Abstract
Background
Porcine islet xenotransplantation is considered a potential cell-based therapy for type 1 diabetes. It is currently being evaluated in diabetic nonhuman primates (NHP) to assess safety and efficacy of the islet product. However, due to a variety of distinct differences between the respective species, including the insulin secretory characteristics of islets, the suitability and predictive value of the preclinical model in the extrapolation to the clinical setting remains a critical issue.
Methods
Islets isolated from human (n=3), NHP (n=2), adult pig (AP, n=3) and juvenile pig (JP, n=3) pancreata were perifused with medium at basal glucose (2.5mM) followed by high glucose (16.7mM) concentrations. The total glucose-stimulated insulin secretion (GSIS) was calculated from generated insulin secretion profiles.
Results
NHP islets exhibited GSIS 3-fold higher than human islets, while AP and JP islets exhibited GSIS 1/3 and 1/16 of human islets, respectively. The insulin content of NHP and AP islets was similar to that of human islets, whereas that of JP islets was 1/3 of human islets.
Conclusion
Despite the fact that human, NHP, and AP islets contain similar amounts of insulin, the much higher GSIS for NHP islets than for human, AP and JP islets suggests the need for increased dosing of islets from JP and AP in pig-to-NHP transplantation which may be substantially higher than that required for humans. Finally, porcine islet xenotransplantation to humans may require significantly higher dosing given the lower GSIS of AP islets compared to human islets.
Keywords: human, islets, nonhuman primate, nutrient-stimulated insulin secretion, pig, species incompatibility
Introduction
The potential of islet cell replacement therapies to cure type1 diabetes has created a considerable demand for transplantable islet preparations. There are over 3 million Americans with type 1diabetes and only about 4,000 human donor pancreata available each year for organ and islet transplantation [1]. Of these, about 20–30% (or 1,300) of the available pancreata are accepted and utilized for pancreas transplantation [2,3]. This low acceptance rate is related to the (poor) quality of most donor organs. Taking into account the failures in islet transplantation, and the number of donor organs per transplant, annually about 50 patients receive an islet transplant [4]. It is thus clear that alternate sources of islets are needed to supply a constant stream of therapeutic cells.
Porcine-derived islets are being investigated for their safety and possible potency and function in human xenotransplantation. Porcine islet xenotransplantation perhaps offers the most immediate solution due to natural similarities in pancreas size, islet numbers, and insulin protein between pig and human species. Regarding preclinical evidence for a therapeutic benefit, a number of groups have reported long-term diabetes reversal and porcine islet graft function in diabetic non-human primate (NHP) recipients of porcine islets [5–12].
Diabetic NHPs represent a good model for studying the immunological aspects associated with islet xenotransplantation. However, NHPs present key metabolic differences when compared to pigs which can significantly influence the outcome of pig-to-NHP islet transplants. Studies of glucose metabolism in cynomolgus (Macaca fascicularis) monkeys indicate that monkeys have high circulating C-peptide and insulin levels associated with low serum glucose levels [13,14]. In response to glucose stimulation in vitro, NHP islets are also able to quickly produce high amounts of insulin [15]. In contrast with NHPs, normal serum C-peptide and insulin levels are lower in the pig, and pig islets are widely known to have a poor insulin secretory response to glucose stimulation in vitro [15]. While porcine and human islets display similar glucose clearance capacities, the glucose clearance in NHPs occurs at a much faster rate, which is supported by a higher basal serum insulin and lower basal serum glucose level. Due to this seemingly higher insulin demand in NHPs, further study into the metabolic characteristics of pig, NHP, and human islets is needed to determine how to best design preclinical islet xenotransplantation studies.
The present study examines the insulin secretion rate of equal numbers of porcine, NHP, and human islets after nutrient stimulation by glucose, a mixture of glutamine and leucine, and potassium chloride (KCl) in a dynamic perifusion system. The results are normalized to the DNA content of the islets used to account for possible differences in islet size and cell packing density within islets [16].
