Abstract
Background
Rapid local drug clearance of antimicrobials is a major drawback for the treatment of chronic periodontitis. In the study reported here, minocycline-loaded poly(ethylene glycol)-poly(lactic acid) nanoparticles were prepared and administered locally for long drug retention and enhanced treatment of periodontitis in dogs.
Methods
Biodegradable poly(ethylene glycol)-poly(lactic acid) was synthesized to prepare nanoparticles using an emulsion/solvent evaporation technique. The particle size and zeta potential of the minocycline-loaded nanoparticles (MIN-NPs) were determined by dynamic light scattering and the morphology of the nanoparticles was observed by transmission electron microscopy. The in vitro release of minocycline from MIN-NPs and in vivo pharmacokinetics of minocycline in gingival crevice fluid, after local administration of MIN-NPs in the periodontal pockets of beagle dogs with periodontitis, were investigated. The anti-periodontitis effects of MIN-NPs on periodontitis-bearing dogs were finally evaluated.
Results
Transmission electron microscopy examination and dynamic light scattering results revealed that the MIN-NPs had a round shape, with a mean diameter around 100 nm. The in vitro release of minocycline from MIN-NPs showed a remarkably sustained releasing characteristic. After local administration of the MIN-NPs, minocycline concentration in gingival crevice fluid decreased slowly and retained an effective drug concentration for a longer time (12 days) than Periocline®. Anti-periodontitis effects demonstrated that MIN-NPs could significantly decrease symptoms of periodontitis compared with Periocline and minocycline solution. These findings suggest that MIN-NPs might have great potential in the treatment of periodontitis.
Keywords: minocycline, nanoparticles, periodontitis, local delivery
Introduction
“Periodontitis” is a common chronic disease that leads to the destruction of tooth-supporting tissues, absorption of alveolar bone, and, finally, tooth loss.1,2 Considerable research has focused on the etiology of human periodontal disease,3 and it has been generally accepted that chronic periodontitis is induced by microorganisms. Periodontal pockets provide a moist, warm, nutritious, and anaerobic environment that profits microbial colonization and multiplication.4 Previous research demonstrated that only 10 to 30 bacteria species, mainly Gram-negative anaerobic bacteria, live here,5 but it has now been found that approximately 500 bacterial taxa sojourn here.4 The amounts and species of the bacteria are variable in different parts of the biofilm and depend on the effectiveness of oral hygiene procedures, depth of pocket, flow of gingival crevice fluid (GCF), type of interacting microbes, and so on.6,7 A recent study has indicated that periodontal disease not only affects human oral health but may also induce several systemic diseases.8
The fundamental treatment of periodontitis is to reduce the pathogenic bacteria by instrumental debridement,9 such as ultrasonic scaling and root planning. However, instrumental debridement cannot completely remove pathogens that have invaded soft tissue and some anatomically inaccessible areas such as furcation areas and root depressions.10 In view of this, antimicrobial therapy by antibiotics is often executed as adjuvant treatment soon after instrumental debridement.11 In order to achieve effective inhibitory concentration in the GCF of the periodontal pockets, large doses must be taken, which may lead to associated side effects and resistance of antibiotics.12 Therefore, localized drug-delivery systems containing antibacterial agents have been developed, including fibers, strips, films, implants, gels, and so forth, and now many products are commercially available.13–15 These are administered in the periodontal pocket after ultrasonic scaling and root planning to enhance the local effect. Compared with the conventional systemic administration of antibiotics, these new formulations reduce the frequency of administration and keep the drug concentrations within the desired range. Further, the side effects are reduced significantly.3 However, some issues still exist. The vehicles of fibers and strips must be removed after complete release of the drug by professionals, and the wound is not conducive to recovery.16 The application of gels is more convenient than fibers and strips, but the issues are burst release and the rapid local drug clearance of antimicrobials.3
Biodegradable nanoparticles (NPs) have been developed rapidly over the past few years since they have shown great potential in drug delivery.17 NPs have more advantages than other new formulations and many of the already-mentioned problems can be avoided. NPs can offer sustained release of drugs and have the ability to adsorb onto polymer gels and act as a glue to bond pieces of hydrogels and tissues together.18 Based on these properties, NPs can adhere to diseased tissues longer and maintain local drug concentration for a long time. Due to the small particle size, NPs are able to penetrate into alveolar bone trabeculae, the underlying connective tissue, and even the periodontal pocket areas below the gum,14,19,20 which can also significantly improve the antibacterial effect. Successful periodontal treatment requires a high initial antibiotic concentration followed by the continued release of the drug at a lower concentration, both of which can be realized with NPs.21
“Minocycline” belongs to the tetracycline family, but it has a broader antibacterial spectrum than other tetracyclines and can be applied in the treatment of periodontitis.21,22 It has been demonstrated that minocycline can affect the immune response caused by cell factors and have a beneficial effect on periodontal health.23 In this study, minocycline was encapsulated in poly(ethylene glycol)-poly(lactic acid) (PEG-PLA) NPs, which are metabolized to nontoxic CO2 and H2O in the body,24 and the pharmacokinetics and pharmacodynamics of this formulation were investigated through a periodontitis model in beagle dogs.
