Synopsis
We present a cost effectiveness analysis of colorectal cancer screening tests which have been recommended by the United States Preventive Services Task Force, American Cancer Sociey-GI Multisocieties-American College of Radiology, or the American College of Gastroenterology. This cost effectiveness analysis supports a common theme of the three Guideline groups that there are multiple acceptable colorectal cancer screening strategies (including colonoscopy). We show which recommended strategies are also cost effective given a range of willingnessto pay.per life-year gained. The set of cost effective strategies include tests which primarily detect cancer early (annual sensitive FOBTs (either guaiac or fecal immunochemical tests, but not Hemocccult II), as well as those which can prevent CRC (flexible sigmoidoscopy every 5 years with a frequent sensitive FOBT (but not flexible sigmoidoscopy as a stand-alone test), and colonoscopy). CT colonography was not a cost effective strategy. Stool DNA testing was not assessed in the analysis for this chapter.
Keywords: colonoscopy, colorectal cancer screening, cost effectiveness analysis, comparative effectiveness research, efficient frontier
Introduction
Colonoscopy was first recommended as a primary screening test for colorectal cancer (CRC) in the 1997 Guidelines of the MultiSociety-GI1 which provided a menu of CRC screening options. This recommendation was based on the ability to use colonoscopy to see and remove the precursor lesion within the same colonoscopic examination across the entire colon and rectum. Clinical evidence for using colonoscopy as a screening tool1 was based on three lines of evidence: 1) the mortality reduction achieved by colonoscopies performed for positive fecal occult blood tests (FOBT) in randomized controlled trials of the Hemoccult II guaiac based fecal occult blood test; 2-4 2) the mortality reduction of rigid sigmoidoscopy in case control studies5-6, and 3) the reduction in colorectal cancer incidence in the National Polyp Study with colonoscopic polypectomy.7 Colonoscopy is now recommended as one of the primary colorectal cancer screening tests by 1.) the United States Preventive Services Task Force (USPSTF),8 2.) the combined organizations of the American Cancer Society, US Multi-Society Task Force on Colorectal Cancer representing multiple gastroenterology societies, and the American College of Radiology 9 (referred to as the Multi-Societies) and 3.) the American College of Gastroenterology (ACG).10 Each organization presents colonoscopy as one option for CRC screening, with the American College of Gastroenterology citing colonoscopy as the primary CRC screening test.10
In this chapter we compare the recommendations for CRC screening tests by the different organizations. In addition we review a cost effectiveness analysis (CEA) of these strategies that we have done for a report to the Center for Medicare and Medicaid Services (CMS) 11 and a recent analysis of the different strategies relative to computed tomographic colonography (CTC).12 This review of the cost-effectiveness results of the different strategies provides further context to understand what are risks and benefits of the recommended strategies in practice.
Recommendations for Colorectal Cancer Screening from three organizations
The recommendations from the three organizations for CRC screening tests are presented in Table 1. Although similar evidence was reviewed by these three groups, there were differences in the test strategies recommended. The USPSTF and the Multi-Societies present a menu of options whereas the ACG presents preferred strategy options. The USPSTF formally evaluated risks and benefits of screening for the average risk patient and concluded that there was insufficient evidence at this time to recommend CT colonography or stool DNA testing for the general population.8 Both of these tests were included in the MultiSociety and ACG recommendations, but with the caveat that the interval of screening with the stool DNA test is not yet established. Furthermore there has been discussion of the minimum size of polyp detected by CTC for referral to colonoscopy. (The MultiSociety did recommend that all CTC polyps of 6 mm or larger would be referred for colonoscopy).
Table 1.
Comparison of Colorectal Cancer Screening Guidelines from the United States Preventive Services Task Force, the American Cancer Society-MultiSociety (Gastroenterology)-American College of Radiology, and the American College of Gastroenterology
| United States Preventive Services Task Force (USPSTF)8 | American Cancer Society-MultiSociety (Gastroenterology)-American College of Radiology (MultiSocieties)9 | American College of Gastroenterology (ACG)10 | |
|---|---|---|---|
| Age to begin, stop | |||
| Age to start screening | 50 | 50 | 50 (begin age 45 for African Americans) |
| Age to stop screening | 75 | None stated | None stated |
| Age to stop surveillance for adenoma and CRC subjects | None | None | None |
| Recommended Tests | |||
| Hemoccult II (annual) | No | No | No |
| Hemoccult SENSA (annual) | Yes | Yes | Yes |
| Fecal immunochemical test (annual) | Yes | Yes | Yes (First preferred alternative test if subject refuses colonoscopy) |
| Flexible Sigmoidoscopy (every 5 years) | No | Yes | Alternative test if colonoscopy refusal |
| Flexible Sigmoidoscopy (every 5 years) with Sensitive FOBT (every 2-to 3 years) | Yes | Not stated | Not stated |
| Colonoscopy (every 10 years) | Yes | Yes | Yes (as first choice) |
| CT colonography (every 5 years for ≥6 mm polyps) | No Insufficient evidence | Yes | Yes (Alternative test if subject refuses colonoscopy) |
| Stool DNA(interval not set) | No Insufficient evidence | Yes | Yes Alternative test if subject refuses colonoscopy |
| Double contrast barium enema | No | Yes | No |
Tests recommended in common by all three groups are colonoscopy and the more sensitive fecal occult blood tests (fecal Immunochemical tests (FIT) (preferred) or guaiac Hemoccult SENSA). Flexible sigmoidoscopy every 5 years in conjunction with a sensitive FOBT (SENSA or FIT) every 2 to 3 years is recommended by the USPSTF, whereas flexible sigmoidoscopy alone or Hemoccult II alone is not recommended. However, the MultiSocieties include flexible sigmoidoscopy and barium enema as individual tests in their recommendations.
The MultiSocieties also classify the screening tests as 1.) those that can prevent cancer through early detection of adenomas as well as detect CRC (recommended tests of colonoscopy every 10 years, flexible sigmoidoscopy every 5 years, CTC every 5 years, and double contrast barium enema every 5 years) and 2.) those that can primarily detect CRC early (recommended tests of annual guaiac-based fecal occult blood test with high test sensitivity for cancer, annual FIT with high test sensitivity for cancer, or stool DNA test with high sensitivity for cancer, interval uncertain. (High test sensitivity for cancer was defined as 50% or greater for testing at one point in time.) Furthermore, the MultiSociety guidelines state that the primary goal of screening should be to prevent CRC.
The ACG recommends that clinicians have access to a preferred strategy as an alternative to a menu of options. The ACG recommends as a first preference the cancer prevention test of colonoscopy, and then the FIT as the first alternative. Additional alternative tests include flexible sigmoidoscopy every 5 to 10 years, CTC every 5 years, Hemoccult SENSA annually, and stool DNA testing every 3 years. The ACG also grades its recommendations on the basis of the amount of evidence supporting the recommendation and the benefit versus risk of the strategies. Colonoscopy, FIT, Annual Hemoccult SENSA, and FIT have recommendation level 1B which denotes strong recommendation with moderate quality evidence.
Thus, recommendations from different organizations vary because the reasoning behind their recommendations differs.
Comparative Effectiveness Research to compare colorectal cancer screening tests
In comparing screening tests we consider both the risk and benefits of screening for each particular test. Risks are commonly represented by the costs and benefits by life years gained. Incidence or mortality reduction obtained with screening can also be used to represent benefits. Comparative Effectiveness Research (CER) is the conduct and synthesis of research comparing the benefits and harms of various interventions and strategies for preventing, diagnosing, treating, and monitoring health conditions in real-world settings. The purpose is to improve health outcomes by developing and disseminating evidence - based information to patients, clinicians, and other decision makers about which interventions are most effective for which patients under specific circumstances. 13
In this chapter we discuss the cost effectiveness of the recommended CRC screening tests, and in particular the relationship of colonoscopy to other tests for screening in the average risk population. We include FOBT (Hemoccult II, Hemoccult SENSA, FIT), flexible sigmoidoscopy alone with and without biopsy, flexible sigmoidoscopy with annual Hemoccult II and with annual Hemoccult SENSA, and colonoscopy. Since the Centers for Medicare and Medicaid Services (CMS) has not approved either CT colonography or stool DNA testing for CRC screening, there are no Medicare reimbursement rates for these tests. We summarize our prior results of cost-effectiveness analyses that we have done for CMS in relationship to CT colonoscopy to assess a potential reimbursement level. 11-12 We do not include stool DNA testing (in this cost-effectiveness analysis. We also do not discuss the capsule endoscopy14 because at this point in time there is insufficient evidence for effectiveness15 and it has not been included in any screening guidelines to date.
