Abstract
Importance
Breast MRI is highly sensitive for detecting breast cancer. Low specificity, high cost and little evidence regarding mortality benefits, however, limit recommendations for its use to high risk women. How breast MRI is actually used in community settings is not known.
Objective
To describe breast MRI trends and indications in a community setting.
Design
Retrospective cohort study.
Settings
Large not-for-profit health plan and multi-specialty group medical practice, both in New England.
Participants
: 10,518 women age 20 and older enrolled in the health plan for at least one year who had ≥ 1 breast MRIs between January 1, 2000 and December 31, 2011.
Main outcomes
Breast MRI counts were obtained from claims data. Clinical indication (screening, diagnostic evaluation, staging/treatment, or surveillance) for each MRI in the claims data was determined by a prediction model developed from electronic medical records on a subset of participants. Breast cancer risk status was assessed using claims data and, for the subset, also through electronic medical record review.
Results
: Breast MRI use increased more than 20-fold from 6.5 per 10,000 women in 2000 to its peak of 130.7 per 10,000 in 2009. Use declined and stabilized to 104.8 per 10,000 by 2011. Screening and surveillance, rare indications in 2000, together accounted for nearly 60% of MRI use by 2011; 30% had claims-based personal and 50% family history of breast cancer, while 4% of women had a genetic mutation noted. In the subset of women who had electronic medical records and received screening or surveillance MRIs, only 21% had evidence of meeting the American Cancer Society's (ACS's) criteria for breast MRI. Conversely, only half of women with documented deleterious genetic mutations received breast MRI screening.
Conclusions
Breast MRI use rose steeply over ten years and then stabilized, especially for screening and surveillance among women with family or personal history of breast cancer; the majority of women receiving screening and surveillance breast MRIs did not have evidence in their medical records meeting ACS criteria and many women with mutations were not screened. Efforts are needed to ensure that breast MRI use and documentation are focused on those women who will benefit most.
Introduction
Breast magnetic resonance imaging (MRI) was introduced into clinical practice in the 1990s 1. Multiple studies have shown that MRI is more sensitive than mammography in detecting breast cancer but has lower specificity, substantially higher cost, and is more invasive, requiring an injection of intravenous contrast material 2-10. Furthermore, there have been no randomized trials to determine whether breast MRI reduces breast cancer mortality more than mammography. Because of breast MRI's limitations, cost, and lack of evidence on patient outcomes, most expert groups have recommended limiting its use to patients at increased substantially risk of breast cancer, such as women who have deleterious genetic mutations or strong family histories for breast cancer, or for selected clinical situations 11-13. Health benefits may not outweigh potential harms for women at lower risk of breast cancer.
Little is known about how breast MRI is used in community practice. In the early 2000s availability of breast MRI services was limited and use was rare 14, 15. By 2007, nearly 75% of a national sample of breast imaging facilities offered breast MRI 16. In our setting, we found a total of 333 documented breast MRIs in a population of over 80,000 adult women from 2000 through 2004, with half performed for diagnostic purposes 15. In a study of Medicare-insured breast cancer patients, use increased from 3% in 2003 to 10% in 2005 although the indication was not specified 17. According to the 2010 U.S. National Health Interview Survey, approximately 2% of US women surveyed (253 of 11,222) self-reported having a recent breast MRI, with the majority (60%) reporting having diagnostic MRI 18. A study in a community hospital found that half of the breast MRIs performed between 2007 and 2009 were for screening or surveillance 19. Long-term trends in rates of MRI use overall and by indication are largely unknown.
We examined the trend in breast MRI use over the past decade in a large not-for-profit health plan covering several states in New England. We also estimated trends in breast MRI use for specific indications – screening, diagnosis, staging/treatment, and surveillance – by applying a prediction model for breast MRI indication developed using clinical notes from a subset of women. For that subset of women, we also examined appropriate use based on the 2007 ACS guidelines among women undergoing screening or surveillance breast MRIs.
Methods
Study settings
Data for this study were drawn from two settings: Harvard Pilgrim Health Care (HPHC) and Atrius Health. HPHC is a large not-for-profit health plan covering more than one million members in Massachusetts, New Hampshire and Maine. Atrius Health is a large non-profit consortium of multi-specialty medical groups in Massachusetts serving over 700,000 patients that share a common electronic medical record system. Approximately 30% of Atrius Health patients were insured by HPHC during the study period. This study was approved by the Institutional Review Board at Harvard Pilgrim Health Care Institute.
Overall breast MRI use
We used automated medical claims data from HPHC to document overall trends in breast MRI use during the study period. We identified women aged 20 and older who had at least one breast MRI performed from January 1, 2000 to December 31, 2011. Bilateral and unilateral breast MRI procedures were identified through Common Procedure Terminology (CPT) codes (76093 or 76094 prior to 12/31/2006; 77058 or 77059 after 1/1/2007) and Healthcare Common Procedure Coding System (HCPCS) procedure codes (C8903 through C8908). We restricted analysis to women continuously enrolled in HPHC for at least six months before and after each MRI procedure.