Materials and Methods
Islet source
Human islets were obtained through the Integrated Islet Distribution Program (n =1) or from islet isolations of local clinical grade pancreata using the standard protocol for the Clinical Islet Transplant Consortium (n=2) [17]. Donors were 39, 24 and 46 years old with a body mass index of 26.9, 21.9 and 29.2, respectively. Pancreata were obtained from two male rhesus monkeys aged 7.3 and 8.1 years weighing 10.4 and 13.0 kg, respectively: these pancreata were subjected to islet cell preparation using a modified Ricordi method [18]. Pancreata were obtained from three Landrace sows aged 3.2, 3.5, and 6.4 years, respectively, and weighing 243, 246, and 255 kg, respectively. Islets were isolated from these pancreata (designated AP) using the standard protocol used at the Schulze Diabetes Institute at the University of Minnesota [5]. Juvenile porcine (JP) islets were obtained from donors less than 3 months of age from Cell and Tissue Systems, South Carolina. A total of of 3 juvenile islet products were used, either freshly prepared after procurement (n=1), or after 24-hour hypothermic machine perfusion preservation (n=2) [19]. This difference in preservation had no apparent effect on the outcome of isolation, so that data were combined.
Media and culture
Following purification, human and NHP islets were cultured in CMRL medium (Mediatech, Manassas, VA), supplemented with heparin (10U/ml, APP Pharmaceuticals, LLC, Schaumburg, IL); for human islets supplementation was performed with human albumin (25% HSA, 10% v/v, Grifols, Los Angeles, CA), and for NHP islets supplementation was done with heat-inactivated fetal bovine serum (10% v/v, Mediatech). Culture was done in gas-permeable culture devices (GRex100, Wilson-Wolf Manufacturing, New Brighton, MN) or T-flasks, in humidified air with 5% CO2, 95% air for 1 day at 37°C and thereafter at 22°C. AP and JP islets were cultured in ME199 culture medium (Mediatech) with 10% heat-inactivated porcine serum (Gibco, Auckland, NZ), L-glutamine (Mediatech) and heparin (10U/ml, APP Pharmaceuticals) in GRex100 at 37°C, in humidified air without CO2 for 6–9 days.
Islet assessments
Macroscopic assessment was performed on a sample of islets stained with dithizone and viewed under the microscope as described previously for visual counting, purity and quality score assessment. The purity of the three human preparations ranged between 70 and 90%, for the two NHP preparations purity ranged between 75 and 90%, for the three AP preparations it was 90% and for the four JP preparations it ranged between 45 and 85% (Table 1).
Table 1.
Characteristics of islet preparations and outcome of dynamic insulin function assay
| NHP n=2 |
Human n=3 |
AP n=3 |
JP n=3 |
|
|---|---|---|---|---|
| Characteristics of islet preparations | ||||
| Purity (%, dithizone stain) |
83 ±11 | 85 ±13 | 90 ±0 | 63 ±19 |
| OCR/DNA (nmol/min.mgDNA) |
221 ±57 | 121 ±12 | 265 ±25 | 188 ±3 |
| Total insulin (pg/ng DNA) |
5.9 ±0.9 | 7.8 ±3.9 | 9.2 ±3.4 | 1.5 ±0.8 |
| Insulin secretory function (AUC , pg/ml.min.ngDNA) | ||||
| Glucose 1st phase | 77.8 ±2.6 | 46.5 ±9.8 | 15.1 ±10.5 | 1.9 ±0.6 |
| Glucose 2nd phase | 258 ±5 | 301 ±76 | 106 ±67 | 9.5 ±3.8 |
| Glutamine and Leucine | 55.4 ±19.5 | 30.5 ±5.4 | 15.1 ±6.3 | 2.3 ±1.5 |
| KCL | 52.4 ±9.9 | 49.1 ±20.8 | 11.9 ±6.9 | 0.6 ±0.3 |
| Ratio of phases in dynamic insulin secretion | ||||
| Glucose 1st+2nd phase/ glutamine and Leucine |
6.5 ±2.4 | 11.3 ±1.3 | 7.7 ±2.1 | 6.0 ±2.3 |
| Glucose 1st+2nd phase/ KCl | 6.5 ±1.4 | 7.9 ±4.0 | 10.4 ±2.7 | 19.1 ±3.7 |
| Glutamine and leucine/ KCL | 1.04 ±0.18 | 0.71 ±0.38 | 1.35 ±0.24 | 3.47 ±1.23 |
Data are presented as arithmetic means values ±SD
Tissue quantity was assessed using the DNA assay and tissue viability was measured by oxygen consumption rate (OCR) as described previously [16,20–23]; samples for OCR were normalized to DNA (OCR/DNA).
