Abstract
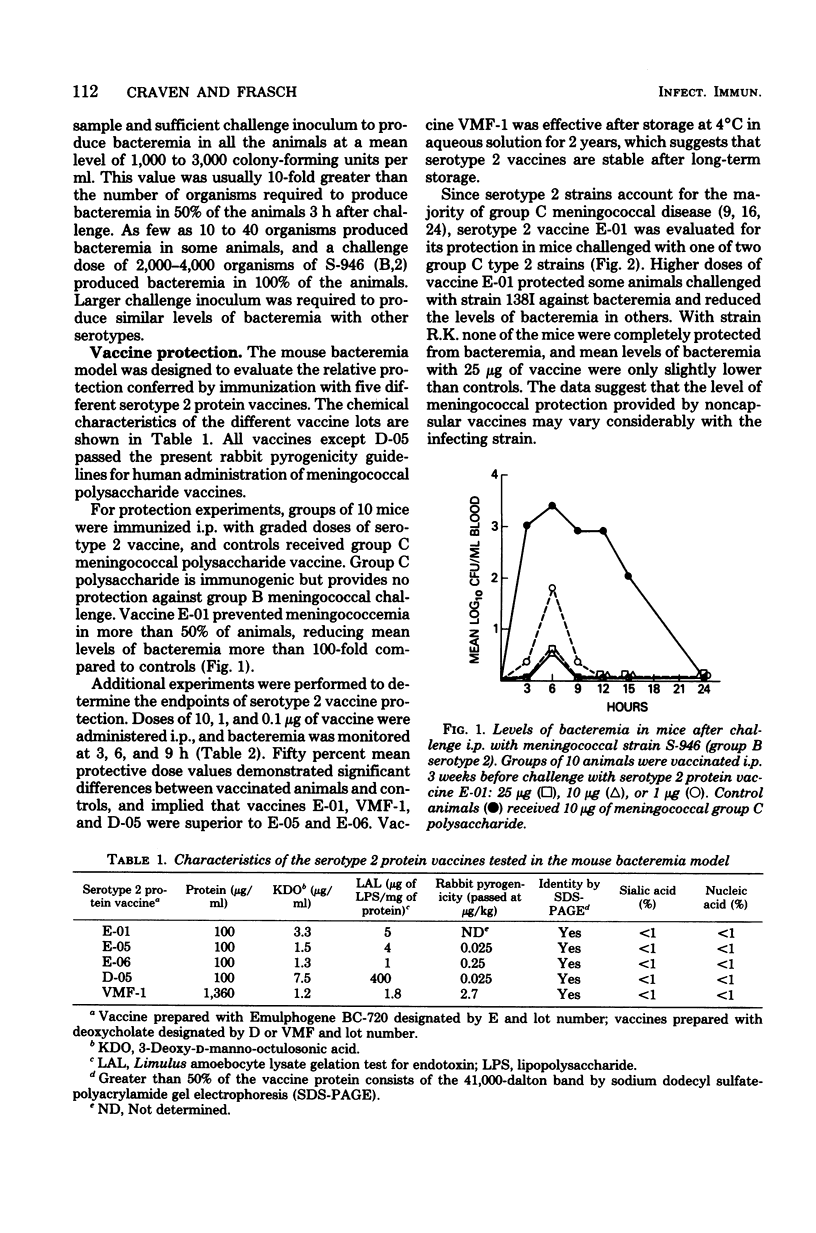
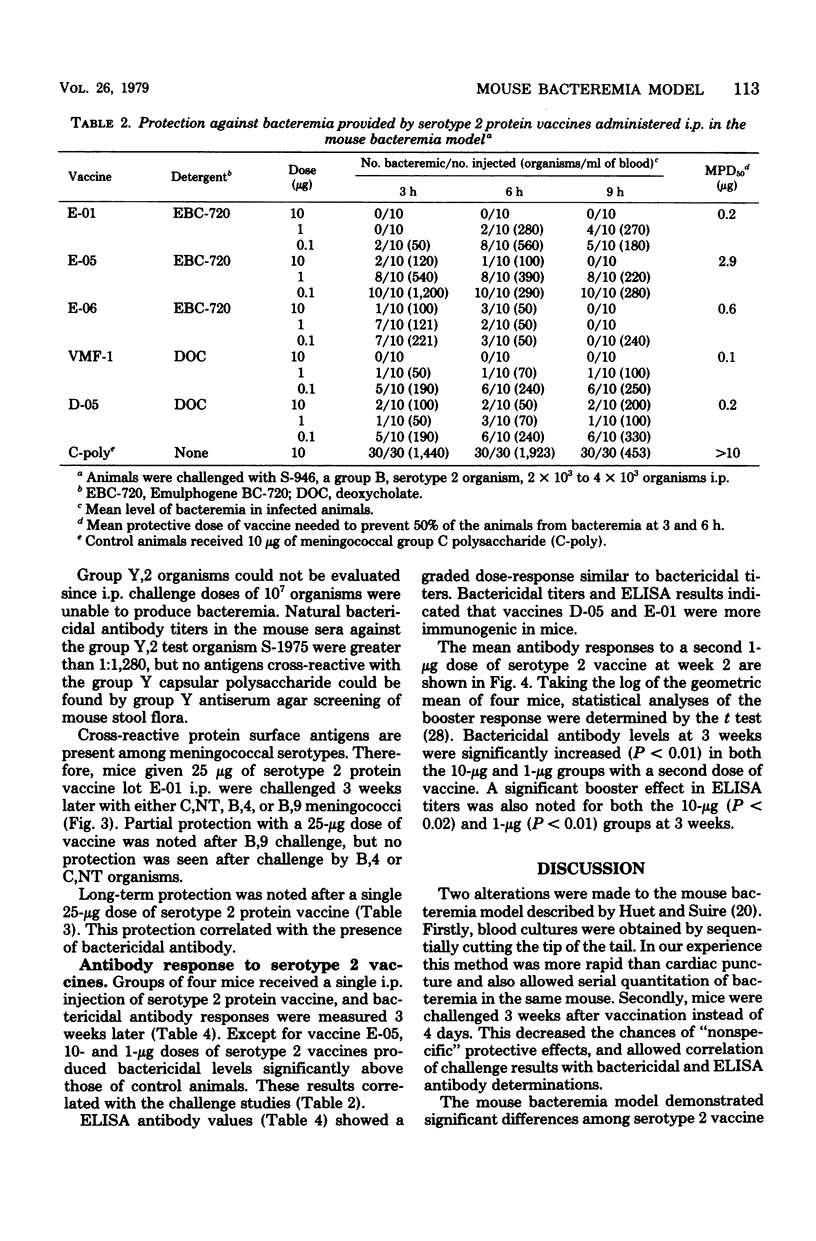
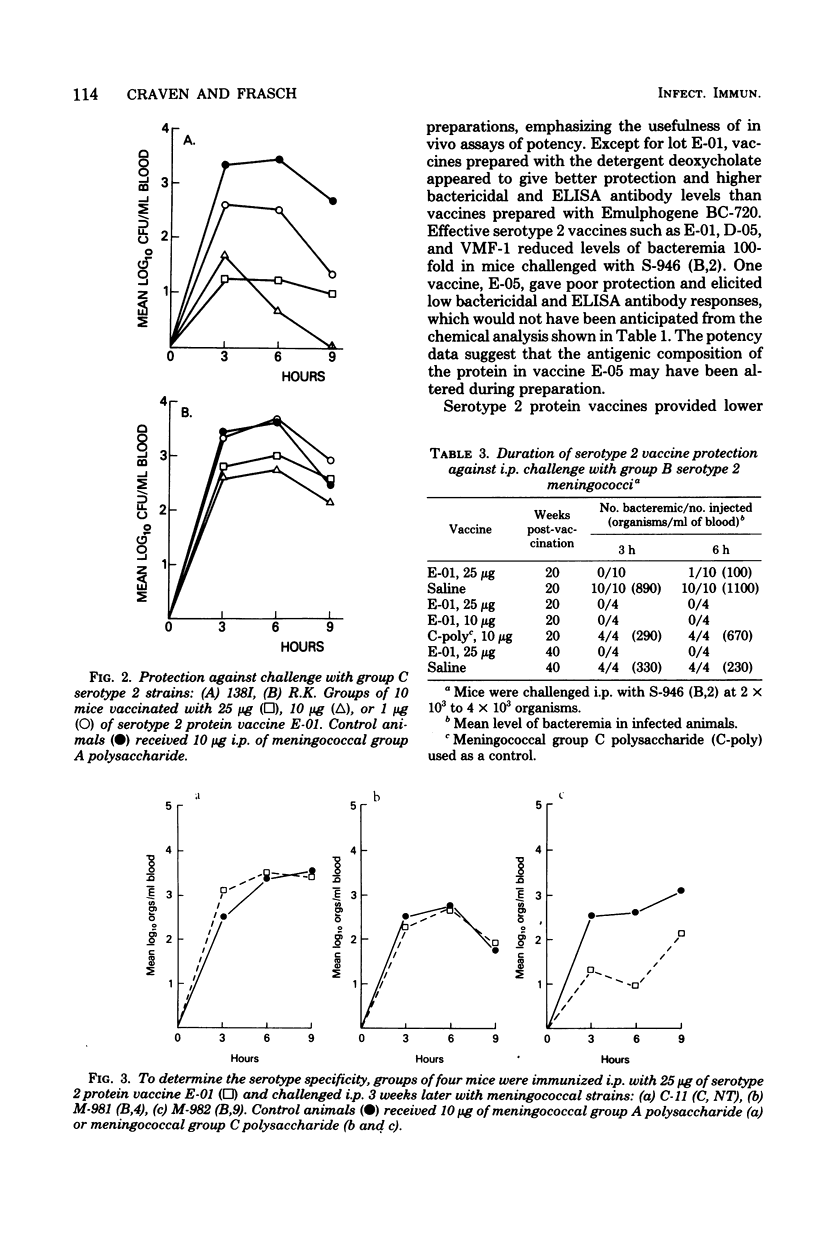
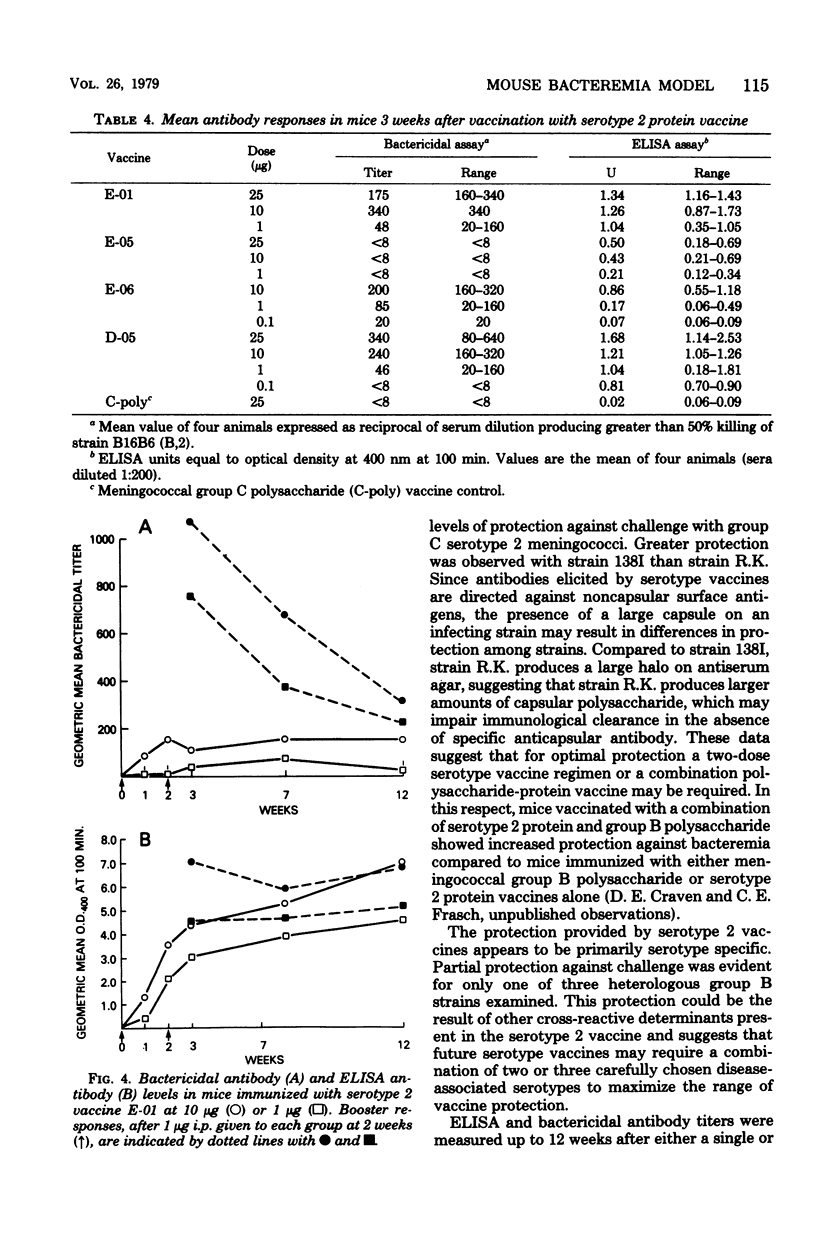
A mouse bacteremia model was used to evaluate the immunogenicity and protection against challenge provided by five different meningococcal serotype 2 vaccines. Mice vaccinated with serotype 2 protein vaccines had levels of bacteremia reduced by at least 100-fold after challenge with group B serotype 2 miningococci. Mice vaccinated with serotype 2 protein vaccine and challenged with group C serotype 2 meningococci showed 10-fold or less reduction in bacteremia. Vaccines were primarily serotype specific since no increase in protection was observed after challenge with either group B serotype 4 or group C nontypable meningococci. Serotype 2 antibody levels, measured by the bactericidal assay and the enzyme-linked immunosorbent assay 3 weeks after immunization, demonstrated a graded dose-response which correlated with protection up to 40 weeks after vaccination with a single 10- or 25-micrograms dose of serotype 2 protein vaccine. A 1-microgram booster dose of serotype 2 vaccine, given 2 weeks after primary immunization, significantly increased both bactericidal (P less than 0.01) and enzyme-linked immunosorbent assay (P less than 0.02) titers. The data obtained from the mouse bacteremia model indicate that serotype 2 protein vaccines are stable and immunogenic, and protect mice against challenge with group B serotype 2 meningococci.