Materials and methods
Materials
Methoxy-poly(ethylene glycol) (MPEG; molecular weight [MW] 3,000 Da) was supplied by NOF Corporation (Tokyo, Japan) and dried under vacuum in a desiccator with P2O5 overnight before polymer synthesis. D,L-lactide (purity: 99.5%) was purchased from Purac Biochem (Gorinchem, The Netherlands) and purified by twice recrystallizing in dried ethyl acetate. Stannous octoate [Sn(Oct)2] (95% pure; Sigma-Aldrich, St Louis, MO, USA) was distilled under vacuum and was used by dissolving it in dry toluene. Minocycline hydrochloride (MIN) and tetracycline (both 99.5% pure) were obtained from the HuBei Xing Galaxy Chemical Corporation Ltd. (Wuhan, People’s Republic of China). MIN ointment (Periocline®) was purchased from Sunstar Inc. (Osaka, Japan). Double distilled water was purified using a Millipore Simplicity System (EMD Millipore, Billerica, MA, USA). All other chemicals were of analytical reagent grade and used without further purification.
Beagle dogs weighing 12–15 kg were obtained from the Shanghai Xingang Laboratory Animal Co Ltd. (Shanghai, People’s Republic of China). The animals used for experiments were treated according to protocols evaluated and approved by the Experimental Animal Ethical Committee of Fudan University.
Methoxy-poly(ethylene glycol) poly(lactic acid) (MPEG-PLA) copolymer synthesis and characterization
MPEG-PLA block copolymer was synthesized by ring-opening polymerization of D,L-lactide, using stannous octoate as the catalyst and MPEG as the initiator. Briefly, 0.4 g of MPEG, 4 g of D,L-lactide, and 50 mg of stannous octoate in dry toluene were added to a round-bottomed flask. The reactants were dried at 70°C under vacuum for 1 hour then polymerized at 160°C under vacuum for 4 hours. The cooled product was dissolved in dichloromethane and precipitated into an excess solvent mixture of ethyl ether and petroleum ether. The precipitant was then redissolved in acetone and precipitated into excess methanol. Finally, the purified MPEG-PLA copolymer was vacuum-dried at 40°C for 24 hours and stored in an electronic dryer. Proton nuclear magnetic resonance (1H NMR) spectroscopy of MPEG-PLA was recorded in CDCl3 with a Varian Mercury Plus-400 MHz (Agilent Technologies, Santa Clara, CA, USA) apparatus operating at 400 MHz at 25°C. Chemical shifts in parts per million (δ) were determined using the chloroform signals at 7.26 ppm as a reference. The integrals of the peaks corresponding to the poly(lactic acid) (PLA) methane protons (δ 5.18 ppm) and the poly(ethylene glycol) (PEG) methylene protons (δ 3.65 ppm) were used to determine the weight ratio of PLA to PEG and to calculate the average number molecular weight (Mn) of the PLA moiety. The molecular weight and molecular distribution of the polymer were also determined by gel permeation chromatography (GPC) as previously described25 using an Agilent 1,100 GPC (Agilent Technologies) with tetrahydrofuran as the solvent.
Preparation and characterization of minocycline-loaded NPs
Preparation of minocycline-loaded NPs
Minocycline-loaded nanoparticles (MIN-NPs) were prepared using an emulsion/solvent evaporation method. In brief, 40 mg of MPEG-PLA and 8 mg of MIN were dissolved in 1 mL of dichloromethane then were added into 5 mL of 0.6% sodium cholate aqueous solution. The mixture was intensively emulsified by sonication (200 W, 5 seconds) 15 times in ice water using a JY92-II probe sonicator (Ningbo Scientz Biotechnology Co Ltd., Ningbo, People’s Republic of China). After evaporating dichloromethane off with a RE-5205 rotary evaporator (Shanghai Yarong Biochemistry Instrument Factory, People’s Republic of China) at 37°C, the obtained NPs were concentrated by centrifugation at 21,000 g for 45 minutes using a TJ-25 centrifuge (Beckman Coulter Inc., Brea, CA, USA). After discarding the supernatant, the NPs were resuspended in 0.3 mL of deionized water. Blank NPs were prepared using the same procedure without the addition of MIN in dichloromethane.
Morphology and particle size
The particle size and zeta potential of the NPs were determined by dynamic light scattering using a Nano-ZS Malvern Zetasizer Nano analyzer (Malvern Instruments, Malvern, UK). The morphology of the NPs was observed using an H-600 transmission electron microscope (Hitachi, Tokyo, Japan) after negative staining with 2% sodium phosphotungstate solution.
Drug encapsulation efficiency and loading capacity
The drug encapsulation efficiency (EE) and drug loading capacity (LC) of the MIN-NPs was investigated as previously described.25 Briefly, the minocycline concentration in the supernatant was determined by high-performance liquid chromatography (HPLC) using a Waters® e2695 Separations Module (Waters Corporation, Milford, MA, USA) equipped with an Agilent Zorbax SB analytical column (150×4.6 mm, pore size 5 μm; Agilent Technologies). The mobile phase was a mixture of methanol, acetonitrile, and 0.01 M KH2PO4 (23:5:22 v/v/v) containing 0.03 mM Na2EDTA and 60% HClO4 (2.9 mL), adjusted to pH 2.5 with 10 M KOH.26 The flow rate was 1.2 mL/min. The sample injection volume was 20 μL, and the detector wavelength was 350 nm. The drug EE was calculated as EE = (MINtotal − MINsupernatant)/MINtotal ×100% and the drug LC was calculated as LC = (MINtotal − MINsupernatant)/materials ×100%.