Cost effectiveness analysis (CEA) of colorectal cancer screening tests has been conducted with varying cost structures and assumptions and has produced varying conclusions. In 2002, Pignone16 provided a systematic review of CEA for CRC screening in the US from 5 models17-21 and reported that the strategies of colonoscopy, sigmoidoscopy with or without FOBT (Hemoccult II), and FOBT alone all provided screening strategies that were less than $50,000 per life-year gained compared to no screening. However there was no screening test strategy that was consistently the most effective CRC strategy when compared to each other across the 5 models. To improve this situation the Institute of Medicine (IOM) convened a conference in 2004 to assess colorectal cancer screening cost effectiveness analyses. Representatives from each of the 5 models originally reviewed by Pignone 22 were asked to present at the January 2004 IOM conference and provide costs and life years gained for 5 screening strategies (colonoscopy, sigmoidoscopy, sigmoidoscopy plus FOBT, FOBT alone, and a test similar to barium enema) under their original assumptions and again with standardized input assumptions concerning test characteristics, adherence to screening (100%) , follow-up, and surveillance as well as for test costs and treatment costs.23 With standardized inputs, the models still had variation in the absolute levels of costs and life years gained, but the relative ordering of strategies with respect to cost-effectiveness results were comparable. Given a willingness to pay of $20,000 or $50,000 per life year saved, The preferred strategy was annual FOBT. However, sigmoidoscopy plus annual FOBT was the preferred strategy in 4 of 5 models, for a willingness to pay of $100,000 per life year saved.
Microsimulation modeling to inform health policy
Although randomized controlled trials are the preferred method for establishing effectiveness of (screening) interventions, they are expensive, require long follow-up, and can include only a few comparison tests. Therefore, well-validated microsimulation models may be used to estimate the required resources and expected benefits from different screening policies and inform decision making. There are 3 colorectal cancer microsimulation models in the National Cancer Institute’s Cancer Intervention and Surveillance Modeling Network (CISNET). The models are based upon clinical incidence data before the introduction of screening (1975-1979 Surveillance, Epidemiology, and End Results (SEER) data24 and upon the size distribution of adenomas in colonoscopy and autopsy studies. 25-34 They have been validated against the long term reductions in incidence and mortality of colorectal cancer with annual FOBT reported in the randomized controlled trial of the Minnesota Colon Cancer Control Study2, 35-36 and show good concordance with the trial results. Transparency of the models is provided through standardized profiles of each model’s structure and underlying model parameters and assumptions are available at http://cisnet.cancer.gov/profiles/ .
In this chapter, we use the results from one of the CISNET models (MISCAN: Microsimulation Screening Analysis, from Erasmus University Medical Center and Memorial – Sloan-Kettering Cancer Center) to provide cost effectiveness analysis of the screening tests recommended by the three guideline groups. The results are from the cost effectiveness analysis for CMS on CT colonography11 and a recent analysis of the different strategies relative to computed tomographic colonography (CTC)12. The primary analysis for this work was for a cohort of those 65 years of age. In this chapter we use the results of the secondary analysis for a cohort of those 50 years of age.
The MISCAN model simulates the life histories of a large population from birth to death under two situations: the natural history of the adenoma-carcinoma sequence and the impact of the screening intervention to detect and remove adenomas or colorectal cancer. As each simulated individual ages, there is a chance that an adenomatous polyp – a benign precursor lesion that may lead to CRC – may develop. One or more adenomas can occur in any individual and each can develop into preclinical CRC. The risk of developing an adenoma may depend on age, sex, genetic and other propensity factors. Adenomas can grow in size over time and eventually some of them can become malignant, transforming to stage I preclinical cancer. A preclinical cancer (i.e., not detected) has a chance of progressing through the stages (from stages I to IV) and may be detected by symptoms at any stage. We assume that adenomas are asymptomatic and can only be detected by a screening test (Figure 1).
Figure 1.
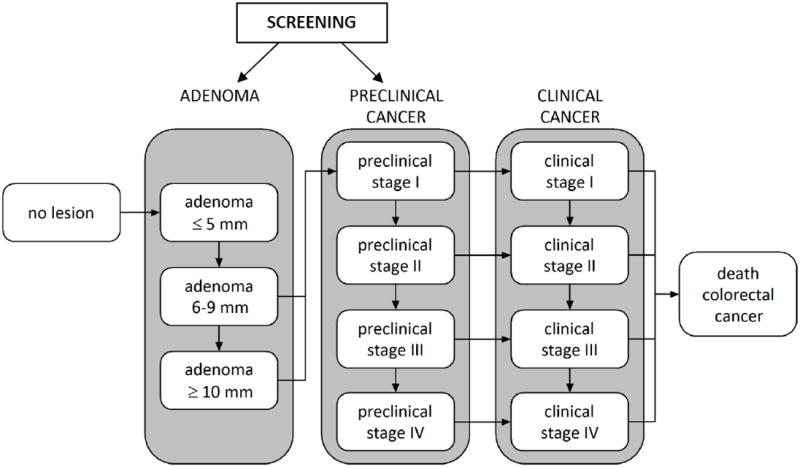
Graphical representation of natural history of colorectal cancer as modeled by MISCAN model. Screening provides the opportunity to intervene in the natural history of the adenoma carcinoma sequence. Screening can either remove a precancerous lesion (i.e., adenoma), thus moving a person to the “No lesion” state, or through early cancer detection, which makes an undiagnosed cancer clinically detected at a potentially earlier stage of disease where it is more amenable to treatment.
The effectiveness of each screening test is modeled through each test’s ability to detect lesions (i.e., adenomas, preclinical cancer). Once screening is introduced, a simulated person who has developed an underlying adenoma or preclinical cancer has a chance of having it detected during a screening episode depending on the sensitivity of the test for that lesion. For screened persons without an underlying lesion we apply the false-positive rate (1 – specificity) to determine whether or not that person will undergo an unnecessary follow-up examination. Hyperplastic polyps are not modeled explicitly but are reflected in the specificity of the test. Furthermore, a percentage of individuals with false-negative test results (i.e., adenoma or preclinical cancer present but not detected) will be referred to colonoscopy because of the detection of a hyperplastic polyp.
Study Population
We used the natural history model to estimate the distribution of underlying disease in terms of the presence, location, size, and type (adenoma vs. preclinical cancer) of lesions. We conducted an analysis of the effect of different screening strategies among a cohort of 50-year old individuals in the US population in 2005 who have never been screened as our base case.
Test strategies
A test strategy includes the initial screening test as well as follow-up diagnosis of a positive test, diagnosis and treatment of colorectal cancer and recurrent cancer, surveillance colonoscopy for those with adenomas, and treatment of any complications from screening. We compared the strategies of screening with FOBT every year, flexible sigmoidoscopy every five years, combinations of annual FOBT and sigmoidoscopy (every 5 years), and colonoscopy every 10 years. Although double contrast barium enema was included in the 2002 screening recommendations for the USPSTF 37; it was not included in the 2008 USPSTF recommendations and is not considered in this analysis. We evaluated three FOBTs (Hemoccult II, Hemoccult SENSA, and FIT) and two strategies for sigmoidoscopy (with and without biopsy and also with and without FOBT), and two representations for CT colonography for a total of 14 screening strategies plus no screening.
We assumed that all individuals begin CRC screening at age 50 as recommended by all three screening guidelines and end at age 80. A subject with a positive screening test is referred for colonoscopy. If adenomas are detected on colonoscopy then the individual begins surveillance with colonoscopy per the 2006 guidelines from the joint publication of the US Multi-Society Task Force and the American Cancer Society. 9, 38 The cohort was followed for their lifetimes to a maximum of age 100. The life-years gained per strategy were derived relative to no screening.