Breast MRI use by clinical indication
Breast MRI procedure codes in medical claims are not specific to clinical indication. Therefore to examine trends by clinical indication in the HPHC population, we developed a model to predict clinical indication. To develop the model, we used electronic medical record data and free-text provider notes from the subset of HPHC members who underwent at least one breast MRI between January 1, 2000 to December 31, 2008 and for whom both HPHC medical claims and Atrius Health electronic medical records for the breast MRI were available (the HPHC-Atrius Health subset). We determined the clinical indication for each MRI in this subset based on the following definitions (16):
Screening - if performed on a woman with no prior diagnosis of invasive or in situ breast cancer, who was asymptomatic at the time of the MRI and who had no physical or breast imaging abnormalities in the prior six months;
Diagnostic - if performed as part of the diagnostic evaluation or follow-up of a breast symptom or physical abnormality noted by the woman or her clinician and/or a radiologic breast abnormality, including one noted on a prior breast MRI, in the prior six months;
Staging/treatment - if performed on a woman who had a new biopsy-proven diagnosis of breast cancer in the prior six months, to examine extent of breast cancer, inform definitive treatment choice, or monitor neo-adjuvant treatment response; and
Surveillance for recurrence – a routine breast MRI performed on a woman with a prior diagnosis of invasive or in situ breast cancer, with no new symptoms or findings and more than six months after definitive surgery or treatment.
The few MRIs performed for the sole purpose of evaluating a symptom or sign related to a breast implant (e.g., suspected rupture) were categorized separately.
Two trained reviewers (NKS, ESM) assessed the clinical indication for each MRI in the subset with disagreements reconciled by independent review by a clinician (SWF, LN). The abstracted clinical indication for each breast MRI was then linked to the corresponding HPHC claim.
Prediction model for clinical indication
With the linked abstracted indication and HPHC claims data from the HPHC-Atrius Health subset, we used multinomial logistic regression to develop a model that predicted the clinical indication from diagnostic and procedure codes in the medical claims. The model also included information about the timing of these diagnoses and procedures relative to each breast MRI (Appendix Table). Predictor variables retained in the final model were chosen based on their relevance to the clinical indication definitions as well as their added contribution to the overall log likelihood of the model. We built the model using a random sample of half of the HPHC-Atrius Health subset, reserving the remaining half for validation and calculating sensitivity and positive predictive value, overall and for each clinical indication.
Analysis
Women's characteristics including age and breast cancer risk were determined using HPHC enrollment data and diagnosis codes available in the medical claims. For those women undergoing screening or surveillance MRIs in the HPHC-Atrius Health subset, we also determined breast cancer risk by review of the clinical notes in the electronic medical records to assess whether the ACS guideline criteria were met.
We calculated overall and indication-specific breast MRI usage rates per 10,000 women in the HPHC population, for calendar years 2000 through 2011. Indication-specific breast MRI use was estimated by applying the prediction model to the HPHC claims data. To avoid over-counting prior to the introduction of breast-specific MRI machines allowing a bilateral MRI to be done as a single procedure, two MRIs within 10 days of each other before 2006 were assumed to be equivalent to one bilateral MRI. For each year, the denominator comprised all women age 20 and older enrolled in HPHC adjusted to reflect the eligibility requirements of one year of continuous enrollment in the numerator.
Annual percent changes in overall and indication-specific rates were estimated using the Joinpoint Regression Program (Version 3.5.4). The Joinpoint program fits line segments to the data and determines statistically significant differences in slope between two connected line segments that indicate points of change in trend 20. All other analyses, including the development of the prediction model, were conducted in STATA Version 10 (Stata Corp, College Station, Texas).
Results
Patient characteristics
The average annual HPHC-insured population of women age 20 and older was over 250,000 during the study period. We identified 13,339 women with one or more breast MRIs, who together had 23,276 breast MRIs. Applying eligibility criteria excluded 2821 women who did not meet the six-month enrollment window before and after the breast MRI, leaving 10,518 women aged 20 and older who had a total of 18,215 breast MRIs for our analysis (Table 1). These women were comparable in demographic characteristics to those who did not meet eligibility requirements (data not shown). Women ranged in age from 20 to 89 and the average age at first MRI was 49.5 years; 65% had only 1 MRI during the study period. Half (5439 women) had a family history of breast cancer while 3.5% (372 women) were identified by claims as having a deleterious BRCA mutation placing them at high risk for breast cancer.
Table 1.
Characteristics ascertained from claims data of HPHC women who had at least one breast MRI from 2000-2009 and the HPHC-Atrius Health subset used in the development of the prediction model for breast MRI clinical indication.
| Characteristics | HPHC cohorta | Subset of HPHC cohort who received care at Atrius Healthb |
|---|---|---|
| Women age 20 and older, n (Reference Year) | 261,943 (2011) | 64,002 (2008) |
| At least one MRI, n | 10,518 | 998 |
| MRIs, n | 18,215 | 1,636 |
| Age at first MRI, n (%) | ||
| 20-29 | 187 (1.8) | 16 (1.6) |
| 30-39 | 1247 (11.9) | 91 (9.2) |
| 40-49 | 3891 (37) | 338 (33.8) |
| 50-59 | 3660 (34.8) | 387 (38.8) |
| 60-69 | 1356 (12.9) | 136 (12.6) |
| 70-79 | 157 (1.5) | 26 (2.6) |
| 80+ | 20 (0.2) | 4 (0.4) |
| MRIs per woman, mean (range) | 1.73 (1-12) | 1.64 (1-9) |
| Multiple MRIs, n (%) | 3709 (35.3) | 338 (32.9) |
| Breast cancer risk, n (%)c | ||
| Personal history | 2369 (22.5) | 275 (27.6) |
| Genetic mutation | 372 (3.5) | 28 (3) |
| Family history of breast cancer | 5469 (51.7) | 422 (42.3) |
MRI = magnetic resonance imaging; HPHC = Harvard Pilgrim Health Care
HPHC cohort is restricted to individuals who were continuously enrolled in HPHC during the 6 month period before and after a breast MRI. If a woman had multiple MRIs, only those MRIs that met the enrollment criteria were included.