In vitro release study
The in vitro release profile of minocycline from the MIN-NPs was investigated by a dialysis method using phosphate-buffered saline (PBS; 0.01 M, pH 7.4) as the release medium. Briefly, 1 mL of MIN solution or MIN-NP suspension in PBS (containing 100 μg of MIN) was introduced into a dialysis bag (MWCO 8,000 Da; Greenbird Inc., Shanghai, People’s Republic of China) and incubated in 10 mL of release medium at 37°C at the shaking speed of 100 rpm. At each setting time point, a 0.2 mL aliquot was withdrawn, and, immediately, an equal volume of fresh release medium was added. The released samples were analyzed by HPLC as already described. Samples stored were away from light throughout the experimental procedure. For the release study, these experiments were performed in quadruplicate.
In vivo pharmacokinetics and pharmacodynamics
Periodontitis modeling in beagle dogs
Periodontitis in beagle dogs was modeled by tying ligatures around the cervical region of the tooth using dental ligature wire (ClassOne Orthodontics, Carlsbad, CA, USA).27 Briefly, 2 weeks after scaling, dogs were anesthetized by intravenous injection of pentobarbital sodium at a dose of 30 mg/kg. Experimental periodontitis was induced on the left upper second premolar (PM2), third premolar (PM3), and fourth premolar (PM4) as well as the left lower PM3, PM4, and first molar (PM1). Prior to ligation, the gingival attachment was incised, and the periodontal ligaments were undermined until a periodontal pocket depth of up to 3 mm was reached with a straight elevator (Osung MND Co Ltd., Gyeonggi-do, Korea). After undermining, a shallow notch was made in the mesial and distal region of each tooth with a round bur to act as a retentive groove for the ligature. After ligature placement, tramadol (4 mg/kg, intramuscular injection) was administered twice a day for 3 days for pain control. To promote plaque formation, soft moistened food was given for the following 8 weeks. The ligatures were checked weekly and any missing ligatures were replaced immediately. To evaluate periodontal status, the clinical periodontal parameters of plaque index (PI), gingival index (GI), periodontal pocket depth (PPD), clinical attachment level, and bleeding on probing were recorded.3
Pharmacokinetics experiments
Pharmacokinetics experiments were performed as previously described.3 In brief, 8 weeks after dental ligature, dogs were anesthetized by intravenous injection of pentobarbital sodium. Fifty microliters of either minocycline solution in N-methyl-2-pyrrolidone (NMP) (2%), MIN-NPs, or Periocline were injected into the periodontal pocket of each beagle dog by a disposable syringe with a 21-gauge needle. No periodontal dressing or adhesive was used to aid in retention of the minocycline formulations. At a set time point after administration, the dogs were anesthetized and GCF samples were collected by positioning an absorbent paper point (Meta, Tianjin, People’s Republic of China) at the orifice of the sulcus for 30 seconds. To avoid contamination with blood and saliva, the paper strips were manipulated gently. The paper strips were weighed and the increase in weight was calculated. GCF volume was measured with a standard curve that was calibrated using bovine serum. Minocycline was immediately eluted from the paper strips by 0.1 M glycine buffer and stored at −80°C. GCF minocycline levels were calculated by dividing the content of each sample pool by total volume. All GCF samples were processed with minimal delay. Minocycline concentrations in GCF were plotted with time and processed by DAS software (v 2.0; Professional Committee of Pharmacomtrics of Chinese Pharmacological Society, People’s Republic of China) to calculate the pharmacokinetics parameters.
Pharmacodynamics experiments
The pharmacodynamics experiments were performed as described in the “Pharmacokinetics experiments” section with a little modification. Eight weeks after dental ligature, 12 dogs were randomly assigned to four groups: saline, free minocycline, MIN-NPs, and Periocline. After anesthetization with pentobarbital sodium, dogs were treated with 50 μL of saline, minocycline solution in NMP (2%), MIN-NPs, or Periocline by injection into the periodontal pocket of each beagle dog with a disposable syringe. The clinical periodontal parameters of PI, GI, PPD, and clinical attachment level were recorded every 3 days to evaluate the therapeutic efficacy of the MIN-NPs.
Statistical analysis
The pharmacokinetics and clinical parameters for each group are expressed as the mean ± standard deviation. Statistical differences were determined by one-way analysis of variance, followed by Dunnett post-hoc analysis for multi-group comparison. Data within the 95% confidence level were considered significant.