CRC Screening Test Characteristics
The sensitivity and specificity of the FOBTs were based on a literature review 39-40 and were consistent with those from the 2008 review of the evidence on CRC screening tests for the USPSTF.41 There are multiple FITs with varying cut points for positivity, number of slides, number of days tested, and preparations reported in the literature.39 Consequently FIT sensitivity and specificity criteria vary widely. The OC-Sensor FIT has recently been used in clinical trials in Holland 42 and Northern California at Kaiser Permanente (TR Levin – personal communication). Sensitivities for colonoscopy were based on a meta-analysis;43 we assumed the same sensitivities for sigmoidoscopy within the reach of the endoscope. We assumed 5% of subjects have more than one colonoscopy to visualize the entire colon and that the cecum is ultimately reached in 98% of subjects. For sigmoidoscopy, we assumed 80% of examinations reach the junction of the sigmoid and descending colon and 40% reach the beginning of the splenic flexure. 44-45 The test characteristics were consistent with the evidence review used in the USPSTF recommendations.41 The test characteristics for CT colonography were based on two clinical trials: Department of Defense (DoD)46 and the National CT Colonography Trial (ACRIN 6664).47
Table 2 contains an overview of test characteristics used in our analyses. Test parameters are given by person for the fecal tests and by lesion for colonoscopy, flexible sigmoidoscopy, and CT-colonography. The sensitivities stated in Table 2 are based on sensitivities of the test at one point in time. However, the life years gained and the costs for each test strategy are based on repeated screening over time.
Table 2.
Test characteristics for colorectal cancer screening tests
| Test | Sensitivity* by adenoma size or CRC (%)
|
Specificity (%) | |||
|---|---|---|---|---|---|
| ≤5 mm | 6-9 mm | ≥10 mm | CRC | ||
| Per Person | |||||
| Hemoccult II | 2.0 | 5.0 | 12.0 | 40.0 | 98.0 |
| Hemoccult SENSA | 7.5 | 12.4 | 23.9 | 70.0 | 92.5 |
| Fecal immunochemical test | 5.0 | 10.1 | 22.0 | 70.0 | 95.0 |
| Stool DNA (version 1.1) | 4.0 | 12.0 | 43.0 | 70.0 | 96.0 |
| Per lesion and within reach | |||||
| Sigmoidoscopy (within reach)† | 75.0 | 85.0 | 95.0 | 95.0 | 92.0‡ |
| Colonoscopy | 75.0 | 85.0 | 95.0 | 95.0 | 90.0‡ |
| CTC DoD 3D 6mm | -- | 83.6** | 92.2 | 92.2+ | 79.6§ |
| CTC ACRIN NCTC 2D/3D 6mm | -- | 57.0** | 84.0 | 84.0+ | 88.0§ |
CTC = computed tomography colonography; DoD = Department of Defense study 46, 79; NCTC = National CT Colonography Trial 47; -- indicates sensitivity is not provided because size is smaller than the colonoscopy referral threshold of 6mm
Sensitivity is provided per individual for stool-based tests and per lesion for endoscopy and CT colonography tests.
Sensitivity for CT colonography for adenomas 6-9 mm was mathematically derived from published tables as
Sensitivity for CRC was assumed to be the same as for adenomas of size ≥10 mm due to the small number of colorectal cancers detected in the DoD and NCTC studies
The lack of specificity with CT colonography reflects the detection of non-adenomatous polyps, artifacts, and adenomas smaller than the colonoscopy referral threshold of 6mm.
Test characteristics for sigmoidoscopy apply only to lesions in the distal colon and rectum.
The lack of specificity with sigmoidoscopy and colonoscopy reflects the detection of non-adenomatous lesions. With sigmoidoscopy, the presence of non-adenomatous lesions induces biopsy costs (in the case of sigmoidoscopy with biopsy) or results in referral for colonoscopy (in the case of sigmoidoscopy without biopsy). With colonoscopy, non-adenomatous lesions are removed and therefore induce polypectomy and biopsy costs.
Costs
Cost reimbursements for the components of screening vary by type of insurance plans, and copayments. In this chapter we present as an example the derivation of costs of a screening strategy based on Medicare reimbursement (without copayments) for each of the recommended screening tests and use these costs in conjunction with the MISCAN microsimulation model for the life years gained with screening for each test. We recognize the actual costs will vary in practice but use Medicare reimbursement without copayments as one standardized measure for costs.
Payer’s perspective
The base-case cost-effectiveness analysis was from the payer’s (CMS) perspective with costs stated as those which Medicare pays and based on Medicare payments of 2007 for procedures and tests associated with CRC screening, complications of screening and treatment.48 These payments reflect approximately 80% of the allowable charge, including the facility charges (as applicable) and physician services charges. (Thus the beneficiary’s co-pay is not reflected in the analysis). We also conducted an analysis from a modified societal perspective by including direct costs borne by beneficiaries as well as estimated patient time costs, but excluding costs due to lost productivity caused by early death or disability.
The screening test costs are provided in Table 3. Briefly, screening-related costs are based on the set of current procedural terminology (CPT) codes relevant to CRC screening in conjunction with the points of service for the procedures: 1) in the Ambulatory Surgery Center (ASC) setting, we include the Medicare ASC facility payment and the payment for physician professional services; 2) in the Outpatient Prospective Payment System (OPPS) setting, we include the Medicare OPPS facility payment and the payment for physician services; and 3) in the office setting, we include the payment to the physician that covers both the professional services and the facility costs of the physician’s office. The total costs per CPT code are weighted by the frequencies for points of service. Then the total costs per screening procedure are based on the total costs per CPT code that are part of the procedure and weighted by the frequencies of the CPT codes. Payments for a procedure across these settings are represented as an average of the three settings weighted by the frequency of which each setting is used for the procedure in 2007. We do not include the cost of a separate office visit for any of the screening strategies as we assume that all recommendations or arrangements for screening would already be associated with a previously-scheduled office visit. Payer cost for Hemoccult II, Hemoccult SENSA, and fecal immunochemical testing do not include additional charges for points of service because these costs are related only to the clinical laboratory fee schedule (http://www.cms.hhs.gov/ClinicalLabFeeSched/).
Table 3.
Screening tests costs based on CMS payment (2007 US dollars)
| Screening test | CMS cost, $* | Modified societal cost,** $ |
|---|---|---|
| Guaiac Hemoccult (II or SENSA) | 4.54 | 21.54 |
| Fecal immunochemical test | 22.22 | 39.22 |
| Flexible sigmoidoscopy | 160.78 | 270.30 |
| Flexible sigmoidoscopy with biopsy | 348.19 | 497.37 |
| Colonoscopy without polypectomy ‡ | 497.59 | 794.94 |
| Colonoscopy with polypectomy or biopsy‡ | 648.52 | 979.28 |
| CT colonography†* | 488.29 | 643.64 |
CMS cost represents approximately 80% of the allowable charge in 2007 dollars.
Modified societal costs include beneficiary costs (co-payments) and time costs in addition to the payer costs
Based on CMS payment for CT of the abdomen (CPT 74150), CT of the pelvis (CPT 72192), and image processing on an independent workstation (CPT 76377). No screening cost for CMS has been established at this time.
Base case cost for colonoscopy does not include additional anesthesia costs. A secondary sensitivity analysis in the CMS report considers an additional $74 cost added to colonoscopy for anesthesia in 29% and 100% of colonoscopies 11
Given that CTC has not been approved for screening in the Medicare population, there is no national CMS payment rate for a screening CTC at this time. Accordingly, we used as a proxy the national average CMS payment for an abdominal CT without contrast (CPT code 74150), a pelvic CT without contrast (CPT code 72192) and image processing on an independent workstation (CPT 76377). This base case cost estimate of CT colonography of $488.29 does not include costs for further radiological evaluations for extracolonic findings. 11
Screening test costs
The costs for colonoscopy without polypectomy were based on CPT codes 45378 (diagnostic colonoscopy), G0105 (colon screen in high risk individuals) and G0121 (colon cancer screening for non high risk individual). Costs for colonoscopy with polypectomy or biopsy were composed of codes 45380 (colonoscopy and biopsy), 45381 (colonoscopy, submucous injection), 45382 (colonoscopy/control bleeding), 45383 (lesion removal colonoscopy – fulguration), 45384 (lesion removal colonoscopy-hot biopsy) and 45385 (lesion removal colonoscopy-snare polypectomy.
We assumed that polypectomy was not performed with flexible sigmoidoscopy screening. However, we distinguished flexible sigmoidoscopy with and without biopsy. For flexible sigmoidoscopy without biopsy we used CPT codes 45330 (diagnostic sigmoidoscopy) and G0104 (CA screen; flexi sigmoidoscope). Flexible sigmoidoscopy with biopsy was based on CPT code 45331 (sigmoidoscopy and biopsy).