The subset of HPHC insured patients receiving care at Atrius Health during 2000-2008 (restricted to those meeting 6 month eligibility criteria) with medical record abstracted clinical indications for each breast MRI.
Risk categories defined by presence of diagnostic or procedure code in HPHC claims data at least 45 days prior to the breast MRI for personal history of breast cancer and in the year prior or subsequent to breast MRI for the remaining.
Categories are not mutually exclusive. Personal history of breast cancer defined by presence of at least one ICD-9 codes: 174, 233.0, V10.3; Deleterious genetic mutation for breast cancer defined by ICD-9 code: V84.01; and family history of breast cancer defined by ICD-9 code: V16.3.
Overall breast MRI use
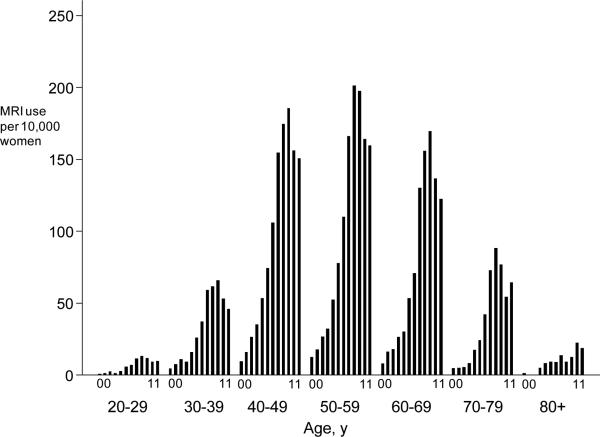
Breast MRI use increased approximately 20-fold from 198 (6.5 per 10,000 women) in 2000 to 2744 (104.8 per 10,000) by 2011. From 2003 through 2011, the average annual percentage increase was 21% (Table 2). However a statistically significant change in trend occurred in 2008; between 2003 and 2008,, use increased annually by 46% (95% CI: 39%; 52%) but and then decreased annually by 10% (95% CI: -19%;-0.5%) from 2009 through 2011. Age-specific rates followed similar time trends; rates rose overtime across all ages through 2009 and showed subsequent declines (Figure 1, panel A). Women in their 40s and 50s consistently had the highest rates of use.
Table 2.
Rates of breast MRI use, overall and by clinical indication, and average annual percent rate increasesa
| MRI Indication | Procedures/10,000 women | Average Annual Increase | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2003 | 2009 | 2011b | 2003-2011 | 2003-2009 | 2009-2011 | |||||||
| Rate | n | Rate | n | Rate | n | % | 95% CI | % | 95% CI | % | 95% CI | |
| Overall | 19.6 | 506 | 130.7 | 3239 | 104.8 | 2744 | 21c | 18;26 | 46c | 39;52 | −10c | −19;1 |
| Screening | 1.5 | 39 | 37.3 | 924 | 32.3 | 846 | 44c | 28;60 | 76c | 55;100 | 2;3 | −18:28 |
| Diagnostic evaluation | 11.6 | 300 | 43.4 | 1076 | 29.8 | 780 | 13c | 8;18 | 22c | 16;27 | −9c | −17;−0.5 |
| Staging/Treatment | 3.3 | 84 | 18.3 | 453 | 14.6 | 382 | 17c | 8;28 | 40c | 25;57 | −13 | −33;13 |
| Surveillance | 2.3 | 58 | 31.7 | 785 | 28.1 | 736 | 37c | 29;47 | 61 c | 49;74 | 5 | −8;20 |
MRI = Magnetic resonance imaging, CI = Confidence Interval
Data shown beginning in 2003 as there were less than 5 breast MRIs for some clinical indications prior to 2003. Average annual percent increase estimated using the Joinpoint software. Overall use includes those with suspected rupture of breast implants. The sum of the four indication-specific rates is therefore less than overall.
The Rates of use in 2011 were extrapolated using the procedure counts from the first half of the year.
P-value<0.05 that the average annual increase is statistically different from zero.
dNo statistically significant difference in rate of increase in use was found for staging/treatment.
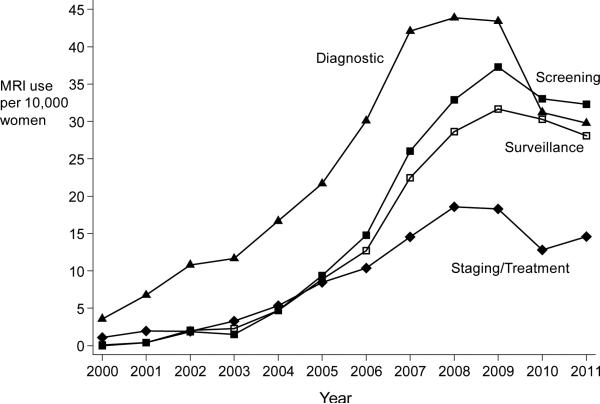
Figure 1.
Panel A: Age-specific rates of breast MRI use from 2000 through 2011. Each bar within an age-group represents a calendar year. Panel B: Indication-specific rates of breast MRI use from 2000-2011 for four primary indications: screening, diagnostic, staging/treatment, surveillance.
Estimation and validation of prediction model for clinical indication
We documented clinical indications for 2072 MRIs from the subset of 1169 HPHC-insured women cared for at Atrius Health who received at least one breast MRI from 2000 through 2008 (225 of whom were included in an earlier study [15]). We were unable to determine indication for 175 breast MRIs. Enrollment eligibility requirements reduced the sample to 1636 breast MRIs from 998 women; 758 were used for model development and 878 for validation. MRIs related to breast implants (36 of 1636) were included in the overall model estimation; however, we excluded them from the indication-specific analysis because we were interested in applications associated with breast cancer.