Results
MPEG-PLA copolymer synthesis and characterization
Block copolymer MPEG-PLA was synthesized with molecular weight of 33,126 Da, as determined by 1H NMR. The typical 1H NMR spectra and chemical shifts (Figure 1) were in agreement with previously published data on MPEG-PLA copolymers. Peaks at 3.38 ppm (a), 3.65 ppm (b), 5.18 ppm (c), and 1.59 ppm (d) confirmed the block nature of the MPEG-PLA copolymer. GPC measurements indicated that the polydispersity index and number-based molecular weight of MPEG-PLA were 1.25 and 28,932 Da, respectively. MPEG-PLA was selected and synthesized for preparing NPs due to its biodegradability. Unlike other nonbiodegradable polymers, PEG-PLA NPs should be fully biodegradable, leaving no potentially toxic by-products upon their degradation, after in vivo application for a long time.
Figure 1.
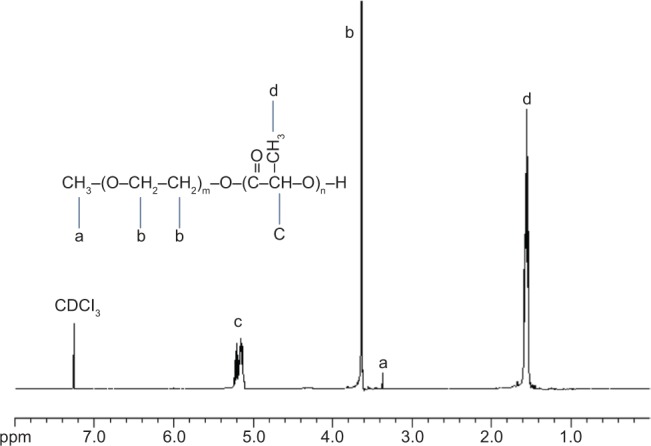
Proton nuclear magnetic resonance spectra of the MPEG-PLA in CDCl3.
Abbreviation: MPEG-PLA, methoxy-poly(ethylene glycol) poly(lactic acid).
Preparation and characterization of MIN-NPs
Morphology, particle size, and zeta potential
As shown in Figure 2, transmission electron microscopy showed that the minocycline-loaded PEG-PLA NPs were spherical with a uniform size and well dispersed without any adhesion or aggregation. The mean particle size of the MIN-NPs was 98±12 nm (Figure 3), which was slightly increased compared with that of blank NPs (90±16 nm). In a previous report,21 poly(lactic-co-glycolic acid) (PLGA)-based NPs were prepared using different methods, but the particle sizes of these PLGA NPs ranged from 85 nm to 7,070 nm, and most of them were larger than 200 nm. Moreover, PLGA NPs without a surface coating or PEG or another hydrophilic component are not stable and prefer to aggregate in physiological conditions.28 Therefore, the local delivery of PLGA NPs in periodontal pockets is limited. Considering the relationship between particle size and tissue absorption and penetration, smaller-size stable PEG-PLA NPs were prepared in this study. A negative surface charge was detected for the MIN-NPs (−24 to −20 mV), which was similar to that of the blank NPs (−23 to −20 mV) (Figure 3). No significant changes in the zeta potential of the NPs were observed after MIN loading.
Figure 2.
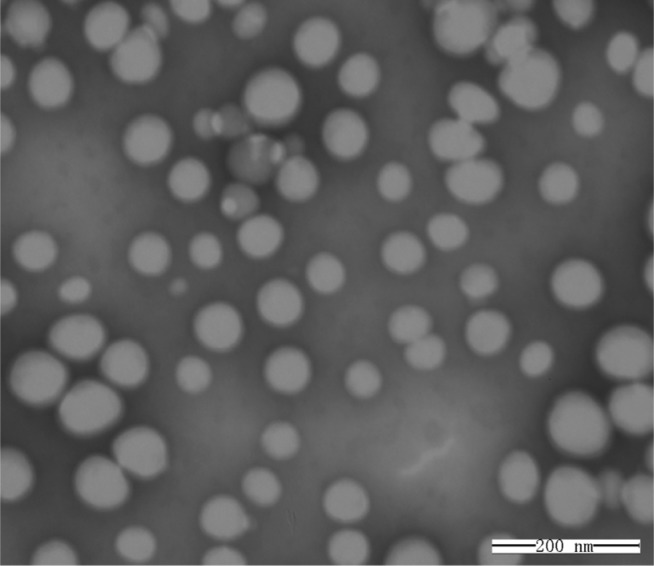
Transmission electron microscopy images of minocycline-loaded nanoparticles negatively stained with phosphotungstic acid solution.
Note: Scale bar 200 nm.
Figure 3.
