Abstract
Purpose
Despite improvements in chemoradiation, local control remains a major clinical problem in locally advanced non-small cell lung cancer. The Hedgehog pathway has been implicated in tumor recurrence by promoting survival of tumorigenic precursors and through effects on tumor-associated stroma. Whether Hedgehog inhibition can affect radiation efficacy in vivo has not been reported.
Methods and Materials
We evaluated the effects of a targeted Hedgehog inhibitor (HhAntag) and radiation on clonogenic survival of human non-small cell lung cancer lines in vitro. Using an A549 cell line xenograft model, we examined tumor growth, proliferation, apoptosis, and gene expression changes after concomitant HhAntag and radiation. In a transgenic mouse model of KrasG12D-induced and Twist1-induced lung adenocarcinoma, we assessed tumor response to radiation and HhAntag by serial micro-computed tomography (CT) scanning.
Results
In 4 human lung cancer lines in vitro, HhAntag showed little or no effect on radio-sensitivity. By contrast, in both the human tumor xenograft and murine inducible transgenic models, HhAntag enhanced radiation efficacy and delayed tumor growth. By use of the human xenograft model to differentiate tumor and stromal effects, mouse stromal cells, but not human tumor cells, showed significant and consistent downregulation of Hedgehog pathway gene expression. This was associated with increased tumor cell apoptosis.
Conclusions
Targeted Hedgehog pathway inhibition can increase in vivo radiation efficacy in lung cancer preclinical models. This effect is associated with pathway suppression in tumor-associated stroma. These data support clinical testing of Hedgehog inhibitors as a component of multimodality therapy for locally advanced non-small cell lung cancer.
Introduction
Lung cancer is the most common cause of cancer mortality in the United States. Non-small cell lung cancer (NSCLC) accounts for 80% of histologically identified cases of lung cancer, and 40% of these cases are unresectable. The standard treatment of unresectable NSCLC is concurrent chemotherapy and thoracic radiation (1). Although systemic failure is common, local recurrence remains a problem in 50% to 75% of patients (2). Research aimed at understanding radioresistance and defining strategies to overcome this problem are paramount to better NSCLC treatment outcomes.
The Hedgehog signaling pathway is an essential developmental pathway that is aberrantly reactivated in some cancers (3, 4). Targeting the Hedgehog pathway has demonstrated promising activity as a single agent in tumor types, including basal cell skin cancer and medulloblastoma (5, 6). PTCH1 loss is implicated in the etiology of these tumors as a gatekeeper mutation; these tumors demonstrate an oncogene-addiction phenotype, with dramatic response to pathway inhibition. By contrast, most solid tumors do not demonstrate constitutive ligand-independent Hedgehog pathway activity. Aberrant Hedgehog signaling has been implicated in the proliferation and spread of other solid tumors, either through an autocrine loop integral to maintenance of a progenitor cell subpopulation or through a paracrine mechanism involving ligand production by cancer cells stimulating pathway activity in adjacent tumor-associated stromal tissues (7, 8). Some recent reports suggest that NSCLC lines demonstrate cytotoxic effects in response to Hedgehog inhibitors in preclinical models (9, 10) and that Hedgehog signaling inhibition can improve cytotoxic response in pancreatic and esophageal cancers (11–13). Whether Hedgehog inhibition can affect radiation response in lung cancer is not known, and this combination has not been extensively explored in any tumor type. We sought to define the in vitro and in vivo effects of Hedgehog signaling in radiation response in preclinical models of NSCLC and to explore the mechanisms contributing to these effects.
Methods and Materials
Cells
Cells were purchased from ATCC (Manassas, VA) and grown in F-12K + 10% FBS + 1% penicillin-streptomycin at 37°C in humidified 5% CO2/95% air.
Xenograft model
Female nude mice 4 to 5 weeks old were purchased from Harlan Laboratories and maintained in accordance with guidelines from our institutional Animal Care and Use Committee. Mice were injected subcutaneously in the right flank with 1.5 × 106 A549 cells in 100 μL of Hank’s solution and Matrigel (BD Biosciences) mixed 1:1.
Autochthonous mouse lung tumor model
The transgenic mouse model has been extensively described elsewhere (14). Briefly, Twist1-tetO7-luc mice were mated with CCSP-rtTA, tetO-KrasG12D mice. Twist1 and KrasG12D expression was activated by administering doxycycline (Sigma) to the drinking water. Autochthonous lung adenocarcinomas developed in the mice by approximately age 15 weeks, visible on micro-computed tomography (CT) imaging.
Hedgehog-pathway inhibitor
HhAntag, a Smoothened antagonist, was provided through a Material Transfer Agreement with Genentech (South San Francisco, CA). HhAntag has been shown to downregulate Shh signaling in vitro and to suppress mRNA levels of Shh pathway genes in vivo, including Gli1 and PTCH1 (15). For in vitro experiments, it was dissolved in 100% DMSO. Control cells received the same volume of DMSO. For in vivo experiments, HhAntag was dissolved in 0.5% (w/v) hydroxyl-propyl methycellulose + 0.2% Tween-80. The mice received 100 mg/kg via gavage daily for 7 consecutive days.