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arko R. J. An immunologic model in laboratory animals for the study of Neisseria gonorrhoeae. J Infect Dis. 1974 Apr;129(4):451–455. doi: 10.1093/infdis/129.4.451. [DOI] [PubMed] [Google Scholar]
- Calver G. A., Kenny C. P., Lavergne G. Iron as a replacement for mucin in the establishment of meningococcal infection in mice. Can J Microbiol. 1976 Jun;22(6):832–838. doi: 10.1139/m76-120. [DOI] [PubMed] [Google Scholar]
- Craven D. E., Frasch C. E., Robbins J. B., Feldman H. A. Serogroup identification of Neisseria meningitidis: comparison of an antiserum agar method with bacterial slide agglutination. J Clin Microbiol. 1978 May;7(5):410–414. doi: 10.1128/jcm.7.5.410-414.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Frasch C. E., Chapman S. S. Classification of Neisseria meningitidis group B into distinct serotypes. 3. Application of a new bactericidal-inhibition technique to distribution of serotypes among cases and carriers. J Infect Dis. 1973 Feb;127(2):149–154. doi: 10.1093/infdis/127.2.149. [DOI] [PubMed] [Google Scholar]
- Frasch C. E., Chapman S. S. Classification of Neisseria meningitidis group B into distinct serotypes. II. Extraction of type-specific antigens for serotyping by precipitin techniques. Infect Immun. 1972 Aug;6(2):127–133. doi: 10.1128/iai.6.2.127-133.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Gotschlich E. C. An outer membrane protein of Neisseria meningitidis group B responsible for serotype specificity. J Exp Med. 1974 Jul 1;140(1):87–104. doi: 10.1084/jem.140.