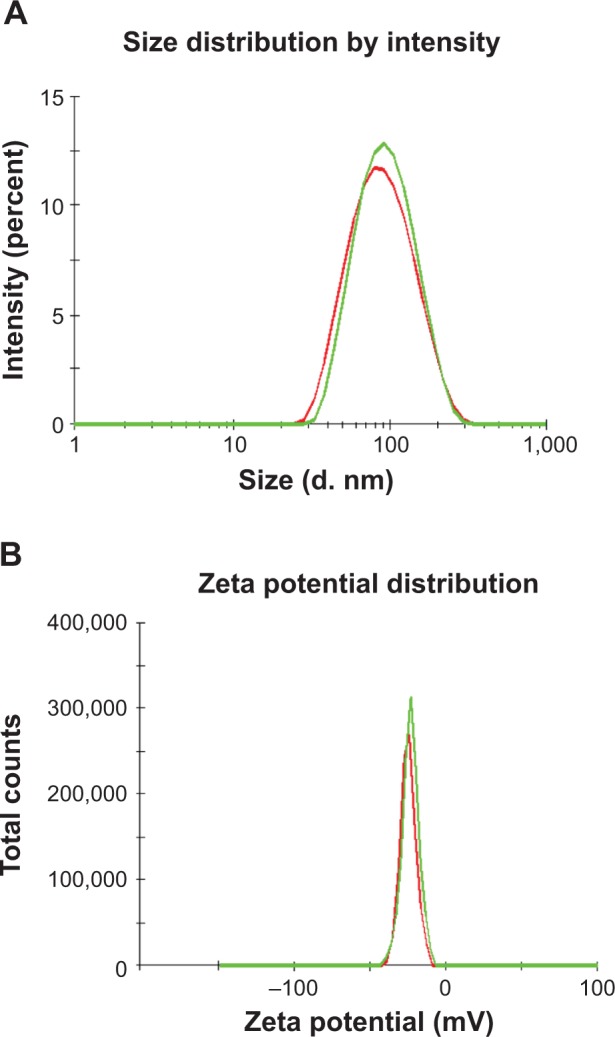
The particle-size distribution and surface zeta potential of minocycline-loaded nanoparticles (NPs) (green line) and blank NPs (red line).
Drug EE and LC
The drug LC and EE of the MIN-NPs were detected by HPLC, and the values of LC and EE were 9.3%±0.2% and 46.5%±0.9%, respectively. The highest LC and EE for PLGA-based NPs prepared using different methods were 1.92%±0.19% and 29.95%±2.47% respectively,21 and both of these two values are lower than those obtained in our study, indicating the superiority of our MIN-NP design.
In vitro release of MIN-NPs
The release profiles of minocycline solution and MIN-NPs in PBS (0.01 M, pH =7.4) at 37°C showed that almost 100% of the minocycline solution was released within 24 hours, while the cumulative release of MIN from the MIN-NPs was approximate 96% after 14 days (Figure 4), which demonstrated the marked sustained-release characteristics of the MIN-NPs. Qin et al prepared a kind of tinidazole in situ forming PLA implant, and the release of tinidazole for local delivery was about 7 days.3 The longest release time obtained with Elyzol was 48 hours.29 Compared with these results, the release of MIN-NPs over 14 days demonstrated a good sustained-release property. The cumulative release of MIN from the MIN-NPs and the release time were fitted with a first-order drug-releasing equation (ln(1–Q) = −Kt), the Higuchi equation (Q = Kt1/2), and Ritger–Peppas equation (Q = Ktn), and the best result (Q =0.47t0.27, r=0.998) was achieved with Ritger–Peppas equation, indicating the release of MIN mainly depended on diffusion.
Figure 4.
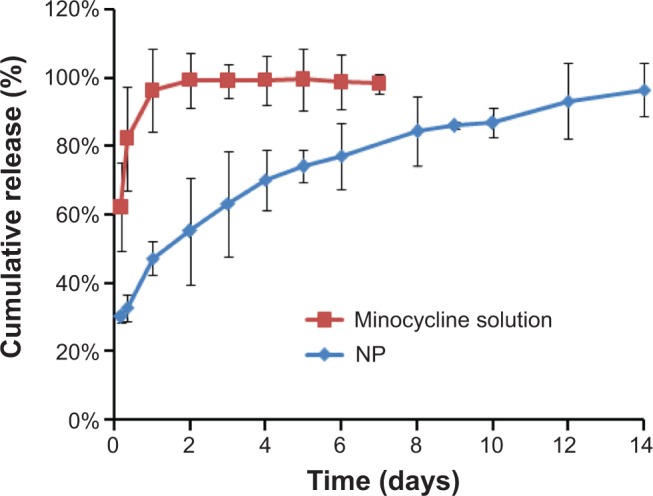
The releasing curve of minocycline hydrochloride from minocycline-loaded nanoparticles (NPs) in phosphate-buffered saline (0.01 M, pH =7.4) at 37°C.
Notes: Data are presented as the mean ± standard deviation; n=4.
Pharmacokinetics of different formulations of MIN in GCF
The concentration of MIN in GCF was determined by HPLC, and the baseline separation of MIN and tetracycline was achieved with the chromatographic conditions outlined. The retention time (RT) for MIN and tetracycline was 7.768 minutes and 9.801 minutes, respectively (Figure 5). The standard curve (y =0.158 × +0.359, R2=0.997) was obtained by the line regression of MIN concentration with the peak area ratio of MIN and tetracycline, while the linearity was given in the range of 0.1–20.0 μg/mL.
Figure 5.
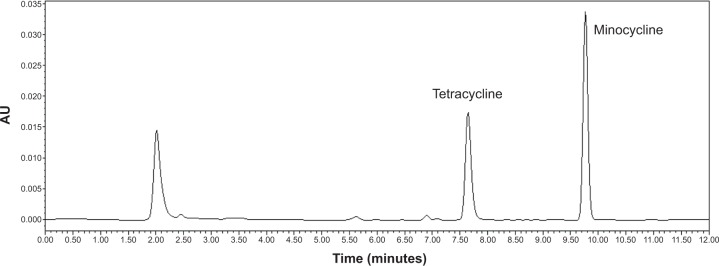
A typical high-performance liquid chromatography figure of a gingival crevice fluid sample.