Polyp removal and pathology review
For the procedures with polypectomy or biopsy we included a pathology charge (CPT code 88305). The Medicare payment rates per jar were $82.40 for the Physician fee schedule for office and ASC settings, and $51.59 for the OPPS setting. We assumed that all biopsies and removed polyps are reviewed by a pathologist and that a separate jar is submitted for each of 4 colon segments so the resection area can be identified should surgery be necessary. Data from the National Colonoscopy Study were used to provide the estimate of 1.38 as the average number of jars per patient with polyps (hyperplastic, other polyps, and adenomas) (personal communication, Ann Zauber, Ph.D.). Consequently, we multiplied the pathology fee by 1.38 to obtain the average pathology cost associated with colonoscopy with polypectomy.
Multiple polyps requiring the same type of polypectomy removal within a single colonoscopy do not add an incremental cost to the procedure. However if different types of polypectomy are required in removing multiple polyps then CMS reimburses 100% for the most expensive procedure and 50% of the facility cost for the second procedure. As a simplifying assumption we use the weights of procedures by CPT type and do not consider different fees for different combinations of endoscopy CPT codes for polyp removal.
Anesthesia cost for colonoscopy
For the base case the cost of moderate sedation was included in the cost of colonoscopy, assuming that it is not administered by an anesthesiologist. Some anesthesia costs such as Monitored Anesthesia Care (MAC) provided by an anesthesia professional are currently being reimbursed in addition to the colonoscopy procedure. The additional CMS payment for the anesthesia was $74 based on an average cost for the CPT code 00810 in 2007 for monitored anesthesia care for lower endoscopy procedures.
Complications of screening
There are essentially no complications from the stool-based screening tests (Hemoccult II, Hemoccult SENSA, or FIT). However patients undergoing colonoscopy and, to a lesser extent, flexible sigmoidoscopy and CT colonography are at risk of experiencing complications from the procedures. Since individuals with a positive FOBT, sigmoidoscopy, or CT colonography are referred for a follow-up colonoscopy, the complications and the associated costs are relevant and accounted for in all of the screening strategies.
The major complications of colonoscopy are perforations, which can occur with or without polypectomy, serosal burns, bleeds requiring transfusion and bleeds not requiring transfusion. 49-53 The costs of complications were based on the relevant diagnosis-related group (DRG) codes (Table 4). Risks of complications reported in organized screening programs 50-51, 54 are lower than those reported for general practice colonoscopies 49, 55 and increase with increasing age. 53, 56 Overall risks of complications of colonoscopy have declined over time. Our estimates for colonoscopy risks are similar to a population based study in Canada 57 with rates of 1.64 per 1000 for bleeding and 0.85 per 1000 for perforation. They are also consistent with the evidence review by Whitlock 41 who stated that complication rates could not be derived for colonoscopies with and without polypectomy because of reporting limitations.
Table 4.
Summary of risks of complications and costs (2007 US dollars)
| Complication | Rate per 1000 | CMS cost, $ | Modified societal cost, $ |
|---|---|---|---|
| With colonoscopy | |||
| Perforation | 0.7 | 12,446 | 12,712 |
| Serosal burn | 0.3 | 5,208 | 5,474 |
| Bleed with transfusion | 0.4 | 5,208 | 5,474 |
| Bleed without transfusion | 1.1 | 320 | 586 |
| With flexible sigmoidoscopy | |||
| Perforation | 0.02 | 12,446 | 12,712 |
| With CT colonography | |||
| Perforation | 0.0456 | 12,446 | 12,712 |
Costs for colorectal cancer treatment
The net costs of CRC treatment are derived from a comparison of costs for CRC cases relative to those of matched controls in the SEER-Medicare files for the years 1998-2003 58 and vary by phase of care (Table 5). The initial phase is the first 12 months following diagnosis, the last-year of life phase is the final 12 moths of life, and the continuing phase is the months between initial and last year. This methodology was used previously by Brown to assess net costs of cancer treatment.59
Table 5.
Net payments for colorectal cancer care during 1998-2003 (in 2007 US dollars)*
| Last Year of Life
|
||||
|---|---|---|---|---|
| AJCC Stage | Initial Phase | Continuing Phase | Died from CRC | Died from Other Causes |
| Direct medical costs | ||||
| I | 25,487 | 2,028 | 45,689 | 11,257 |
| II | 35,173 | 1,890 | 45,560 | 9,846 |
| III | 42,885 | 2,702 | 48,006 | 13,026 |
| IV | 56,000 | 8,375 | 64,428 | 34,975 |
| Modified societal costs | ||||
| I | 32,720 | 2,719 | 56,640 | 17,408 |
| II | 43,752 | 2,561 | 56,417 | 15,740 |
| III | 53,003 | 3,573 | 59,481 | 19,413 |
| IV | 68,853 | 10,743 | 78,227 | 44,384 |
The initial phase of care is the first 12 months following diagnosis, the last-year–of-life phase is the final 12 months of life, and the continuing phase is all the months between the initial and last-year-of-life phases. Cancer-related costs in the continuing phase of care are an annual estimate.
Cost-effectiveness analysis
In order to conduct a cost effectiveness analysis we use the MISCAN microsimulation model to calculate the lifetime costs and life expectancy for a previously unscreened cohort of 50-year-old individuals residing in the US under different CRC screening strategies. Cost effective analysis does not select which strategy is economically preferred overall, but only which strategy is the most effective, in terms of life-years saved, for a given level of desired (or possible) expenditure.60
The first consideration in a cost-effectiveness analysis is whether a strategy is effective as represented by the life years gained (LYG) with screening without regard for the relative costs of the strategies (Table 6). We use an illustration from our analysis for the CMS for CT colonography 11 to demonstrate that the life years gained (per 1000) with screening (ie effectiveness) is the lowest for the strategies of annual Hemoccult II only and flexible sigmoidoscopy only every 5 years (214 and 222 per 1000 screened respectively) but higher and at approximately the same level of effectiveness for the strategies of sensitive FOBT’s with or without flexible sigmoidoscopy every 5 years (238-250 LYG) and colonoscopy every 10 years (243 LYG) under the assumption of 100% adherence for all aspects of testing and follow-up. CTC has a range of 231 to 241 LYG which is close to range of the sensitive FOBT’s without flexible sigmoidoscopy (238-240 LYG).
Table 6.
Cost-effectiveness analysis for 14 colorectal cancer screening strategies considered by one or more of the three guideline organizations: Life years gained, discounted and net costs, average and incremental cost effectiveness ratios (ACER and ICER)
| Strategy * | MISCAN model for life years gained and strategy costs per 1000 screened
|
|||||
|---|---|---|---|---|---|---|
| Life years gained | Discounted costs, $ | Net Discounted costs, $ | Discounted life-years gained | ACER $/LYG | ICER $/LYG | |
| No Screening | 0 | 2,320,612 | 0 | 0.0 | --- | |
| HII | 207 | 2,369,426 | 48,814 | 85.3 | 572 | 572 |
| HS | 240 | 2,615,292 | 294,680 | 100.2 | 2,941 | 16,605 |
| FIT | 238 | 2,688,092 | 367,480 | 99.7 | 3,686 | Dominated |
| SIGB | 222 | 2,725,559 | 404,947 | 89.2 | 4,540 | Dominated |
| SIG | 214 | 2,759,328 | 438,716 | 92.2 | 4,758 | Dominated |
| HII + SIGB | 246 | 2,760,602 | 439,990 | 103.0 | 4,272 | Dominated |
| HII + SIG | 246 | 2,823,342 | 502,730 | 102.9 | 4,886 | Dominated |
| HS + SIGB | 248 | 2,952,372 | 631,760 | 104.8 | 6,028 | 73,336 |
| HS + SIG | 249 | 2,933,686 | 613,074 | 104.4 | 5,872 | Dominated |
| FIT + SIGB | 250 | 3,151,945 | 831,333 | 105.6 | 7,872 | 272,160 |
| FIT + SIG | 251 | 3,058,485 | 737,873 | 105.0 | 7,027 | Dominated |
| COL | 243 | 3,011,165 | 690,553 | 101.8 | 6,783 | Dominated |
|
| ||||||
| CTC DoD | 241 | 3,685,253 | 1,364,641 | 100.6 | 13,565 | Dominated |
| CTC ACRIN | 231 | 3,751,074 | 1,430,462 | 96.1 | 14,885 | Dominated |
The bolded strategies represent the acceptable strategies with respect to life years gained and willingness to pay
LYG = life-years gained vs. no screening
ICER = incremental cost-effectiveness ratio
ACER = average cost-effectiveness ratio compared with no screening
HII = Hemoccult II
HS = Hemoccult SENSA
FIT= immunochemical fecal occult blood test;
SIG = sigmoidoscopy without biopsy
SIGB = sigmoidoscopy with biopsy
COL = colonoscopy
d = dominated; --- indicates default strategy (i.e., the least costly and least effective non-dominated strategy)
The two CT colonography strategies are not competing options. They are shown here together for comparison purposes only. The ICERs are assessed separately using each CT colonoscopy strategy in turn.