Islet products were assayed in vitro for dynamic insulin secretory function (GSIS) using various stimulating factors with the following rationale: at first glucose because this is the physiologic initiator of insulin secretion by pancreatic β-cells; then glucose with a combination of amino acids leucine and glutamine because amino acids play a role in glucose homeostatis and insulin release in vivo; and finally potassium chloride (KCl) because this acts as non-specific membrane stimulator. The method used is described elsewhere [24], with a modification to allow a larger number of islets (approximately 75–80IEs for human islets, and 300IEs for NHP, AP and JP islets). The base media used was Krebs Ringer’s bicarbonate. The basal glucose concentration was maintained at 2.5mM, with a glucose stimulus consisting of a single-step increase in glucose concentration to 16.7mM. After reduction to 2.5 mM glucose, a second stimulation was performed with a combination of 2.5mM glucose with amino acids 10mM leucine and 4mM glutamine. Finally, after reduction to 2.5mM glucose, a final stimulation was done with 30mM KCl alone. The supernatant was assessed for insulin concentration using an enzyme-linked immunosorbent assay (ELISA) for human insulin (80-INSHU-E01, ALPCO Diagnostics, Salem, NH) for human and NHP islets or porcine insulin (80-INSPO-E01, ALPCO Diagnostics) for porcine islets. Most measurements were performed in triplicate. Insulin levels in the supernatant were normalized to DNA. The area under the curve (AUC) was calculated using the linear trapezoidal rule. Insulin content measurement was determined by ELISA.
Statistical analysis
Data are presented as mean ±SD. Statistical analysis for differences between groups was analyzed using the parametric t-test. Differences were considered statistically significant in case of p<0.05.
Results
The reproducibility in the yield of islet preparations was acceptable, enabling the evaluation of a small number of preparations per group. The same was observed regarding the quality assessments. Fractional viability (±SD) measured by OCR/DNA in the four groups was 121±12 nmol/min.mg DNA in the human islet preparations, 188±3 nmol/min.mg DNA in the JP preparations, 221±57 nmol/min.mg DNA in the NHP preparations, and 265±25 nmol/min.mg DNA in the AP islet preparations. The difference between individual groups was statistically significant, except for the difference between NHP and JP islet preparations, and between NHP and AP islet preparations (Table 1).