The final prediction model contained 15 variables (Appendix Table) describing the timing of breast imaging, diagnostic and treatment procedures, prior breast MRIs, family and genetic risk for breast cancer, benign breast disease and breast cancer diagnoses. The model had an overall prediction accuracy of 92% in the development set (data not shown) and 82% in the validation set (Table 3). Positive predictive values in the validation set ranged from 76% to 88% for the four clinical indications. The prediction model misclassified approximately 16% of the breast MRIs performed for screening and surveillance as diagnostic, primarily due to the timing of a prior abnormal mammogram that required additional breast imaging other than MRI to resolve.
Table 3.
Discrimination ability of the clinical indication prediction model to predict the four breast cancer specific clinical indications for breast MRI in the validation data set from the HPHC-Atrius Health subset.
| Abstracted Indication | |||||
|---|---|---|---|---|---|
| Model Predicted Indication | Screening | Diagnostic | Staging/Treatment | Surveillance | Total |
| Screening | 131 | 21 | 1 | 0 | 153 |
| Diagnostic | 26 | 263 | 17 | 38 | 344 |
| Staging/Treatment | 0 | 15 | 111 | 10 | 136 |
| Surveillance | 1 | 17 | 8 | 188 | 214 |
| Total | 158 | 316 | 137 | 236 | 847 |
| Prediction Accuracy | |||||
| “Sensitivity”a | 0.83 | 0.83 | 0.81 | 0.80 | 0.82 |
| PPVb | 0.86 | 0.76 | 0.82 | 0.88 | |
MRI = magnetic resonance imaging; HPHC = Harvard Pilgrim Health Care
Sensitivity = number correctly predicted by the model/total number of MRIs assigned after medical record abstraction, for each indication and overall.
Positive Predictive Value = number of MRIs correctly predicted by model /total number assigned after medical record abstraction, for each indication and overall.
Breast MRI use by clinical indication
Similar to the trends in overall use, we found that rates for all four indications increased over time and peaked between 2008 and 2009 and then declined or stabilized.(Figure 1, panel B). The greatest increase was in screening and surveillance. From 2003 through 2008, use for screening and surveillance increased an average of 76% and 61%, respectively, per year (Table 2). Diagnostic breast MRI comprised 60% of all breast MRIs in 2003 but only 28% in 2011. By 2011, screening was the leading breast MRI indication, at 32 per 10,000 women (31% of total use); use for diagnosis and surveillance followed at 30 and 28 per 10,000, respectively.
Only 3.5% women had a deleterious genetic mutation for breast cancer noted in medical claims (Table 4). Women undergoing screening and surveillance MRI had higher frequency of this marker compared with the other indications (5-6% versus 2-3%). Nearly half of the women with a mutation noted (180 of 372) underwent MRI for screening. Approximately 80% of women undergoing screening MRIs had a family history of breast cancer noted in their medical claims compared with only 40% of those undergoing MRI for other indications. Among women in the HPHC-Atrius Health subgroup who underwent at least one screening or surveillance breast MRI, we found that 21% (90 of 429) had family history documentation and/or positive mutation status in their medical records supporting a lifetime risk greater than 20%.
Table 4.
Women's use of breast MRI by breast cancer risk and number
| Women undergoing breast MRI | Women undergoing breast MRI | By Clinical Indicationa | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Screening | Diagnostic | Staging/Treatment | Surveillance | |||||||
| no. women | Col % | no. women | Col % | no. women | Col % | no. women | Col % | no. women | Col % | |
| Total | 10518 | 100 | 3049 | 100 | 5330 | 100 | 2113 | 100 | 2116 | 100 |
| Breast Cancer Riskb | ||||||||||
| Personal history | 3167 | 30 | 0 | 0 | 1077 | 20.2 | 2113 | 100 | 2086 | 98.5 |
| Genetic Mutation | 372 | 3.5 | 180 | 6 | 107 | 2.0 | 66 | 3.1 | 108 | 5.1 |
| Family History | 5439 | 52 | 2501 | 82 | 2283 | 43 | 863 | 41 | 905 | 43 |
| Number of MRIsc | ||||||||||
| Only one breast MRI | 6810 | 65 | 1687 | 55 | 3204 | 61 | 1143 | 54 | 688 | 33 |
| More than one breast MRI | 3708 | 35 | NA | NA | NA | NA | ||||
| More than one breast MRI for same indication | 2937 | 28 | 846 | 28 | 1120 | 21 | 403 | 19 | 941 | 44 |
MRI = Magnetic resonance imaging; Col = Column; NA = not applicable
Sum across clinical indications is greater than total as women may have multiple MRIs, each with a different clinical indication.
Risk categories defined by presence of diagnostic or procedure code in HPHC claims data at least 45 days prior to the breast MRI for personal history of breast cancer and in the year prior or subsequent to breast MRI for the remaining. For staging/treatment breast MRI, no restriction on timing was used in defining personal history of breast cancer. Categories are not mutually exclusive. Personal history of breast cancer defined by presence of at least one ICD-9 codes: 174, 233.0, V10.3; Deleterious genetic mutation for breast cancer defined by ICD-9 code: V84.01; and family history of breast cancer defined by ICD-9 code: V16.3.
Categories are not mutually exclusive.
More women undergoing breast MRI for screening or surveillance purposes had multiple MRIs (28% and 44%, respectively) compared with those undergoing MRI for diagnostic or staging/treatment purposes (21% and 19%) (Table 4). Further, while only 11% of all women had three or more breast MRIs for the same clinical indication, over 70% were women undergoing screening or surveillance MRI. Women undergoing MRI for screening purposes were younger on average than women receiving MRI for other reasons.