The next step in a cost effectiveness analysis is assessing the ratio of risks (generally in costs) to benefits (in life years gained) with screening. Cost effectiveness analysis generally discounts its costs by 3% to account for the up-front costs of screening while the benefits arise in the future. Given that costs are typically discounted (3%) the life years gained are also discounted by 3% in analyses comparing costs and life years gained. This discounting reflects the general societal preference to have a dollar in the present rather than in the future.
Cost effectiveness ratios (CER) are derived as average or incremental (marginal) measures. 61 An average CER (ACER) is derived without regard to other screening alternatives. It is the discounted cost of the strategy relative to no screening divided by the life years gained with screening and shows whether the net benefits of the strategy are a good value for the resources required among individuals who would not be screened at all without the availability of that strategy (Table 6). All the screening options considered have an average cost effectiveness ratio less than $15,000 which is considerably lower than the commonly accepted level of $50,000 per life year saved as an acceptable intervention. The strategies, excluding CTC, all had ACER’s even less than $8,000 per life year gained.
An incremental analysis is recommended by the Panel on Cost-Effectiveness in Health and Medicine 62 for competing strategies and shows whether the net benefits of a strategy are a good value for the resources required compared with the other currently available CRC screening strategies; this is the preferred approach in comparative effectiveness research. We conducted an incremental cost-effectiveness analysis from the perspective of CMS and discounted future costs and life years 3% annually for our report to CMS on CTC. We did not use quality adjusted life years gained because we did not have good measures on the effects of screening on quality of life particularly as quality relates to the anxiety of waiting for the results of the screening tests.
The discounted life years gained (on the y-axis) plotted against the discounted costs (on the x-axis) for each strategy provide descriptive and quantitative measures of the comparisons of risk to benefit (Figure 2). The higher the point, the more effective the screening strategy.60 As for the undiscounted analysis, all strategies except Hemoccult II and sigmoidoscopy alone have relatively high life years gained. The more to the left, the lower is the cost. The more to the right, the more expensive is the screening strategy. The strategies towards the upper left hand corner are those with the higher life years gained relative to lower costs per life year gained. Costs for strategies that include endoscopy are more expensive than those using FOBT’s only. Cost is $3,011,165 per 1000 screened for colonoscopy and $3,151,945 for flexible sigmoidoscopy plus FIT. The Hemoccult II strategy is the lowest cost of $2,369,426 per 1000 screened and is almost cost savings compared to the cost of not screening at all of $2,320,612. The highest cost strategy is CTC ($3,685,253 to $3,751,074).
Figure 2.
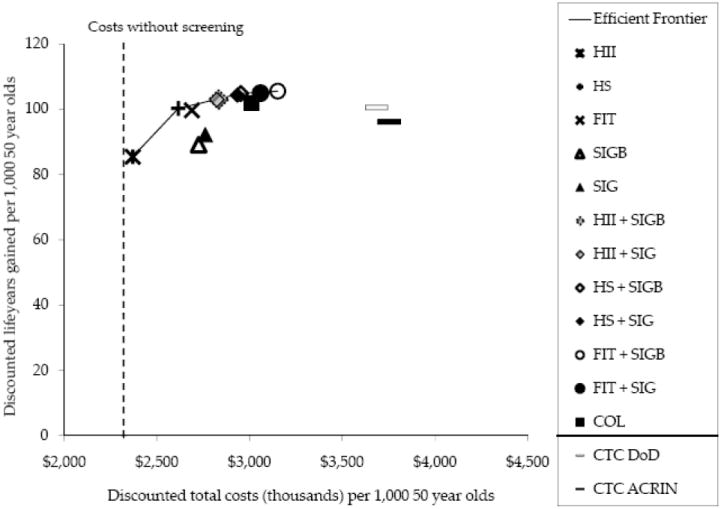
Cost effectiveness analysis for 14 colorectal cancer screening strategies.
For a quantitative comparison of life years gained per cost for multiple strategies, we ranked the screening strategies by increasing effectiveness (i.e., discounted number of life-years gained compared with no screening) from annual Hemoccult II with the lowest life years saved (85.3 per 1000 screened) to flexible sigmoidoscopy with biopsy every 5 years plus annual FIT (105.6 per 1000 screened) and compared their life years saved relative to the cost of the strategy. In figure 2 for the plot of costs versus life years gained, the black line links the strategies with the most life-years gained relative to a given level of costs and is called the efficient frontier. These strategies represent the set of efficient options and include Hemoccult II, Hemoccult SENSA, flexible sigmoidoscopy with biopsy and annual Hemoccult SENSA, and flexible sigmoidoscopy with biopsy and annual FIT. Strategies which are more costly and less effective (fewer life years gained) than another strategy are below the efficient frontier and are considered dominated by the more efficient strategies. These dominated strategies include FIT, sigmoidoscopy alone, 4 of the flexible sigmoidoscopy and FOBT combinations, colonoscopy, and CTC. However the only strategies relatively far off the efficient frontier (ie a dominated strategy) are flexible sigmoidoscopy alone and CTC. The other dominated strategies (including colonoscopy) are close to that of the efficient frontier and could be considered in the set of acceptable cost effective screening options. Based on this analysis Hemoccult II and flexible sigmoidoscopy are less attractive screening options because of the lower life years gained with Hemoccult II and the lower life years gained as well as the higher costs per life year gained than other options with flexible sigmoidoscopy. CTC also is a less attractive strategy with higher costs than other strategies which provide comparable or higher life years gained at lower costs.
This cost effective analysis is also used to visualize and quantify the increase in costs per life year gained when moving from one efficient strategy to the next highest strategy. The slope of the efficient frontier changes markedly going from Hemoccult II to the Hemoccult SENSA strategy. Then there is a relatively flat line with only slight increase in life years saved relative to increasing costs for the remaining strategies. The inverse of the slope is used as the measure of the relative performance of the efficient strategies and is the incremental cost-effectiveness ratio (ICER), defined as the additional cost of a specific strategy, divided by its additional clinical benefit, compared with the next least expensive strategy. For example the incremental cost per life year gained for the Hemoccult SENSA strategy relative to that of Hemoccult II is $16,605 per 1000 screened (Table 6) . The incremental cost per life year gained is even higher going from the Hemoccult SENSA alone to the flexible sigmoidoscopy plus Hemoccult SENSA strategy ($73,336) and markedly higher in going from there to the flexible sigmoidoscopy plus FIT ($272,160). Those strategies on the flat of the efficient frontier curve represent diminishing returns of effectiveness per expenditure. 60
The two strategies (DoD and ACRIN ) for CTC are far from the efficient frontier when using a cost of $488 per scan for CTC which is very close to the $498 cost for colonoscopy without polypectomy. However this value was a place holder cost for CTC when assessing the conditions under which CTC could be cost effective with the CRC screening tests currently reimbursed by CMS.11-12 uses threshold analysis to determine that the cost per scan for CTC would have to be $108 to $122 (for the CTC ACRIN and DoD strategies respectively) in the 65 year old cohort to place the CTC strategy on the efficient frontier (ie thus cost effective) relative to the life years gained with the CTC strategy for the base case analysis. In a previous analysis using slightly different CTC test characteristics, Lansdorp-Vogelaar 63 determined that CTC would need to be at a cost approximately 40% lower per scan than colonoscopy procedure with referral of CTC lesions 6 mm or larger and repeat CTC every 5 years to be cost effective.
Limitations of Cost Estimates
The costs of the screening tests, as well as the costs of complications associated with screening (primarily colonoscopy), were based on 2007 Medicare payment rates. To the extent that these rates change differentially in the future (e.g., a decrease in the payment rate for colonoscopy) our results will change.