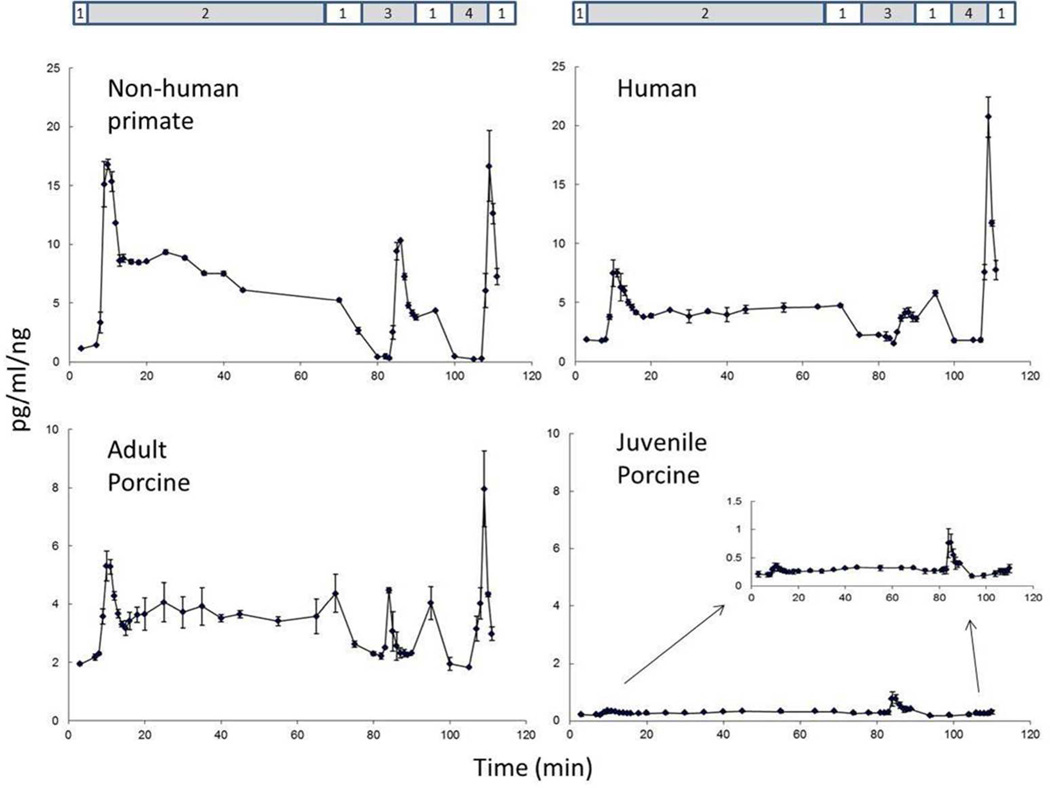
The results on the insulin secretory function showed such a low variation in each individual group (Table 1), that the combined evaluation of a small number of samples per group gave informative data. A representative graph of the profiles of insulin secretion upon nutrient stimulation for each species is shown in Fig. 1. The first stimulation at 16.7mM insulin induced a biphasic response, a first immediate and high response peaking at around 9–10 min followed by a lower response peaking at around 25 min. This was most clear for NHP and AP islets, but also apparent for human islets. The combination of leucine and glutamine stimulation resulted in a quick insulin secretory response, which was in the same range or somewhat lower than the first peak after glucose stimulation. Finally, KCl stimulation resulted in a quick insulin secretory response that peaked at a higher level than any of the preceding responses.
Fig 1.
Representative glucose-stimulated insulin secretion profiles in islet preparations from nonhuman primates, human, adult porcine and juvenile porcine pancreata. Stimulation is done in successive order with (1) 2.5mM glucose (basal), (2) 16.7mM glucose (1st and 2nd phase), (3) leucine+gleucine, and (4) potassium chloride.
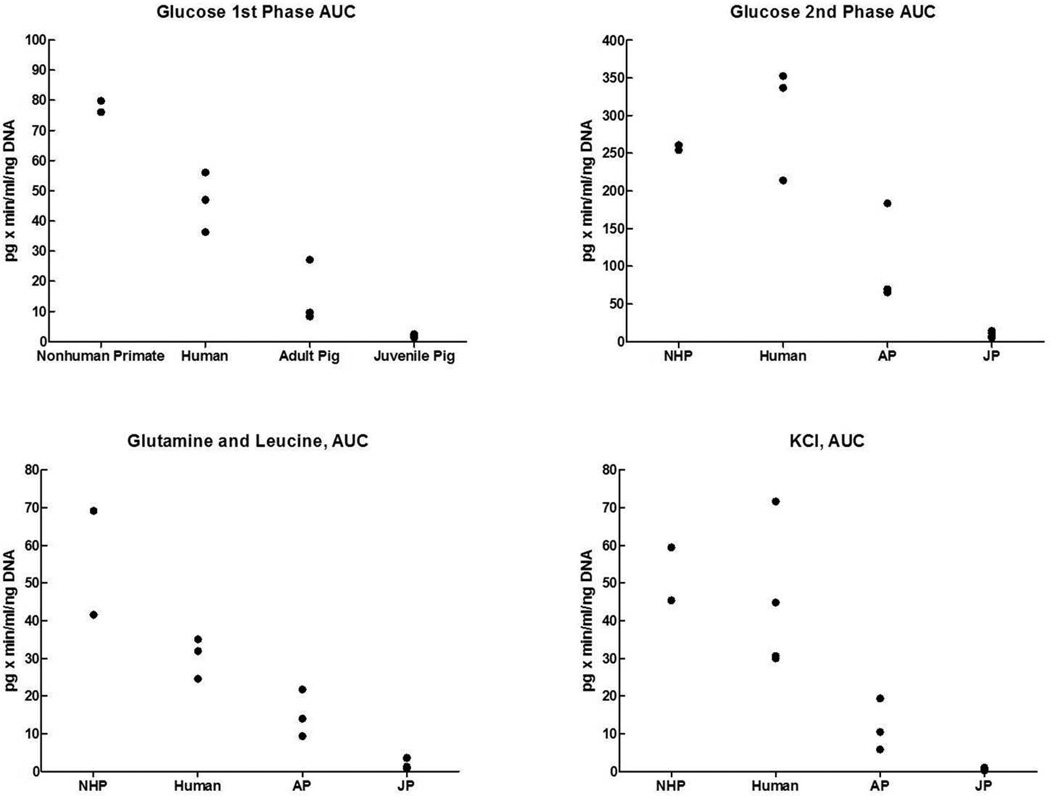
The graphs also show a relevant difference between the four species. The highest insulin secretory response was seen for islets from NHP, followed by the response of human islets. Islets from AP islets showed a much lower response, while the response from JP islets was very low. These differences between the groups are shown for the AUC values of all individual islet preparations in Fig. 2. Highest values are evident for the NHP islets, followed by human islets: for the 2nd phase glucose stimulation and KCl stimulation values for NHP islets were similar to those of human islets (Table 1). The outcome was lower for AP islets and lowest for JP islets. In statistical analysis the difference in AUC values between JP islets and each of the other groups was significant. The difference between AP islets and human islets reached statistical significance for AUC values of the glucose 1st stimulation phase and glutamine/leucine stimulation, but not for AUC values of glucose 1st stimulation phase and KCl. The difference between NHP and human islets reached statistical significance for the AUC values of glucose 1st stimulation phase but not for any other AUC value.
Fig. 2.
Responses of islets preparations from nonhuman primates, human, adult pig and juvenile pig pancreata during the different phases of the glucose-stimulated insulin secretion assay. AUC, area-under-the curve.
The data were also expressed in ratios of glucose (1st and 2nd phase combined) over glutamine/leucine stimulation and KCl stimulation, and for the ratio between glutamine/leucine stimulation and KCl stimulation (Table 1). Regarding the glucose over glutamine/leucine stimulation ratio, the difference between AP and NHP islets was statistically significant, and regarding the glucose over KCl or glucose or glutamine/leucine over KCl ratio the difference between JP islets and all other groups was statistically significant. Considering the very low AUC values of JP islets, this latter observation might have little physiological relevance.
There were some differences in purity between the various islet preparations, but differences between groups did not reach statistical significance. This aside, we repeated the statistical evaluation after correction of the AUC values for purity of the islets. The outcome of this evaluation was the same as that of evaluation of AUC values without this correction for purity: this included the significant differences in insulin secretory responses. This indicates that there was no unequivocal effect of purity on the difference in outcome between the various groups. It should be noted that lowest purity of islet preparations was observed for the preparations from juvenile pigs (63%, compared with purity between 83 and 90% for preparations from the other three sources).