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Parkes L., McNelis R. M., Gotschlich E. C. Protection against group B meningococcal disease. I. Comparison of group-specific and type-specific protection in the chick embryo model. J Exp Med. 1976 Aug 1;144(2):319–329. doi: 10.1084/jem.144.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Robbins J. D. Protection against group B meningococcal disease. II. Infection and resulting immunity in a guinea pig model. J Exp Med. 1978 Mar 1;147(3):619–628. doi: 10.1084/jem.147.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Robbins J. D. Protection against group B meningococcal disease. III. Immunogenicity of serotype 2 vaccines and specificity of protection in a guinea pig model. J Exp Med. 1978 Mar 1;147(3):629–644. doi: 10.1084/jem.147.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E. Role of protein serotype antigens in protection against disease due to Neisseria meningitidis. J Infect Dis. 1977 Aug;136 (Suppl):S84–S90. doi: 10.1093/infdis/136.supplement.s84. [DOI] [PubMed] [Google Scholar]
- Gold R., Lepow M. L., Goldschneider I., Gotschlich E. C. Immune Response of human infants of polysaccharide vaccines of group A and C Neisseria meningitidis. J Infect Dis. 1977 Aug;136 (Suppl):S31–S35. doi: 10.1093/infdis/136.supplement.s31. [DOI] [PubMed] [Google Scholar]