Costs for CRC treatment in this analysis were for the period 1998 to 2003 when the use of the expensive biological therapies cetuximab and bevacizumab was limited 64. We have used the MISCAN microsimulation modeling to project that with the increase in chemotherapy costs for treating advanced colorectal cancer, most CRC screening strategies will provide cost savings by screening. (Colonoscopy does not become cost saving; however the net costs of this strategy approach cost savings. 65 )
Sensitivity analysis
An important component of cost-effectiveness analysis is the inclusion of sensitivity analysis on the base case assumptions of the model and costs. Lansdorp-Vogelaar 66 provides an example of a one-way and multivariate uncertainty analysis using a similar analysis as this presented here. An important aspect of sensitivity analysis includes assessing the assumptions on adherence to the test strategy requirements(e.g. annual FOBT or colonoscopy every 10 years).40 The results given in this chapter are based on 100% adherence for all tests.
In this chapter we present the results from only one of the CISNET models as an example of CEA for these screening recommendations. A strength of the CISNET program is comparative modeling to provide a special type of sensitivity analysis of independently developed models addressing the same analysis. Relatively similar results, and thus conclusions, were obtained by all three models although the absolute values and rank orderings do differ.11-12
Cost-effectiveness summary
In summary the cost effective analysis suggests that annual high sensitive FOBT’s (guaiac and FIT), flexible sigmoidoscopy every 5 years with a sensitive FOBT, and colonoscopy are reasonable cost effective screening strategies for CRC. Hemoccult II only and flexible sigmoidoscopy only would not be included in this set of acceptable tests. Similarly at this date with current levels of test costs based on diagnostic procedures, CTC would not be a cost effective choice.
Do we achieve these benchmarks in community practice?
In order to compare the common effectiveness of the screening procedures we first compared these tests with all having 100 percent adherence to all aspects of screening. However in practice only 50% of those 50 and older are compliant with current screening recommendations.67-68 Those with no medical insurance, no regular source of care, and less education are less likely to get CRC screening. 69 Furthermore, even in those having a positive screening test, there is non-adherence with follow-up diagnostic colonoscopy 70 and with surveillance colonoscopy for those with adenomas or CRC. 71 Also there is a decline in adherence for repeat FOBT testing.72 In the decision analysis for the USPSTF reducing adherence for colonoscopy screening from 100% to 80% reduced life years saved by 20% and reduction to 50% reduced colonoscopy life years saved 40% .40 Non-adherence for initial screening, follow-up of positive tests or surveillance, and regular repeat screening affects the impact of screening interventions. To increase screening adherence we need interventions to increase both patient willingness to be screened as well as organizational structures within the medical system to facilitate identifying subjects in need of initial screening and to provide effective reminders for screening tests and follow-up. Physician recommendation is one of the most powerful incentives for subjects to get screened. 68
The analysis presented here assumes that colonoscopy for primary screening or for follow-up for a positive screening test is of high quality. This includes a thorough examination of the colon based on good bowel preparation and reaching the cecum in 98% of cases. Effectiveness of the colonoscopy to prevent CRC can be compromised if colonoscopies do not achieve the standards as given by CO-RAD73-74 which include a benchmark of detection adenomas in of 25% of men and 15% of women on screening colonoscopy. Kaminski 75 has demonstrated that endoscopists with higher adenoma detection rates had lower rates of interval cancers. The ACG has established quality indicators for colonoscopy with a major focus on the quality of mucosal inspection and on obtaining safe and effective bowel preparation. Guidelines are given for an average of 6 minutes withdrawal time from time of reaching the cecum. Attention to preventing complications is stressed with recommendations for hydration before, during, and after the procedure. Furthermore continuing quality evaluation programs are recommended for multiple specialty practices. A large GI group in Minnesota has demonstrated that the quality measures for CO-RAd’s can be monitored in the general GI practice using electronic records.76
New evidence from a randomized controlled trial of flexible sigmoidoscopy 77 shows that flexible sigmoidoscopy reduces the incidence for rectal and left sided colon cancer but not for right sided colon cancer. A case-control study from Canada 78 suggests that colonoscopy did not reduce right sided colon cancer mortality. Further research is needed to determine the efficacy of colonoscopy for reducing right sided disease and whether the biology or the possibilities of polypectomy intervention differ for the right and left colon.
Current Screening Guidelines – differences and cost implications
This cost effectiveness analysis supports a common theme of the three Guideline groups that there are multiple acceptable colorectal cancer screening strategies. This analysis also identifies which screening tests are also cost effective given a range of willingness to pay per life year gained. Furthermore it is important that the subject have a choice in the decision of what test to use. The set of cost effective strategies are annual sensitive FOBT ‘s (either guaiac or FIT), flexible sigmoidoscopy every 5 years with a frequent sensitive FOBT, and colonoscopy( which is near the efficiency frontier). The cost effectiveness analysis suggests that Hemoccult II, even though cost effective, has a low life years gained compared to the other screening strategies and would not be recommended given the willingness to pay in the US. Also flexible sigmoidoscopy only every 5 years had lower life years gained than other strategies with similar costs (and was dominated) and also would not be included as a recommended strategy. The MultiSocieties and the ACG did not consider the combination of flexible sigmoidoscopy and sensitive FOBT which was cost effective and included in the menu of acceptable options by the USPSTF. Colonoscopy was only slightly more costly for its level of life years gained than the flexible sigmoidoscopy plus FOBT strategies which were on the efficient frontier. However colonoscopy is one of the more expensive tests per life year gained in the recommended cost efficient screening strategies. In contrast CTC would not be among the cost effective strategies unless the cost per testing time was considerably less than that of colonoscopy and with referral of 6 mm polyps to colonoscopy with a 5 year repeat CTC.
The cost effectiveness analysis was based on 100% adherence for all aspects of screening. In reality adherence is less overall and varies by screening test. Screening colonoscopy has increased rapidly in the past 5 years whereas flexible sigmoidoscopy and FOBT have markedly decreased. 68 Availability of high quality flexible sigmodioscopies could be an issue if its use continues to diminish. The cost effectiveness analysis suggested flexible sigmoidoscopy plus a sensitive FOBT would be cost effective; however, this strategy requires the most test completion (considering flexible sigmoidoscopy every 5 years and a FOBT annually or biennially). Consequently if adherence is low for either test, the overall impact of this strategy is affected.
The MultiSocieties suggest favoring the cancer prevention CRC screening methods over those that primarily detected CRC’s early. However the cost effectiveness analysis suggest that the sensitive FOBT’s had life years saved almost comparable to those with colonoscopy given high adherence. If there is differential adherence with lower levels of adherence, including regular repeat screening for FOBT’s , then the sensitive FOBT’s would not attain similar life years gained as those strategies that could prevent cancer. The role of adherence is key to understanding which CRC tests would provide higher life years gained at reasonable resource utilization and cost.
These results indicate that adherence to a strategy of screening is a critical component of saving life years. The currently available screening tests provide substantial prevention for life years gained and can be delivered within reasonable economic levels. Newer CRC screening tests need to be able to provide similar or higher levels of prevention, comparable levels of costs for screening, and high adherence.
Acknowledgments
Dr. Zauber is supported in part by Cancer Intervention and Surveillance Modeling Network grants from the National Cancer Institute: (U01-CA-097426 and U01-CA-115953.)
Footnotes
Conflict of interest: None.