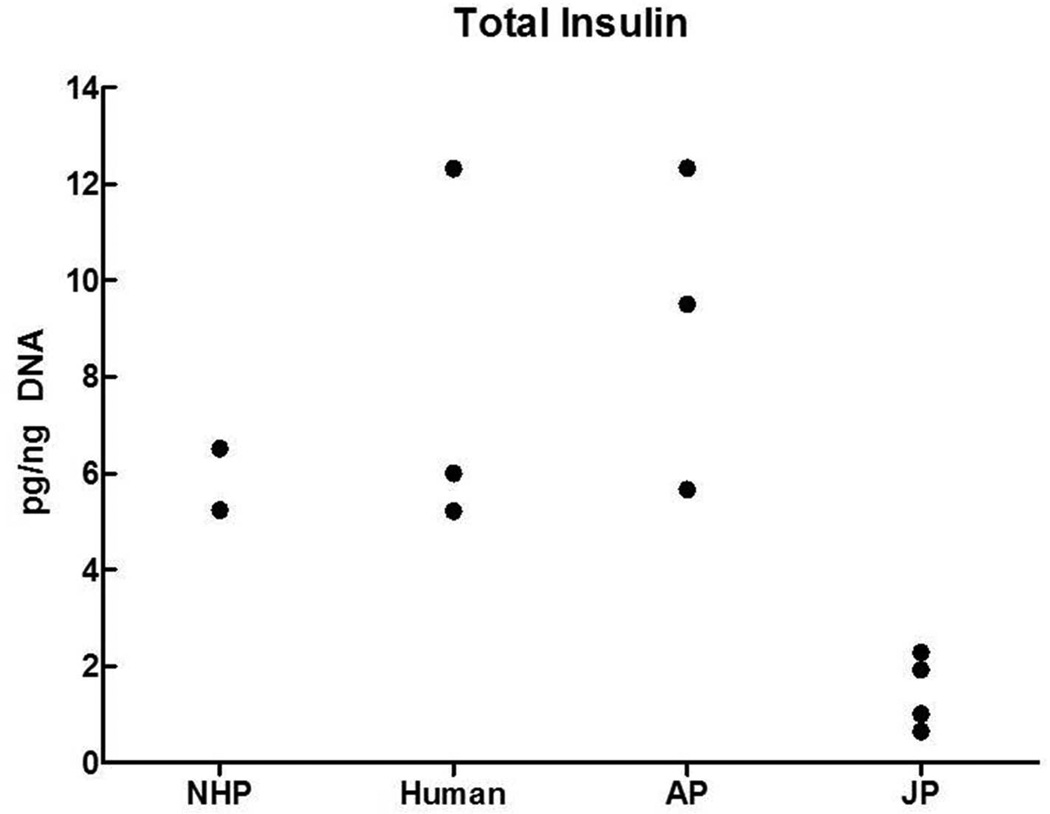
Finally, the data on insulin content of the various preparations are presented in Fig. 3. The levels were in the same range for islets from NHP, human and AP pancreata (differences were not statistically significant), but significantly lower in islet preparations from JP pancreata (P<0.02 or less).
Fig. 3.
Insulin content of islet preparations from nonhuman primates (NHP), human, adult pig (AP) and juvenile pig (JP) pancreata.
Discussion
Despite the fact that AP and NHP islets contain similar amounts of insulin, the GSIS for NHP islets is much higher than that of AP and JP islets. These differences in vitro agree with published data regarding in vivo incompatibilities between species in insulin secretion after stimulation with either glucose or arginine [13,14]: we have summarized these data in the Introduction and have described these in detail elsewhere [14]. This may indicate the need for increased numbers of islets from JP and AP in pig-to-NHP transplantation. Since GSIS from NHP islets is 2-fold higher than that of human islets, as evidenced by the AUC comparison of the 1st phase of glucose stimulation, the islet dosing requirement in NHPs may be substantially higher than what is required for humans. The differences in insulin secretory capacity between porcine and NHP islets may explain in part the higher porcine islet dose requirement for diabetes reversal in NHP (25,000 IE/Kg BW) [5] as opposed to 5,000–10,000 IE/Kg BW for islet allotransplantation in either human or NHP [14,18]. The very low insulin secretory activity of JP islets could in part be ascribed to the lower purity of the preparations (63% versus 83–90%), but even more by the more immature status of pancreatic β-cells in juvenile pigs.
These results are also relevant in the area of tissue engineering for islet xenotransplantation where current research is being pursued towards constructs which would allow for islets transplanted in biocompatible, immunoisolating implantable devices [25,26]. The size, as determined by the surface area of the device needed for efficacy, is a function of the number of islets placed in the device (IE/cm2), which in turn is strongly dependent on the oxygen consumption rate and insulin secretory capacity of the islets [25–30]. Here we report that porcine islets secrete half as much insulin with twice as much demand for oxygen (OCR) than human islets – this difference is more pronounced when compared with NHP islets. These differences are expected to have a profound impact on the design of devices for tissue engineering applications such that substantially larger devices will be required, given that everything else is constant, if porcine (xeno) instead of allo islets were to be used in humans or NHPs, as well as the choice of the animal model used.
Acknowledgement
The authors would like to acknowledge the Isolation core at the Schulze Diabetes Institute at the University of Minnesota for the technical expertise and support in isolations of islets from different species.