- Gold R., Winklehake J. L., Mars R. S., Artenstein M. S. Identification of an epidemic strain of group C Neisseria meningitidis by bactericidal serotyping. J Infect Dis. 1971 Dec;124(6):593–597. doi: 10.1093/infdis/124.6.593. [DOI] [PubMed] [Google Scholar]
- Gold R., Wyle F. A. New Classification of Neisseria meningitidis by Means of Bactericidal Reactions. Infect Immun. 1970 May;1(5):479–484. doi: 10.1128/iai.1.5.479-484.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969 Jun 1;129(6):1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein H. D., Elfin R. J., Cooper J. F., Seligmann E. B., Jr, Wolff S. M. Further developments of Limulus Amebocyte Lysate test. Bull Parenter Drug Assoc. 1973 May-Jun;27(3):139–148. [PubMed] [Google Scholar]
- Huet M., Suire A. Mise en évidence de la valeur protectrice des vaccins poly-saccharidiques méningococciques par la réduction de la bactéríemie chez la Souris. C R Acad Sci Hebd Seances Acad Sci D. 1976 Sep 13;283(4):421–422. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Munford R. S., Patton C. M., Gorman G. W. Epidemiologic studies of serotype antigens common to groups B and C Neisseria meningitidis. J Infect Dis. 1975 Mar;131(3):286–290. doi: 10.1093/infdis/131.3.286. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltola H., Mäkelä H., Käyhty H., Jousimies H., Herva E., Hällström K., Sivonen A., Renkonen O. V., Pettay O., Karanko V. Clinical efficacy of meningococcus group A capsular polysaccharide vaccine in children three months to five years of age. N Engl J Med. 1977 Sep 29;297(13):686–691. doi: 10.1056/NEJM197709292971302. [DOI] [PubMed] [Google Scholar]