Discussion
Breast MRI use rose more than 20-fold, from 6 to 131 per 10,000 women between 2000 and 2009 and then declined slightly to 105 per 10,000 by 2011 in a large multi-state health plan in New England. Rates of breast MRI use by age followed the same trends,, with the highest use among women aged 40 to 59. From 2000 through 2009, breast MRI use rose for all indications – screening, diagnostic workup, staging/treatment, or surveillance for recurrence – with use for screening and surveillance showing the greatest increases, then declining for diagnostic workup and stabilizing for the other indications.
While rare early in the decade, screening and surveillance together accounted for nearly 60% of all breast MRIs by 2011. To our knowledge, this is the first study to document trends in breast MRI rates over more than a decade in a large community-based population with commercial insurance.
In 2007, the American Cancer Society released guidelines recommending routine use of breast MRI as an adjunct screening tool for women with at least a 20% lifetime risk of breast cancer due to genetic mutations and/or strong family history 11. In our study, screening was the fastest growing indication for a breast MRI but only about half of 372 women with claims-based history of genetic mutations had screening breast MRI and of those, half had multiple screening MRIs. This pattern suggests that recommendations for screening high risk women with breast MRI are beginning to be followed in community practice, but the fact that only half of those eligible for screening breast MRI had one suggests more should be done. Also, according to several estimates as many as 6% of all women may be eligible for screening MRI 21, 22. Of approximately 250,000, only 3049 women (1.2%) underwent screening MRI in our study and of those, very few (180 women) had a claims-based history of a genetic mutation
Most women undergoing screening breast MRI (approximately 80%) had a family history of breast cancer noted in their medical claims. Since claims lack detailed information about family history, it is unclear whether use of screening breast MRI is appropriate for all of these women; based on our review of medical records for the HPHC-Atrius Health subgroup, we found only 21% undergoing screening or surveillance MRI had evidence to meet the ACS criteria, partly because family history was rarely detailed. Breast MRI has a high false positive rate and broadening the scope of use to women with lower risk would reduce the positive predictive value of the test 23, 24. Implications of this ‘indication creep’ include increased downstream resource use and cost as well as potential harm to women through the cascade of additional imaging and invasive procedures for diagnostic resolution 25.
Surveillance was the second fasting growing indication for breast MRI. Patterns of use were similar to those for screening yet surveillance breast MRI is generally recommended only for women who have a genetic mutation and/or strong family history and is not recommended for most women who have a personal history of breast cancer 11, 26, 27. We found that 4% of women receiving breast MRIs for surveillance had genetic mutations and 43% had family history of breast cancer noted in claims.
Breast MRI was originally used for diagnostic evaluation in the decision whether to biopsy a suspicious lesion (5) and for treatment to assess disease extent or monitor response to neo-adjuvant therapy. MRI use for these indications is not routinely recommended in part because of mixed evidence about benefits 6, 12, 28, 29 and concerns about increases in the use of mastectomy when MRI is used to assess the extent of disease 30, 31. The declining use of breast MRI for these indications that we estimated in recent years may reflect increasing consensus about the limited benefit.
We found overall use rose 46% per year on average through 2009 and began to decline and stabilize thereafter. These trends are consistent with predictions about the diffusion of new technology 32. The stabilization of rates following 2009 is also consistent with use of noninvasive diagnostic imaging in general, although our observed annual increases in breast MRI use prior to 2009 were much larger than those for all MRIs regardless of location within the body 33, 34. For example, in similar settings as ours, use of MRI overall grew between 11-26% on average early in the decade and slowed to 6.5% 33, 35, 36.
Our study was conducted in a large health plan that spans multiple states. Nevertheless, community practice in New England may not be generalizable to other regions in the US. High regional variation in use of diagnostic imaging has been reported in the Medicare population although use in the New England region was similar to the national average 37. Our findings also may not be generalizable to practices with different availability of the technology and/or the policies of the insurer. HPHC's coverage policies, as of 2011, allowed use of breast MRI for all of the indications studied. Populations of women with other forms of insurance may have experienced different rates and trends in use.
Our indication-specific rates from 2000 through 2011 in the HPHC population were based on a classification of procedures derived from a multinomial logistic prediction model. This model was based on information abstracted from physician notes regarding the purpose of the MRI in combination with medical claims data. The model showed reasonable prediction accuracy of over 80% and 76%-88% positive predictive values, although some screening and surveillance MRIs were misclassified as diagnostic. The rates of screening and surveillance breast MRIs we report may underestimate actual use. As our prediction model was constructed and validated using data through 2008, trends in use following 2008 are an extrapolation. Finally, in our HPHC-Atrius Health subset, we found that information about genetic markers in claims corresponded with documentation in medical records only about half of the time and were under-reported in claims compared with medical records. Therefore our estimate of less than 1% of women undergoing MRI had a genetic mutation is low. However, the percentage of women with any evidence of genetic mutations was so low - 4% that it is clear most women had no evidence of genetic mutations. Overall, we found evidence meeting ACS guidelines for screening MRI in only 21% of those undergoing screening or surveillance MRI.
Breast MRI use has expanded rapidly over the past decade. We found that increases occurred for all indications, but especially screening and surveillance. Use has stabilized and begun to decline since 2009. Although benefits for some indications have been inferred from observational and modeling studies 10, 38-40, the increases in use have occurred without strong evidence of long-term mortality benefits. The overall impact on health outcomes and medical care costs from breast MRI use in the community remains unknown. As with most medical technologies, indication creep is likely inevitable 41, 42. Our data suggest that the majority of women undergoing screening breast MRI did not meet the recommended criteria for appropriate use, while many who did meet the criteria were not. However, to clearly understand appropriateness of use, better documentation of breast cancer risk is needed. Understanding who is receiving breast MRI and the downstream consequences of this use should be a high research priority to ensure that the limited health care funds are used wisely to maximize population health.