Abbreviations
- AP
adult pig
- AUC
area under the curve
- ELISA
enzyme-linked immunosorbent assay
- GSIS
glucose-stimulated insulin secretion
- IE
islet equivalent
- JP
juvenile pig
- NHP
nonhuman primate
- OCR
oxygen consumption rate
Footnotes
Conflict of interest: Michael J Taylor PhD, is Vice President Research and Development of Cell & Tissue Systems, Inc, a company with the mission “Preservation of biological materials for restoration of patient health".
References
- 1.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) OPTN / SRTR 2010 Annual Data Report. [Accessed 29 June 12];Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation. 2011 Available at http://www.srtr.org/annual_reports/2010.
- 2.Tuttle-Newhall JE, Krishnan SM, Levy MF, et al. Organ donation and utilization in the United States, 1998–2007. Am J Transplant. 2009;9:879–893. doi: 10.1111/j.1600-6143.2009.02565.x. 2009. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe RA, Merion RM, Roys EC, Port FK. Trends in organ donation and transplantation in the United States, 1998–2007. Am J Transplant. 2009;9:869–878. doi: 10.1111/j.1600-6143.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- 4.Citr Annual Report. 2011 Dec 20; https://web.emmes.com/study/isl/reports/reports.htm.
- 5.Hering BJ, Wijkstrom M, Graham ML, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12:301–303. doi: 10.1038/nm1369. [DOI] [PubMed] [Google Scholar]
- 6.Cardona K, Korbutt GS, Milas Z, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12:304–306. doi: 10.1038/nm1375. [DOI] [PubMed] [Google Scholar]
- 7.Thompson P, Cardona K, Russell M, et al. CD40-specific costimulation blockade enhances neonatal porcine islet survival in nonhuman primates. Am J Transplant. 2011;11:947–957. doi: 10.1111/j.1600-6143.2011.03509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardona K, Milas Z, Strobert E, et al. Engraftment of adult porcine islet xenografts in diabetic nonhuman primates through targeting of costimulation pathways. Am J Transplant. 2007;7:2260–2268. doi: 10.1111/j.1600-6143.2007.01933.x. [DOI] [PubMed] [Google Scholar]
- 9.Thompson P, Badell IR, Lowe M, et al. Islet xenotranplantation using Gal-deficient neonatal donors improves engraftment and function. Am J Transplant. 2011;11:2593–2602. doi: 10.1111/j.1600-6143.2011.03720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van der Windt DJ, Bottino R, Casu A, et al. Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. Am J Transplant. 2009;9:2716–2726. doi: 10.1111/j.1600-6143.2009.02850.x. [DOI] [PubMed] [Google Scholar]
- 11.Hecht G, Eventov-Friedman S, Rosen C, et al. Embryonic pig pancreatic tissue for the treatment of diabetes in a nonhuman primate model. Proc Natl Acad Sci USA. 2009;106:8659–8664. doi: 10.1073/pnas.0812253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dufrane D, Goebbels RM, Gianello P. Alginate macroencapsulation of pig islets allows correction of streptozotocin-induced diabetes in primates up to 6 months without immunosuppression. Transplantation. 2010;90:1054–1062. doi: 10.1097/TP.0b013e3181f6e267. [DOI] [PubMed] [Google Scholar]
- 13.Casu A, Bottino R, Balamurugan AN, et al. Metabolic aspects of pig-to-monkey (Macaca fascicularis) islet transplantation: implications for translation into clinical practice. Diabetologia. 2008;51:120–129. doi: 10.1007/s00125-007-0844-4. [DOI] [PubMed] [Google Scholar]
- 14.Graham ML, Bellin MD, Papas KK, Hering BJ, Schuurman H-J. Species incompatibilities in the pig-to-macaque islet xenotransplant model affect transplant outcome: a comparison with allotransplantation. Xenotransplantation. 2011;18:328–342. doi: 10.1111/j.1399-3089.2011.00676.x. [DOI] [PubMed] [Google Scholar]
- 15.Balamurugan AN, Ramakrishna B, Gunasekaran S. Insulin secretory characteristics of monkey pancreatic islets: a simple method of islet isolation and the effect of various density gradients on separation. Diabetes Res Clin Pract. 2004;66:13–21. doi: 10.1016/j.diabres.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Colton CK, Papas KK, Pisania A, et al. Characterization of islet preparations. In: Halberstadt C, Emerich DF, editors. Cell transplantation from laboratory to clinic. New York: Elsevier; 2007. pp. 85–132. [Google Scholar]
- 17.Purified Human Pancreatic Islets Master Production Batch Record (Product Code Phpi-A-01) (Cit Protocols 03 – 07) [Accessed September 13, 2012]; http://www.isletstudy.org/CITDocs/SOP%203101,%20B01,%20MPBR%20v05,%20October%2028,%202010.pdf.