- Ueda K., Diena B. B., Greenberg L. The chick embryo neutralization test in the assay of meningococcal antibody. 1. Infection of the embryo with Neisseria meningitidis. Bull World Health Organ. 1969;40(2):235–240. [PMC free article] [PubMed] [Google Scholar]
- Wyle F. A., Artenstein M. S., Brandt B. L., Tramont E. C., Kasper D. L., Altieri P. L., Berman S. L., Lowenthal J. P. Immunologic response of man to group B meningococcal polysaccharide vaccines. J Infect Dis. 1972 Nov;126(5):514–521. doi: 10.1093/infdis/126.5.514. [DOI] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E., Altieri P., Berman S., Lowenthal J., Artenstein M. S. Safety and immunogenicity of a Neisseria meningitidis type 2 protein vaccine in animals and humans. J Infect Dis. 1978 Jun;137(6):728–739. doi: 10.1093/infdis/137.6.728. [DOI] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E., Griffiss J. M., Altieri P., Berman S. Complex of meningococcal group B polysaccharide and type 2 outer membrane protein immunogenic in man. J Clin Invest. 1979 May;63(5):836–848. doi: 10.1172/JCI109383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Pennington C. L., Artenstein M. S. Human antibody response to three meningococcal outer membrane antigens: comparison by specific hemagglutination assays. Infect Immun. 1974 Nov;10(5):975–984. doi: 10.1128/iai.10.5.975-984.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