The pharmacokinetics curve of MIN in GCF (Figure 6) showed that the concentration of MIN in GCF declined rapidly after the administration of MIN solution and was lower than the median effective concentration (1 μg/mL) after 2 days. The concentration of MIN in GCF declined slowly after administration of Periocline, and the action of the drug lasted 8 days (>1 μg/mL), suggesting good sustained release of Periocline. However, after administration of the MIN-NPs, the concentration of MIN in GCF declined slowly and was still higher than 1 μg/mL (1.28 μg/mL) 12 days later, representing the longest action time among three groups. The long action time of the MIN-NPs may be related to either the drug’s sustained release or the adherence and penetration of MIN-NPs into inflammation tissues. The pharmacokinetics parameters of MIN in GCF, as calculated using DAS pharmacokinetics software, are shown in Table 1. The elimination of MIN in each group was well fitted to two-compartment open models. There were significant differences in the area under the curve (AUC) from zero to infinity (0–∞), mean retention time (MRT) (0–∞), half-life (t1/2), elimination rate constant (k), and clearance (Cl) between groups (P<0.01). The AUC(0–∞), MRT(0–∞), and t1/2 of the MIN-NP group were 4.62-fold, 2.21-fold, and 3.74-fold, respectively, those of the MIN solution group. The AUC(0–∞) and MRT(0–∞) of the MIN-NP group were 1.42-fold and 1.21-fold, respectively, higher than those of the Periocline group, indicating higher local bioavailability and the better effect of the former.
Figure 6.
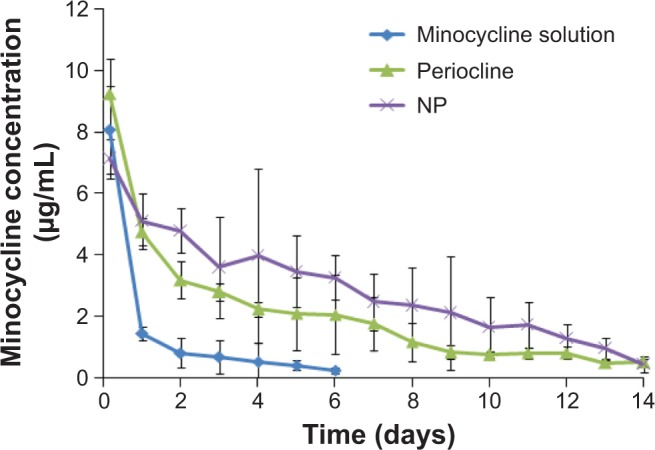
Pharmacokinetics curve of minocycline hydrochloride (MIN) in gingival crevice fluid after local administration of different MIN formulations.
Notes: Data presented as the mean ± standard deviation; n=6.
Table 1.
Pharmacokinetics parameters of minocycline hydrochloride (MIN) in gingival crevice fluid after local administration of different MIN formulations (n=6)
| Parameter | MIN-NPs | Periocline® | MIN solution |
|---|---|---|---|
| AUC(0–t) (mg/L*d) | 40.060±4.120a,b | 28.140±3.350b | 7.890±1.200 |
| AUC(0–∞) (mg/L*d) | 42.470±4.530a,b | 32.220±3.630b | 9.190±1.420 |
| MRT(0–t) (d) | 4.990±0.520b | 4.130±0.420b | 1.330±0.150 |
| MRT(0–∞) (d) | 5.690±0.630b | 6.490±0.690b | 2.570±0.290 |
| t1/2 (d) | 3.460±0.360a,b | 2.860±0.290b | 0.920±0.100 |
| k (d−1) | 0.201±0.021a,b | 0.242±0.025b | 0.750±0.084 |
| Tmax (d) | 0.167 | 0.167 | 0.167 |
| V (L) | 0.124±0.025a | 0.147±0.029 | 0.169±0.015 |
| Cl (L/d) | 0.025±0.005a,b | 0.036±0.007b | 0.127±0.011 |
| Cmax (mg/L) | 7.130±1.220a | 9.230±1.350 | 8.060±1.390 |
Notes: Data are presented as the mean ± standard deviation
P<0.05 vs Periocline
P<0.01 vs MIN solution.
Abbreviations: 0–∞, time zero to infinity; 0–t, zero to time t; AUC, area under the curve; Cl, clearance; Cmax, peak concentration; k, elimination rate constant; MIN-NPs, minocycline-loaded nanoparticles; MRT, mean retention time; t1/2, half-life; Tmax, time to peak concentration; V, apparent volume of distribution; d, days.