References
- 1.Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology. 1997 Feb;112(2):594–642. doi: 10.1053/gast.1997.v112.agast970594. [DOI] [PubMed] [Google Scholar]
- 2.Mandel J, Bond J, Church T, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993 May 13;328(19):1365–1371. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 3.Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996 Nov 30;348(9040):1472–1477. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 4.Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996 Nov 30;348(9040):1467–1471. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- 5.Selby JV, Friedman GD, Quesenberry CP, Jr, Weiss NS. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992 Mar 5;326(10):653–657. doi: 10.1056/NEJM199203053261001. [DOI] [PubMed] [Google Scholar]
- 6.Newcomb PA, Norfleet RG, Storer BE, Surawicz TS, Marcus PM. Screening sigmoidoscopy and colorectal cancer mortality. Journal of the National Cancer Institute. 1992 Oct 21;84(20):1572–1575. doi: 10.1093/jnci/84.20.1572. [DOI] [PubMed] [Google Scholar]
- 7.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993 Dec 30;329(27):1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 8.Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008 Nov 4;149(9):627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 9.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008 May;134(5):1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] The American journal of gastroenterology. 2009 Mar;104(3):739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 11.Zauber AG, Knudsen AB, Rutter CM, et al. Cost-Effectiveness of CT Colonography to Screen for Colorectal Cancer. 2009 Available from http://www1.cms.hhs.gov/mcd/viewtechassess.asp?from2=viewtechassess.asp&where&=index&tid&=58&. [PubMed]
- 12.Knudsen AB, Lansdorp-Vogelaar I, Rutter CM, Savarino JE, Van Ballegooijen M, Kuntz KM. Cost-effectiveness of CT colonography screening for colorectal cancer in the Medicare population. J Natl Cancer Institute. doi: 10.1093/jnci/djq242. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iglehart JK. Prioritizing comparative-effectiveness research--IOM recommendations. N Engl J Med. 2009 Jul 23;361(4):325–328. doi: 10.1056/NEJMp0904133. [DOI] [PubMed] [Google Scholar]
- 14.Van Gossum A, Munoz-Navas M, Fernandez-Urien I, et al. Capsule endoscopy versus colonoscopy for the detection of polyps and cancer. N Engl J Med. 2009 Jul 16;361(3):264–270. doi: 10.1056/NEJMoa0806347. [DOI] [PubMed] [Google Scholar]
- 15.Bretthauer M. The capsule and colorectal-cancer screening--the crux of the matter. N Engl J Med. 2009 Jul 16;361(3):300–301. doi: 10.1056/NEJMe0903924. [DOI] [PubMed] [Google Scholar]
- 16.Pignone M, Saha S, Hoerger T, Mandelblatt J. Cost-effectiveness analyses of colorectal cancer screening: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2002 Jul 16;137(2):96–104. doi: 10.7326/0003-4819-137-2-200207160-00007. [DOI] [PubMed] [Google Scholar]
- 17.Loeve F, Boer R, van Oortmarssen GJ, van Ballegooijen M, Habbema JDF. The MISCAN-COLON simulation model for the evaluation of colorectal cancer screening. Comput Biomed Res. 1999;32:13–33. doi: 10.1006/cbmr.1998.1498. [DOI] [PubMed] [Google Scholar]
- 18.Frazier AL, Colditz GA, Fuchs CS, Kuntz KM. Cost-effectiveness of screening for colorectal cancer in the general population. JAMA. 2000 Oct 18;284(15):1954–1961. doi: 10.1001/jama.284.15.1954. [DOI] [PubMed] [Google Scholar]
- 19.Ness RM, Holmes AM, Klein R, Dittus R. Cost-utility of one-time colonoscopic screening for colorectal cancer at various ages. The American journal of gastroenterology. 2000 Jul;95(7):1800–1811. doi: 10.1111/j.1572-0241.2000.02172.x. [DOI] [PubMed] [Google Scholar]
- 20.Vijan S, Hwang EW, Hofer TP, Hayward RA. Which colon cancer screening test? A comparison of costs, effectiveness, and compliance. Am J Med. 2001 Dec 1;111(8):593–601. doi: 10.1016/s0002-9343(01)00977-9. [DOI] [PubMed] [Google Scholar]
- 21.Khandker RK, Dulski JD, Kilpatrick JB, Ellis RP, Mitchell JB, Baine WB. A decision model and cost-effectiveness analysis of colorectal cancer screening and surveillance guidelines for average-risk adults. Int J Technol Assess Health Care. 2000 Summer;16(3):799–810. doi: 10.1017/s0266462300102077. [DOI] [PubMed] [Google Scholar]
- 22.Pignone M, Rich M, Teutsch SM, Berg AO, Lohr KN. Screening for colorectal cancer in adults at average risk: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002 Jul 16;137(2):132–141. doi: 10.7326/0003-4819-137-2-200207160-00015. [DOI] [PubMed] [Google Scholar]
- 23.Pignone M, Russell LB, Wagner JL. Economic Models of Colorectal Cancer Screening in Average-risk. Washington, DC: National Academies Press; 2005. [PubMed] [Google Scholar]
- 24.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Mortality- All COD, Public-Use With State, Total U.S. (1969-2003), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2006. Underlying mortality data provided by NCHS (www.cdc.gov/nchs).
- 25.Clark JC, Collan Y, Eide TJ, et al. Prevalence of polyps in an autopsy series from areas with varying incidence of large-bowel cancer. International journal of cancer. 1985 Aug 15;36(2):179–186. doi: 10.1002/ijc.2910360209. [DOI] [PubMed] [Google Scholar]
- 26.Blatt LJ. Polyps of the colon and rectum: incidence and distribution. Dis Colon Rectum. 1961;4:277–282. [Google Scholar]
- 27.Arminski TC, McLean DW. Incidence and distribution of adenomatous polyps of the colon and rectum based on 1.000 autopsy examinations. Dis Colon Rectum. 1964 Jul-Aug;7:249–261. doi: 10.1007/BF02630528. [DOI] [PubMed] [Google Scholar]
- 28.Vatn MH, Stalsberg H. The prevalence of polyps of the large intestine in Oslo: an autopsy study. Cancer. 1982 Feb 15;49(4):819–825. doi: 10.1002/1097-0142(19820215)49:4<819::aid-cncr2820490435>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 29.Jass JR, Young PJ, Robinson EM. Predictors of presence, multiplicity, size and dysplasia of colorectal adenomas. A necropsy study in New Zealand. Gut. 1992;33:1508–1514. doi: 10.1136/gut.33.11.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johannsen LG, Momsen O, Jacobsen NO. Polyps of the large intestine in Aarhus, Denmark. An autopsy study. Scand J Gastroenterol. 1989 Sep;24(7):799–806. doi: 10.3109/00365528909089217. [DOI] [PubMed] [Google Scholar]
- 31.Bombi JA. Polyps of the colon in Barcelona, Spain. Cancer. 1988;61(7):1472–1476. doi: 10.1002/1097-0142(19880401)61:7<1472::aid-cncr2820610734>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 32.Williams AR, Balasooriya BA, Day DW. Polyps and cancer of the large bowel: a necropsy study in Liverpool. Gut. 1982 Oct;23(10):835–842. doi: 10.1136/gut.23.10.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rickert RR, Auerbach O, Garfinkel L, Hammond EC, Frasca JM. Adenomatous lesions of the large bowel. Cancer. 1979;43:1847–1857. doi: 10.1002/1097-0142(197905)43:5<1847::aid-cncr2820430538>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 34.Chapman I. Adenomatous polypi of large intestine: incidence and distribution. Ann Surg. 1963;157:223–226. doi: 10.1097/00000658-196302000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000 Nov 30;343(22):1603–1607. doi: 10.1056/NEJM200011303432203. [DOI] [PubMed] [Google Scholar]
- 36.Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. Journal of the National Cancer Institute. 1999 Mar 3;91(5):434–437. doi: 10.1093/jnci/91.5.434. [DOI] [PubMed] [Google Scholar]
- 37.U.S. Preventive Services Task Force. Screening for colorectal cancer: recommendation and rationale. Ann Intern Med. 2002 Jul 16;137(2):129–131. doi: 10.7326/0003-4819-137-2-200207160-00014. [DOI] [PubMed] [Google Scholar]
- 38.Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Published jointly in Gastroenterology. 2006;130:1872–85. doi: 10.1053/j.gastro.2006.03.012. [DOI] [PubMed] [Google Scholar]; CA Cancer J Clin. 56:143–59. [Google Scholar]
- 39.van Ballegooijen M, Habbema JDF, Boer R, Zauber AG, Brown ML. Report to the Agency for Healthcare Research and Quality: a comparison of the cost-effectiveness of fecal occult blood tests with different test characteristics in the context of annual screening in the Medicare population. 2003 Aug; Available from: http://www/cms.hhs.gov/coverage/download/id87.zip. [PubMed]
- 40.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2008 Nov 4;149(9):659–669. doi: 10.7326/0003-4819-149-9-200811040-00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the u.s. Preventive services task force. Ann Intern Med. 2008 Nov 4;149(9):638–658. doi: 10.7326/0003-4819-149-9-200811040-00245. [DOI] [PubMed] [Google Scholar]