Acknowledgments
The authors thank Fang Zhang, PhD for his valuable consultation on this project.
This research was supported by Mentored Research Scholar Grant 118223-MRSG-10-002-01-CPHPS from the American Cancer Society (NKS) and by grant 1UL1RR025011 from the Clinical and Translational Science Award program of the National Center for Research Resources, National Institutes of Health (MAR). The funding agreements ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
Dr. Stout had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Each author has made substantial contribution to the intellectual content of the paper, and each has reviewed the final version and approved it for publication. None of the authors have any conflicts of interest associated with this research.
Appendix
Appendix Table.
Variable definitions based on procedure and diagnoses available in claims used to develop the clinical indication prediction model for clinical indication.
| Variable Category | Procedure and Diagnosis Codesa | Format | Inclusion in final model?b | If included, timing relative to breast MRI |
|---|---|---|---|---|
| Breast MRI | CPT: 76093-76094; 77058-77059 HCPCS: C8903-C8908 |
Indicator | Yes | Within 250 days before |
| Age | 5 year age groups | Yes | Concurrent | |
| Calendar year | Continuous | Yes | Concurrent | |
| Breast cancer (invasive or in situ) | ICD-9 Dx: 174; 233.0; V10.3 | Indicator | Yes | Anytime before MRI |
| Other breast disease | ICD-9 Dx: 217; 610; 611(except 611.7) | Indicator | Yes | Within 45 days after |
| Screening | CPT: 76083; 76085; 77057; | |||
| Mammogram | HCPCS: G0202 ICD-9 Dx: V76.1 |
Indicator | No | |
| Breast Cancer Risk Markers | Indicator | Yes | Concurrent | |
| Genetics | 96040; 99401 HCPCS: S3818-23 |
|||
| BRCA or Family History | ICD-9 Dx:V10.43; V16.3; V16.41; V84.01-V84.02 | |||
| Other | ICD-9 Dx: 183; 201 | |||
| Sign, Symptom or Abnormal image of breast | ICD-9 Dx: 611.7; 793.8 | Indicator | Yes, with age interaction | Current or within 45 days before or after (as 3 variables) |
| Other Breast Disorders | ICD-9 Dx: 611.0-611.9 except 611.7 | Indicator | Yes, with age interaction | Within 45 days after |
| Diagnostic Work-up | Indicator | Yes | Within 6 months before | |
| Imaging | CPT: 76082-91; 76645; 77051-6 HCPCS: G0203-G0207; G0236 |
|||
| Biopsy | CPT: 19100-19103; 19120-19126; 76095-76096; 77031-77032 ICD-9 Px: 85.11-85.12; 85.19-85.20 |
|||
| Other diagnostic px | CPT: 19000-19001; 19105; 76086-76089; 76939; 77053-77054 ICD-9 Px: 85.91; 87.35-87.37; 88.73 |
|||
| Treatment | Indicator | Yes | Within 6 months before or after (as 2 variables) | |
| Chemotherapy | ICD-9 Px: 85.92; 99.25 CPT: 96400-96549 CPT II: 0519F HCPCS: C9280; J1100, J8520-J8540; J9000-J9100; J9200-J9209; J9211; J9230-J9249; J9260-J9399; J9999 |
|||
| Radiation | CPT: 77261-77499 | |||
| Surgery | ICD-9 Px: 40.22-40.23; 40.3; 40.51; 85.22-85.25; 85.41-85.48 CPT: 19160-19162; 19180-19182; 19200-19272; 19301-19307; 19340; 19361-19369; 38525; 38740-38745; 76998 HCPCS: S3854 |
|||
| Breast implant complication | ICD-9 Dx: 996.54; 996.79 | Indicator | No |
Code Types: CPT = Common Procedure Terminology; HCPCS = Healthcare Common Procedure Coding System; ICD-9 Dx = International Classification of Diseases Version 9 Diagnoses; ICD-9 Px = ICD-9 Procedures
Predictor variables for the occurrence at 15, 30, 45, 60, 90 and 180 days before and after the breast MRI were examined during model development.
Footnotes
Contributions and Conflict of Interest Disclosure: Each author has made substantial contribution to the intellectual content of the paper, and each has reviewed the final version and approved it for publication. None of the authors have any conflicts of interest associated with this research.
References
- 1.Harms SE, Flamig DP. Breast MRI. Clin Imaging. 2001;25(4):227–246. doi: 10.1016/s0899-7071(01)00279-0. [DOI] [PubMed] [Google Scholar]
- 2.DeMartini W, Lehman C, Partridge S. Breast MRI for Cancer Detection and Characterization: A Review of Evidence-Based Clinical Applications. Acad Radiol. 2008;15(4):408–416. doi: 10.1016/j.acra.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Warner E, Messersmith H, Causer P, Eisen A, Shumak R, Plewes D. Systematic Review: Using Magnetic Resonance Imaging to Screen Women at High Risk for Breast Cancer. Ann Int Med. 2008;148(9):671–679. doi: 10.7326/0003-4819-148-9-200805060-00007. 2008. [DOI] [PubMed] [Google Scholar]
- 4.Lord SL, Lei W, Craft P, et al. A systematic review of the effectiveness of magnetic resonance imaging (MRI) as an addition to mammography and ultrasound in screening young women at high risk of breast cancer. Eur J Cancer. 2007;43:1905–1917. doi: 10.1016/j.ejca.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Bruening W, Uhl S, Fontanarosa J, Reston J, Treadwell J, Schoelles K. Comparative Effectiveness Review No. 47. Agency for Healthcare Research and Quality; Rockville, MD: Feb, 2012. Noninvasive diagnostic tests for breast abnormalities: Update of a 2006 review. www.effectivehealthcare.ahrq.gov/reports/final.cfm (Prepared by the ECRI Institute Evidence-based Practice Center under Contract No. 290-02-0019.) AHRQ Publication No. 12-EHC014-EF.