Pharmacodynamics of MIN-NPs
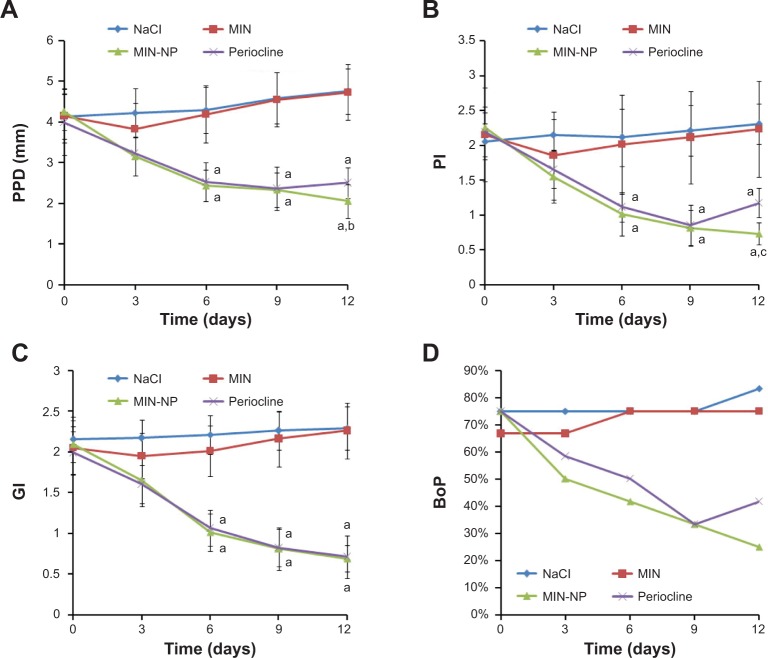
As shown in Figure 7, there were no significant differences in clinical periodontal parameters between the MIN solution group and saline group after the local delivery of MIN solution in the dog periodontitis models, indicating the MIN solution had no obvious treatment efficacy for periodontitis. However, for Periocline and MIN-NPs, 6 days after local administration, clinical periodontal parameters (PPD, PI, GI) were significantly improved, and significantly better than those of the MIN solution group. In addition, 9 days after administration, the PPD, PI, and bleeding on probing of the Periocline group increased again, which may be because an effective drug concentration of Periocline could only be maintained for 8 days (Figure 6). Twelve days after the administration, the PPD and PI of the MIN-NP group were still significantly lower than those of the MIN solution and Periocline groups, suggesting that MIN has a long treatment efficacy, which agreed well with pharmacokinetics results.
Figure 7.
Changes in clinical periodontitis parameters after local administration of different minocycline hydrochloride (MIN) formulations.
Notes: Data presented as the mean ± standard deviation; n=6. aP<0.01 vs MIN solution; bP<0.05, cP<0.01 vs Periocline®.
Abbreviations: BoP, bleeding on probing; GI, gingival index; MIN-NP, minocycline-loaded nanoparticles; PI, plaque index; PPD, periodontal pocket depth.
Conclusion
In order to maintain the effective concentration of MIN in GCF for a longer period, MIN-NPs were prepared for evaluation in the study. Different forms of MIN were placed into the periodontal pockets of beagle dogs with periodontitis. GCF samples were collected after drug delivery and quantified by HPLC. After local administration of MIN-NPs, the minocycline concentration in GCF decreased slowly and an effective drug concentration was retained for a longer time than with Periocline. The MIN-NPs demonstrated anti-periodontitis effects that MIN-NPs could significantly decrease the symptoms of periodontitis, compared with Periocline and MIN solution. These findings suggest that MIN-NPs might have great potential in the treatment of periodontitis.
Acknowledgments
This work was supported by the Shanghai Municipal Public Health Bureau Research Grant, National Science and Technology Major Project no. 2012ZX09304004, National Natural Science Foundation of China no. 81001404, the Doctoral Fund of the Ministry of Education of China no. 20100071120050, and the Zhuoxue Plan of Fudan University.
Footnotes
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Socransky SS, Haffajee AD. Evidence of bacterial etiology: a historical perspective. Periodontol 2000. 1994;5:7–25. doi: 10.1111/j.1600-0757.1994.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 2.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4(1):1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Qin Y, Yuan M, Li L, Li W, Xue J. Formulation and evaluation of in situ forming PLA implant containing tinidazole for the treatment of periodontitis. J Biomed Mater Res B Appl Biomater. 2012;100(8):2197–2202. doi: 10.1002/jbm.b.32788. [DOI] [PubMed] [Google Scholar]
- 4.Tezel A, Yucel O, Orbak R, et al. The gingival crevicular fluid ciprofloxacin level in subjects with gingivitis and periodontitis, and its effects on clinical parameters. J Periodontal Res. 2005;40(5):395–400. doi: 10.1111/j.1600-0765.2005.00820.x. [DOI] [PubMed] [Google Scholar]
- 5.Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 6.Slots J, Chen C. The oral microflora and human periodontal disease. In: Tannock GW, editor. Medical Importance of the Normal Microflora. Dordrecht: Kluwer; 1999. pp. 101–127. [Google Scholar]
- 7.Marsh PD. Dental plaque: biological significance of a biofilm and community life-style. J Clin Periodontol. 2005;32(Suppl 6):7–15. doi: 10.1111/j.1600-051X.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 8.Scannapieco FA, Dasanayake AP, Chhun N. “Does periodontal therapy reduce the risk for systemic diseases?”. Dent Clin North Am. 2010;54(1):163–181. doi: 10.1016/j.cden.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Apatzidou DA, Kinane DF. Nonsurgical mechanical treatment strategies for periodontal disease. Dent Clin North Am. 2010;54(1):1–12. doi: 10.1016/j.cden.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Lu SH, Huang RY, Chou TC. Magnolol ameliorates ligature-induced periodontitis in rats and osteoclastogenesis: in vivo and in vitro study. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/634095. Article ID 634095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Winkelhoff AJ, Rams TE, Slots J. Systemic antibiotic therapy in periodontics. Periodontol 2000. 1996;10:45–78. doi: 10.1111/j.1600-0757.1996.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 12.van Winkelhoff AJ, Herrera Gonzales D, Winkel EG, Dellemijn-Kippuw N, Vandenbroucke-Grauls CM, Sanz M. Antimicrobial resistance in the subgingival microflora in patients with adult periodontitis. A comparison between The Netherlands and Spain. J Clin Periodontol. 2000;27(2):79–86. doi: 10.1034/j.1600-051x.2000.027002079.x. [DOI] [PubMed] [Google Scholar]