- 42.Hol L, van Leerdam ME, van Ballegooijen M, et al. Screening for colorectal cancer: randomised trial comparing guaiac-based and immunochemical faecal occult blood testing and flexible sigmoidoscopy. GUT. 2010 Jan;59(1):62–68. doi: 10.1136/gut.2009.177089. [DOI] [PubMed] [Google Scholar]
- 43.Mulhall BP, Veerappan GR, Jackson JL. Meta-analysis: computed tomographic colonography. Ann Intern Med. 2005 Apr 19;142(8):635–650. doi: 10.7326/0003-4819-142-8-200504190-00013. [DOI] [PubMed] [Google Scholar]
- 44.Doria-Rose VP, Levin TR, Selby JV, Newcomb PA, Richert-Boe KE, Weiss NS. The incidence of colorectal cancer following a negative screening sigmoidoscopy: implications for screening interval. Gastroenterology. 2004 Sep;127(3):714–722. doi: 10.1053/j.gastro.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 45.Adam I, Ali Z, Shorthouse A. How accurate is the endoscopist’s assessment of visualization of the left colon seen at flexible sigmoidoscopy? Colorectal Dis. 22001 doi: 10.1046/j.1463-1318.2000.00091.x. [DOI] [PubMed] [Google Scholar]
- 46.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003 Dec 4;349(23):2191–2200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 47.Johnson CD, Chen MH, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008 Sep 18;359(12):1207–1217. doi: 10.1056/NEJMoa0800996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zauber AG, Lansdorp-Vogelaar I, Wilschut J, Knudsen AB, van Ballegooijen M, Kuntz KM. Cost-Effectiveness of DNA Stool Testing to Screen for Colorectal Cancer: Report to AHRQ and CMS from the Cancer Intervention and Surveillance Modeling Network (CISNET) for MISCAN and SimCRC Models. 2007 Dec 20; Available from: https://www.cms.hhs.gov/mcd/viewtechassess.asp?from2=viewtechassess.asp&id&=212&. [PubMed]
- 49.Levin TR, Zhao W, Conell C, et al. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006 Dec 19;145(12):880–886. doi: 10.7326/0003-4819-145-12-200612190-00004. [DOI] [PubMed] [Google Scholar]
- 50.Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000 Jul 20;343(3):162–168. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 51.Pox C, Schmiegel W, Classen M. Current status of screening colonoscopy in Europe and in the United States. Endoscopy. 2007 Feb;39(2):168–173. doi: 10.1055/s-2007-966182. [DOI] [PubMed] [Google Scholar]
- 52.Klabunde CN, Warren JL, Ransohoff DF, Brown ML. Complications of colonoscopy in the Medicare population. Gastroenterology. 2007;132(Supplement 2):A149. (995) [Google Scholar]
- 53.Warren JL, Klabunde CN, Mariotto AB, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med. 2009 Jun 16;150(12):849–857. W152. doi: 10.7326/0003-4819-150-12-200906160-00008. [DOI] [PubMed] [Google Scholar]
- 54.Regula J, Rupinski M, Kraszewska E, et al. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006 Nov 2;355(18):1863–1872. doi: 10.1056/NEJMoa054967. [DOI] [PubMed] [Google Scholar]
- 55.Levin TR, Conell C, Shapiro JA, Chazan SG, Nadel MR, Selby JV. Complications of screening flexible sigmoidoscopy. Gastroenterology. 2002 Dec;123(6):1786–1792. doi: 10.1053/gast.2002.37064. [DOI] [PubMed] [Google Scholar]
- 56.Ko CW, Riffle S, Michaels L, et al. Serious complications within 30 days of screening and surveillance colonoscopy are uncommon. Clin Gastroenterol Hepatol. 2010 Feb;8(2):166–173. doi: 10.1016/j.cgh.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rabeneck L, Paszat LF, Hilsden RJ, et al. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology. 2008 Dec;135(6):1899–1906. 1906 e1891. doi: 10.1053/j.gastro.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 58.Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States. Journal of the National Cancer Institute. 2008 May 7;100(9):630–641. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 59.Brown ML, Riley GF, Schussler N, Etzioni R. Estimating health care costs related to cancer treatment from SEER-Medicare data. Med Care. 2002 Aug;40(8 Suppl):IV-104–117. doi: 10.1097/00005650-200208001-00014. [DOI] [PubMed] [Google Scholar]
- 60.Mark DH. Visualizing cost-effectiveness analysis. Jama. 2002 May 8;287(18):2428–2429. doi: 10.1001/jama.287.18.2428. [DOI] [PubMed] [Google Scholar]
- 61.Petitti DB. Meta-Analysis, Decision Analysis, and Cost-Effectiveness Analysis: Methods for Quantitative Synthesis in Medicine. 2. Vol. 31. New York: Oxford University Press; 2000. [Google Scholar]
- 62.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine (NY) New York: Oxford University Press; 1996. [Google Scholar]
- 63.Lansdorp-Vogelaar I, van Ballegooijen M, Zauber AG, Boer R, Wilschut J, Habbema JD. At what costs will screening with CT colonography be competitive? A cost-effectiveness approach. International journal of cancer. 2009 Mar 1;124(5):1161–1168. doi: 10.1002/ijc.24025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schrag D. The price tag on progress--chemotherapy for colorectal cancer. N Engl J Med. 2004 Jul 22;351(4):317–319. doi: 10.1056/NEJMp048143. [DOI] [PubMed] [Google Scholar]
- 65.Lansdorp-Vogelaar I, van Ballegooijen M, Zauber AG, Habbema JD, Kuipers EJ. Effect of Rising Chemotherapy Costs on the Cost Savings of Colorectal Cancer Screening. Journal of the National Cancer Institute. 2009 Sep 24; doi: 10.1093/jnci/djp319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lansdorp-Vogelaar I, van Ballegooijen M, Zauber AG, et al. Individualizing colonoscopy screening by sex and race. Gastrointest Endosc. 2009 Jul;70(1):96–108. 108 e101–124. doi: 10.1016/j.gie.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shapiro JA, Seeff LC, Thompson TD, Nadel MR, Klabunde CN, Vernon SW. Colorectal cancer test use from the 2005 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2008 Jul;17(7):1623–1630. doi: 10.1158/1055-9965.EPI-07-2838. [DOI] [PubMed] [Google Scholar]
- 68.Enhancing Use and Quality of Colorectal Cancer Screening. Paper presented at: NIH State-of-the-Science Conference; Bethesda, Maryland. 2010. [Google Scholar]
- 69.Coups EJ, Manne SL, Meropol NJ, Weinberg DS. Multiple behavioral risk factors for colorectal cancer and colorectal cancer screening status. Cancer Epidemiol Biomarkers Prev. 2007 Mar;16(3):510–516. doi: 10.1158/1055-9965.EPI-06-0143. [DOI] [PubMed] [Google Scholar]
- 70.Miglioretti DL, Rutter CM, Bradford SC, et al. Improvement in the diagnostic evaluation of a positive fecal occult blood test in an integrated health care organization. Med Care. 2008 Sep;46(9 Suppl 1):S91–96. doi: 10.1097/MLR.0b013e31817946c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schoen RE, Pinsky PF, Weissfeld JL, et al. Utilization of surveillance colonoscopy in community practice. Gastroenterology. 2010 Jan;138(1):73–81. doi: 10.1053/j.gastro.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weller D, Coleman D, Robertson R, et al. The UK colorectal cancer screening pilot: results of the second round of screening in England. Br J Cancer. 2007 Dec 17;97(12):1601–1605. doi: 10.1038/sj.bjc.6604089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. The American journal of gastroenterology. 2006 Apr;101(4):873–885. doi: 10.1111/j.1572-0241.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 74.Lieberman D. A call to action--measuring the quality of colonoscopy. N Engl J Med. 2006 Dec 14;355(24):2588–2589. doi: 10.1056/NEJMe068254. [DOI] [PubMed] [Google Scholar]
- 75.Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010 May 13;362(19):1795–1803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 76.Shaukat A, Oancea C, Bond JH, Church TR, Allen JI. Variation in detection of adenomas and polyps by colonoscopy and change over time with a performance improvement program. Clin Gastroenterol Hepatol. 2009 Dec;7(12):1335–1340. doi: 10.1016/j.cgh.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 77.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010 May 8;375(9726):1624–1633. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 78.Baxter N, Goldwasser M, Paszat L, Saskin R, Urbach D, Rabeneck L. Supplement to Gastroenterology, Digestive Disease Week. Washington, DC: 2007. A population-based case control study of the effectiveness of colonoscopy for prevention of death from CRC. [Google Scholar]
- 79.Pickhardt PJ, Lee AD, Taylor AJ, et al. Primary 2D versus primary 3D polyp detection at screening CT colonography. AJR Am J Roentgenol. 2007 Dec;189(6):1451–1456. doi: 10.2214/AJR.07.2291. [DOI] [PubMed] [Google Scholar]


