi: 10.1128/jcm.35.11.2996-2998.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khalili B, Cuevas LE, Reisi N, Dove W, Cunliffe NA, Hart CA. Epidemiology of rotavirus diarrhea in Iranian children. Journal of Medical Virology. 2004;73(2):309–312. doi: 10.1002/jmv.20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kazemi A, Tabatabaie F, Agha-Ghazvini MR, Kelishadi R. The role of rotavirus in acute pediatric diarrhea in Isfahan, Iran. Pakistan Journal of Medical Sciences. 2006;22(3):282–285. [Google Scholar]
- 27.Bresee JS, Glass RI, Ivanoff B, Gentsch JR. Current status and future priorities for rotavirus vaccine development, evaluation and implementation in developing countries. Vaccine. 1999;17(18):2207–2222. doi: 10.1016/s0264-410x(98)00376-4. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen TV, Le Van P, Le Huy C, Weintraub A. Diarrhea caused by rotavirus in children less than 5 years of age in Hanoi, Vietnam. Journal of Clinical Microbiology. 2004;42(12):5745–5750. doi: 10.1128/JCM.42.12.5745-5750.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nimri LF, Hijazi S. Rotavirus-associated diarrhoea in children in a refugee camp in Jordan. Journal of Diarrhoeal Diseases Research. 1996;14(1):1–4. [PubMed] [Google Scholar]
- 30.Hassine-Zaafrane M, Sdiri-Loulizi K, Salem IB, et al. The molecular epidemiology of circulating rotaviruses: three-year surveillance in the region of Monastir, Tunisia. BMC Infectious Diseases. 2011;11(article 266):1–6. doi: 10.1186/1471-2334-11-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benhafid M, Youbi M, Klena JD, et al. Epidemiology of rotavirus gastroenteritis among children <5 years of age in morocco during 1 year of sentinel hospital surveillance, June 2006–May 2007. Journal of Infectious Diseases. 2009;200(1):S70–S75. doi: 10.1086/605048. [DOI] [PubMed] [Google Scholar]
- 32.Benhafid M, Elomari N, Elqazoui M, et al. Diversity of rotavirus strains circulating in children under 5 years of age admitted to hospital for acute gastroenteritis in Morocco, June 2006 to May 2009. Journal of Medical Virology. 2013;85(2):354–362. doi: 10.1002/jmv.23445. [DOI] [PubMed] [Google Scholar]
- 33.Puel JML, Orillac MS, Bauriaud RM, Boughermouh R, Akacem O, Lefevre-Witier P. Occurrence of viruses in human stools in the Ahaggar (Algeria) Journal of Hygiene. 1982;89(1):171–174. doi: 10.1017/s0022172400070674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Mougi M, Amer A, El-Abhar A, Hughes J, El-Shafie A. Epidemiological and clinical features of rotavirus associated acute infantile diarrhoea in Cairo, Egypt. Journal of Tropical Pediatrics. 1989;35(5):230–233. doi: 10.1093/tropej/35.5.230. [DOI] [PubMed] [Google Scholar]
- 35.Trung Vu N, le Van P, le Huy C, Weintraub A. Diarrhea caused by rotavirus in children less than 5 years of age in Hanoi, Vietnam. Journal of Clinical Microbiology. 2004;42(12):5745–5750. doi: 10.1128/JCM.42.12.5745-5750.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bok K, Castagnaro N, Borsa A, et al. Surveillance for rotavirus in Argentina. Journal of Medical Virology. 2001;65(1):190–198. [PubMed] [Google Scholar]
- 37.Chang HH, Glass RI, Smith PF, Cicirello HG, Holman RC, Morse DL. Disease burden and risk factors for hospitalizations associated with rotavirus infection among children in New York State, 1989 through 2000. Pediatric Infectious Disease Journal. 2003;22(9):808–814. doi: 10.1097/01.inf.0000086404.31634.04. [DOI] [PubMed] [Google Scholar]
- 38.Kramer MS, Chalmers B, Hodnett ED, et al. Promotion of breastfeeding intervation trial (PROBIT): a randomized trial in the Republic of Belarus. The Journal of the American Medical Association. 2001;285(4):413–420. doi: 10.1001/jama.285.4.413. [DOI] [PubMed] [Google Scholar]
- 39.Heinig MJ. Host defense benefits of breastfeeding for the infant: effect of breastfeeding duration and exclusively. Pediatric Clinics of North America. 2001;48(1):105–123. doi: 10.1016/s0031-3955(05)70288-1. [DOI] [PubMed] [Google Scholar]
- 40.Misra S, Sabui TK, Basu S, Pal N. A prospective study of rotavirus diarrhea in children under 1 year of age. Clinical Pediatrics. 2007;46(8):683–688. doi: 10.1177/0009922807300700. [DOI] [PubMed] [Google Scholar]
- 41.Al-Wardi HA, Al Obaidi A, Al Hadithi TS, Omar AR. Rotavirus gastroenteritis in infants and young children in Sadam City in Baghdad. Saudi Medical Journal. 1990;11(6):457–459. [Google Scholar]
- 42.El Baroudi R, Chaker M, Eissa SK, Bader A, Safouh H. Comparative study between ELISA and EM . In the diagnosis of rotavirus as a cause of diarrhea. Journal of the Arabic Child. 1994;5(1):417–422. [Google Scholar]
- 43.Weinberg RJ, Tipton G, Klish WJ, Brown MR. Effect of breast-feeding on morbidity in rotavirus gastroenteritis. Pediatrics. 1984;74(2):250–253. [PubMed] [Google Scholar]
- 44.Robert Y, Jerry P, Steven V, Erik FF, Karean M, David N. Human milk mucin inhibits rotavirus replication and prevents experimental gastroenteritis. The Journal of Clinical Investigation. 1992;90(5):1984–1991. doi: 10.1172/JCI116078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al Kerwi AA, Al-Samaria AG, Yacoub AA. Rotavirus infection among children under the age of five in Basra: a community based survey. WHO. 1993;7:41–47. [Google Scholar]
- 46.Fragoso M, Kumar A, Murray DL. Rotavirus in nasopharyngeal secretions of children with upper respiratory tract infections. Diagnostic Microbiology and Infectious Disease. 1986;4(1):87–88. doi: 10.1016/0732-8893(86)90062-3. [DOI] [PubMed] [Google Scholar]
- 47.Jian Zheng B, Xu Chang R, Zhang Ma G, et al. Rotavirus infection of the oropharynx and respiratory tract in young children. Journal of Medical Virology. 1991;34(1):29–37. doi: 10.1002/jmv.1890340106. [DOI] [PubMed] [Google Scholar]
- 48.World Health Organization (WHO)/CDR 95.3. The Treatment of Diarrhea a Manual for Physician and Other Senior Health Worker. Division of Diarrhea and Acute Respiratory Tract Disease Control; 1995. [Google Scholar]