- 13.Kelly HM, Deasy PB, Ziaka E, Claffey N. Formulation and preliminary in vivo dog studies of a novel drug delivery system for the treatment of periodontitis. Int J Pharm. 2004;274(1):167–183. doi: 10.1016/j.ijpharm.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 14.Jain N, Jain GK, Javed S, et al. Recent approaches for the treatment of periodontitis. Drug Discov Today. 2008;13(21):932–943. doi: 10.1016/j.drudis.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Kenawy el-R, Bowlin GL, Mansfield K, et al. Release of tetracycline hydrochloride from electrospun poly(ethylene-co-vinylacetate), poly(lactic acid), and a blend. J Control Release. 2002;81(1–2):57–64. doi: 10.1016/s0168-3659(02)00041-x. [DOI] [PubMed] [Google Scholar]
- 16.Tonetti MS, Pini-Prato G, Cortellini P. Principles and clinical applications of periodontal controlled drug delivery with tetracycline fibers. Int J Periodontics Restorative Dent. 1994;14(5):421–435. [PubMed] [Google Scholar]
- 17.Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev. 2012;41(7):2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rose S, Prevoteau A, Elzière P, Hourdet D, Marcellan A, Leibler L. Nanoparticle solutions as adhesives for gels and biological tissues. Nature. 2014;505(7483):382–385. doi: 10.1038/nature12806. [DOI] [PubMed] [Google Scholar]
- 19.Piñón-Segundo E, Ganem-Quintanar A, Alonso-Pérez V, Quintanar-Guerrero D. Preparation and characterization of triclosan nanoparticles for periodontal treatment. Int J Pharm. 2005;294(1):217–232. doi: 10.1016/j.ijpharm.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Chwalibog A, Sawosz E, Hotowy A, et al. Visualization of interaction between inorganic nanoparticles and bacteria or fungi. Int J Nanomedicine. 2010;5:1085–1094. doi: 10.2147/IJN.S13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kashi TSJ, Eskandarion S, Esfandyari-Manesh M, et al. Improved drug loading and antibacterial activity of minocycline-loaded PLGA nanoparticles prepared by solid/oil/water ion pairing method. Int J Nanomedicine. 2012;7:221–234. doi: 10.2147/IJN.S27709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu D, Yang PS. Minocycline hydrochloride nanoliposomes inhibit the production of TNF-α in LPS-stimulated macrophages. Int J Nanomedicine. 2012;7:4769–4775. doi: 10.2147/IJN.S34036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gopinath V, Ramakrishnan T, Emmadi P, Ambalavanan N, Mammen B, Vijayalakshmi Effect of a controlled release device containing minocycline microspheres on the treatment of chronic periodontitis: a comparative study. J Indian Soc Periodontol. 2009;13(2):79–84. doi: 10.4103/0972-124X.55844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kulkarni RK, Pani KC, Neuman C, Leonard F. Polylactic acid for surgical implants. Arch Surg. 1966;93(5):839–843. doi: 10.1001/archsurg.1966.01330050143023. [DOI] [PubMed] [Google Scholar]
- 25.Pang Z, Gao H, Yu Y, et al. Brain delivery and cellular internalization mechanisms for transferrin conjugated biodegradable polymersomes. Int J Pharm. 2011;415(1–2):284–292. doi: 10.1016/j.ijpharm.2011.05.063. [DOI] [PubMed] [Google Scholar]
- 26.Colovic M, Caccia S. Liquid chromatographic determination of minocycline in brain-to-plasma distribution studies in the rat. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;791(1–2):337–343. doi: 10.1016/s1570-0232(03)00247-2. [DOI] [PubMed] [Google Scholar]
- 27.Kim SE, Lee ER, Lee Y, et al. A modified method for inducing periodontitis in dogs using a silk-wire twisted ligature. J Vet Sci. 2012;13(2):193–197. doi: 10.4142/jvs.2012.13.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263(5153):1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 29.Norling T, Lading P, Engström S, Larsson K, Krog N, Nissen SS. Formulation of a drug delivery system based on a mixture of monoglycerides and triglycerides for use in the treatment of periodontal disease. J Clin Periodontol. 1992;19(9):687–692. doi: 10.1111/j.1600-051x.1992.tb02529.x. [DOI] [PubMed] [Google Scholar]