- 18.Wijkstrom M, Kenyon NS, Kirchhof N, et al. Islet allograft survival in nonhuman primates immunosuppressed with basiliximab, RAD, and FTY720. Transplantation. 2004;77:827–835. doi: 10.1097/01.tp.0000116390.76425.20. [DOI] [PubMed] [Google Scholar]
- 19.Taylor MJ, Baicu S, Greene E, Vazquez A, Brassil J. Islet Isolation from juvenile porcine pancreas after 24-h hypothermic machine perfusion preservation. Cell Transplant. 2010;19:613–628. doi: 10.3727/096368910X486316. [DOI] [PubMed] [Google Scholar]
- 20.Papas KK, Pisania A, WU H, Weir GC, Colton Ck. A stirred microchamber for oxygen consumption rate measurements with pancreatic islets. Biotechnol Bioeng. 2007;98:1071–1082. doi: 10.1002/bit.21486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papas KK, Colton CK, Nelson RA, et al. Human islet oxygen consumption rate and DNA measurements predict diabetes reversal in nude mice. Am J Transplant. 2007;7:707–713. doi: 10.1111/j.1600-6143.2006.01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papas KK, Suszynski TM, Colton CK. Islet assessment for transplantation. Curr Opin Organ Transplant. 2009;14:674–682. doi: 10.1097/MOT.0b013e328332a489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papas KK, Colton CK, Qipo A, et al. Prediction of marginal mass required for successful islet transplantation. J Invest Surg. 2010;23:28–34. doi: 10.3109/08941930903410825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pongratz RL, Kibbey RG, Kirkpatrick CL, et al. Mitochondrial dysfunction contributes to impaired insulin secretion in INS-1 cells with dominant-negative mutations of HNF-1alpha and in HNF-1alpha-deficient islets. J Biol Chem. 2009;284:16808–16821. doi: 10.1074/jbc.M807723200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colton CK. Implantable biohybrid artificial organs. Cell Transplant. 1995;4:415–436. doi: 10.1177/096368979500400413. [DOI] [PubMed] [Google Scholar]
- 26.Ludwig B, Rotem A, Schmid J, et al. Improvement of islet function in a bioartificial pancreas by enhanced oxygen supply and growth hormone releasing hormone agonist. Proc Natl Acad Sci USA. 2012;109:5022–5027. doi: 10.1073/pnas.1201868109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Avgoustiniatos ES, Colton CK. Effect of external oxygen mass transfer resistances on viability of immunoisolated tissue. Ann NY Acad Sci. 1997;831:145–167. doi: 10.1111/j.1749-6632.1997.tb52192.x. [DOI] [PubMed] [Google Scholar]
- 28.Wu H, Avgoustiniatos ES, Swette L, et al. In situ electrochemical oxygen generation with an immunoisolation device. Ann NY Acad Sci. 1999;875:105–125. doi: 10.1111/j.1749-6632.1999.tb08497.x. [DOI] [PubMed] [Google Scholar]
- 29.Avgoustiniatos ES, Colton CK. Design considerations in immunoisolation. In: Lanza RP, Langer R, Chick WL, Landes RG, editors. Principles of Tissue Engineering. San Diego: Academic Press; 1997. pp. 333–346. [Google Scholar]
- 30.Avgoustiniatos ES, Wu H, Colton CK. Engineering in immunoisolation device development. In: Lanza RP, Langer R, Chick WL, editors. Principles of Tissue Engineering. 2nd Ed. San Diego: Academic Press; 2000. pp. 331–350. [Google Scholar]