- 6.Houssami N, Hayes DF. Review of Preoperative Magnetic Resonance Imaging (MRI) in Breast Cancer: Should MRI Be Performed on All Women with Newly Diagnosed, Early Stage Breast Cancer? CA Cancer J Clin. 2009;59(5):290–302. doi: 10.3322/caac.20028. [DOI] [PubMed] [Google Scholar]
- 7.Brennan ME, Houssami N, Lord S, et al. Magnetic resonance imaging screening of the contralateral breast in women with newly diagnosed breast cancer: systematic review and meta-analysis of incremental cancer detection and impact on surgical management. J Clin Oncol. 2009;27(33):5640–5649. doi: 10.1200/JCO.2008.21.5756. [DOI] [PubMed] [Google Scholar]
- 8.Bigenwald RZ, Warner E, Gunasekara A, et al. Is Mammography Adequate for Screening Women with Inherited BRCA Mutations and Low Breast Density? Cancer Epidemiol Biomarkers Prev. 2008;17(3):706–711. doi: 10.1158/1055-9965.EPI-07-0509. [DOI] [PubMed] [Google Scholar]
- 9.Griebsch I, Brown J, Boggis C, et al. Cost-effectiveness of screening with contrast enhanced magnetic resonance imaging vs X-ray mammography of women at a high familial risk of breast cancer. Br J Cancer. 2006;95(7):801–810. doi: 10.1038/sj.bjc.6603356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plevritis SK, Kurian AW, Sigal BM, et al. Cost-effectiveness of Screening BRCA1/2 Mutation Carriers With Breast Magnetic Resonance Imaging. JAMA. 2006;295(20):2374–2384. doi: 10.1001/jama.295.20.2374. [DOI] [PubMed] [Google Scholar]
- 11.Saslow D, Boetes C, Burke W, et al. American Cancer Society Guidelines for Breast Screening with MRI as an Adjunct to Mammography. CA Cancer J Clin. 2007;57(2):75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 12.Lehman CD, DeMartini W, Anderson BO, Edge SB. Indications for breast MRI in the patient with newly diagnosed breast cancer. J Natl Compr Canc Netw. 2009;7(2):193–201. doi: 10.6004/jnccn.2009.0013. [DOI] [PubMed] [Google Scholar]
- 13.Lee CH, Dershaw DD, Kopans D, et al. Breast cancer screening with imaging: recommendations from the Society of Breast Imaging and the ACR on the use of mammography, breast MRI, breast ultrasound, and other technologies for the detection of clinically occult breast cancer. J Am Coll Radiol. 2010;7(1):18–27. doi: 10.1016/j.jacr.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 14.Hendrick RE, Cutter GR, Berns EA, et al. Community-Based Mammography Practice: Services, Charges, and Interpretation Methods. Am J Roentgenol. 2005;184(2):433–438. doi: 10.2214/ajr.184.2.01840433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stout NK, Nekhlyudov L. Early Uptake of Breast Magnetic Resonance Imaging in a Community-Based Medical Practice, 2000-2004. J Womens Health. 2011;20(4):631–634. doi: 10.1089/jwh.2010.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bassett LW, Dhaliwal SG, Eradat J, et al. National Trends and Practices in Breast MRI. Am J Roentgenol. 2008;191(2):332–339. doi: 10.2214/AJR.07.3207. [DOI] [PubMed] [Google Scholar]
- 17.Sommer CA, Stitzenberg KB, Tolleson-Rinehart S, Carpenter WR, Carey TS. Breast MRI utilization in older patients with newly diagnosed breast cancer. J Surg Res. 2011;170(1):77–83. doi: 10.1016/j.jss.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller JW, Sabatino SA, Thompson TD, et al. Breast MRI Use Uncommon among U.S. Women. Cancer Epidemiology Biomarkers & Prevention. 2013 Jan 1;22(1):159–166. doi: 10.1158/1055-9965.EPI-12-0967. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lobrano MB, Stolier A, L'Hoste R, Luttrell CA. Breast MRI: patterns of utilization and impact on patient management in the community hospital setting. J La State Med Soc. 2012;164(1):38–42. [PubMed] [Google Scholar]
- 20.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 21.Ozanne E, Drohan B, Bosinoff P, et al. Which risk model to use? Clinical implications of the ACS MRI screening guidelines. Cancer Epidemiol Biomarkers Prev. 2012;22(1):146–9. doi: 10.1158/1055-9965.EPI-12-0570. [DOI] [PubMed] [Google Scholar]
- 22.US Preventive Services Task Force Genetic Risk Assessment and BRCA Mutation Testing for Breast and Ovarian Cancer Susceptibility: Recommendation Statement. Ann Int Med. 2005;143(5):355–361. doi: 10.7326/0003-4819-143-5-200509060-00011. [DOI] [PubMed] [Google Scholar]
- 23.Port ER, Park A, Borgen PI, Morris E, Montgomery LL. Results of MRI screening for breast cancer in high-risk patients with LCIS and atypical hyperplasia. Ann Surg Oncol. 2007;14(3):1051–1057. doi: 10.1245/s10434-006-9195-5. [DOI] [PubMed] [Google Scholar]
- 24.Yu J, Park A, Morris E, Liberman L, Borgen PI, King TA. MRI screening in a clinic population with a family history of breast cancer. Ann Surg Oncol. 2008;15(2):452–461. doi: 10.1245/s10434-007-9622-2. [DOI] [PubMed] [Google Scholar]
- 25.Deyo RA. Cascade effects of medical technology. Annu Rev Public Health. 2002;23(1):23–44. doi: 10.1146/annurev.publhealth.23.092101.134534. [DOI] [PubMed] [Google Scholar]
- 26.Quinn EM, Coveney AP, Redmond HP. Use of Magnetic Resonance Imaging in Detection of Breast Cancer Recurrence: A Systematic Review. Ann Surg Oncol. 2012 doi: 10.1245/s10434-012-2341-3. [DOI] [PubMed] [Google Scholar]
- 27.Khatcheressian JL, Wolff AC, Smith TJ, et al. American Society of Clinical Oncology 2006 Update of the Breast Cancer Follow-Up and Management Guidelines in the Adjuvant Setting. J Clin Oncol. 2006;24(31):5091–5097. doi: 10.1200/JCO.2006.08.8575. [DOI] [PubMed] [Google Scholar]
- 28.Lehman CD, Gatsonis C, Kuhl CK, et al. MRI Evaluation of the Contralateral Breast in Women with Recently Diagnosed Breast Cancer. N Engl J Med. 2007;356(13):1295–1303. doi: 10.1056/NEJMoa065447. [DOI] [PubMed] [Google Scholar]
- 29.Morrow M, Waters J, Morris E. MRI for breast cancer screening, diagnosis, and treatment. Lancet. 2011;378(9805):1804–1811. doi: 10.1016/S0140-6736(11)61350-0. [DOI] [PubMed] [Google Scholar]
- 30.Katipamula R, Degnim AC, Hoskin T, et al. Trends in Mastectomy Rates at the Mayo Clinic Rochester: Effect of Surgical Year and Preoperative Magnetic Resonance Imaging. J Clin Oncol. 2009;27(25):4082–4088. doi: 10.1200/JCO.2008.19.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrow M, Harris JR. More mastectomies: Is this what patients really want? J Clin Oncol. 2009;27(25):4038–4040. doi: 10.1200/JCO.2009.23.0078. [DOI] [PubMed] [Google Scholar]
- 32.Greenhalgh T, Robert G, Bate P, Macfarlane F, Kyriakidou O. Diffusion of Innovations of Health Service Organizations: A Systematic Literature Review. Blackwell Publishing, LTD; Malden: 2005. [Google Scholar]
- 33.Smith-Bindman R, Miglioretti DL, Johnson E, et al. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996-2010. JAMA. 2012;307(22):2400–2409. doi: 10.1001/jama.2012.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith-Bindman R, Miglioretti DL, Larson EB. Rising Use Of Diagnostic Medical Imaging In A Large Integrated Health System. Health Aff. 2008;27(6):1491–1502. doi: 10.1377/hlthaff.27.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell JM. Utilization trends for advanced imaging procedures: evidence from individuals with private insurance coverage in California. Med Care. 2008;46(5):460–466. doi: 10.1097/MLR.0b013e31815dc5ae. [DOI] [PubMed] [Google Scholar]
- 36.Dinan MA, Curtis LH, Hammill BG, et al. Changes in the Use and Costs of Diagnostic Imaging Among Medicare Beneficiaries With Cancer, 1999-2006. JAMA. 2010;303(16):1625–1631. doi: 10.1001/jama.2010.460. [DOI] [PubMed] [Google Scholar]
- 37.Parker L, Levin DC, Frangos A, Rao VM. Geographic Variation in the Utilization of Noninvasive Diagnostic Imaging: National Medicare Data, 1998-2007. Am J Roentgenol. 2010;194(4):1034–1039. doi: 10.2214/AJR.09.3528. [DOI] [PubMed] [Google Scholar]
- 38.Lee JM, Kopans DB, McMahon PM, et al. Breast Cancer Screening in BRCA1 Mutation Carriers: Effectiveness of MR Imaging--Markov Monte Carlo Decision Analysis. Radiology. 2008;246(3):763–771. doi: 10.1148/radiol.2463070224. [DOI] [PubMed] [Google Scholar]
- 39.Heijnsdijk EA, Warner E, Gilbert FJ, et al. Differences in natural history between breast cancers in BRCA1 and BRCA2 mutation carriers and effects of MRI screening - MRISC, MARIBS and Canadian studies combined. Cancer Epidemiol Biomarkers Prev. 2012;21(9):1458–1468. doi: 10.1158/1055-9965.EPI-11-1196. [DOI] [PubMed] [Google Scholar]
- 40.Passaperuma K, Warner E, Causer PA, et al. Long-term results of screening with magnetic resonance imaging in women with BRCA mutations. Br J Cancer. 2012;107(1):24–30. doi: 10.1038/bjc.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Djulbegovic B, Paul A. From Efficacy to Effectiveness in the Face of Uncertainty. JAMA. 2011;305(19):2005–2006. doi: 10.1001/jama.2011.650. [DOI] [PubMed] [Google Scholar]
- 42.Qaseem A, Alguire P, Dallas P, et al. Appropriate Use of Screening and Diagnostic Tests to Foster High-Value, Cost-Conscious Care. Ann Int Med. 2012;156(2):147–149. doi: 10.7326/0003-4819-156-2-201201170-00011. [DOI] [PubMed] [Google Scholar]