Radiation therapy
In vitro, cells were irradiated with 0 to 10 Gy at room temperature by the use of GammaCell 137Cs source, dose rate 50 cGy/min. In vivo, mice were treated using the Small Animal Radiation Research Platform described in a previous publication (16). Hind-flank tumors received 5 Gy with a circular 1-cm beam. Transgenic mice received whole lung radiation by use of a posterior beam to 15 Gy under image guidance. When fractionated radiation was given, mice were treated with 3 Gy whole lung radiation daily for 10 consecutive days.
Clonogenic assay
Cells were counted, plated, and allowed to attach overnight. HhAntag at specified doses was added 24 hours after plating. Twenty-four hours after HhAntag was added, radiation was delivered. Colonies were stained and counted approximately 14 days after irradiation. Surviving fraction was calculated by dividing the number of colonies formed by the number of cells plated times the plating efficiency.
Hind-flank tumor growth delay experiments
Once hind-flank tumors reached 100 mm3, the mice were randomly assigned to 1 of 4 treatment arms: (1) no treatment; (2) HhAntag 100 mg/kg only; (3) radiation 5 Gy only; and (4) HhAntag + radiation. Tumor measurements were taken until they were more than 4 times the pretreatment volume. Tumor volume was calculated using length × width × height × π/6. Tumor growth delay is the difference between the quadrupling times of untreated versus treated tumors.
Tumor immunohistochemistry and immunofluorescence
Xenografts were established as just described. The mice received either no treatment or daily HhAntag gavage for 3 days, and then were killed. The tumors were removed, fixed, and sectioned. The sections were stained for cleaved caspase-3 (Cell Signaling #9661) and Ki-67 (Abcam ab15580).
Reverse transcription polymerase chain reaction
Total RNAwas isolated from tissue by the use of Qiaprep RNAeasy Kit (Qiagen) and treated with RQ1 RNase-Free DNase (Promega). cDNA was generated by the use of Superscript II kit (Invitrogen Technologies) and amplified in ABI-prism 7700 (Perkin Elmer Applied Biosystems) for 40 cycles by the use of SYBR green polymerase chain reaction (PCR) Master mix (Perkin Elmer Applied Biosystems). Primers for mouse and human PTCH1 were purchased from TaqMan Gene Expression Assays (Applied Biosystems). RNA level was standardized to β-actin. For additional broad-based analysis of the Hedgehog pathway gene expression, the Human Hedgehog Signaling Pathway PCR Array was used (Qiagen).
Small animal imaging
The mice underwent micro-CT imaging by use of the Small Animal Radiation Research Platform irradiator described earlier. The uncollimated primary beam was used for imaging. A total of 1800 projections were acquired at approximately 0.2° angular increments. The Feldkamp CBCT algorithm was used for reconstruction. Tumor volumes were quantified from the CT dataset with Pinnacle3 software v.8.1y (Philips Inc, Madison, WI).
Statistics
Clonogenic survival curve was fitted with a linear quadratic model by the use of GraphPad Prism using a least squares fit, weighted to minimize the relative distances squared, and compared by use of the extra-sum of squares F test. The mean inactivation dose was calculated by the method of Fertil et al (17), and the cell survival enhancement ratio was calculated as described by Morgan et al (18). A value significantly exceeding 1 indicates radio-sensitization. For experiments with 4 treatment arms (apoptosis assay and tumor growth delay assay), 1-way analysis of variance was used to analyze the results. For comparison of 2 arms (with and without HhAntag), unpaired, 2-tailed t tests were used.
Results
HhAntag demonstrated minimal radiosensitization in NSCLC lines in vitro
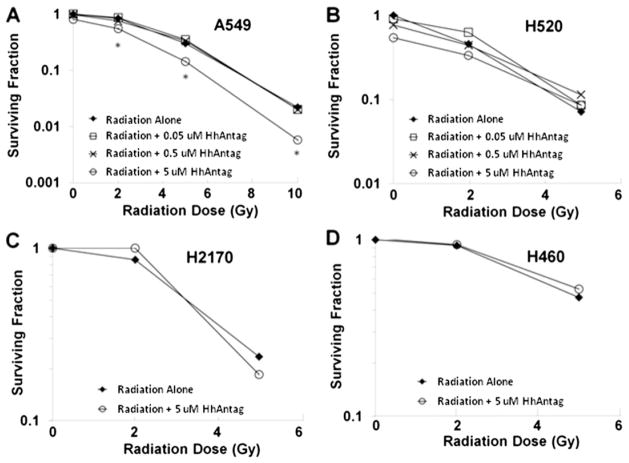
To determine whether Hedgehog pathway suppression would alter radiosensitivity in NSCLC in a cell-autonomous, tumor cell-specific manner, we evaluated the effect of HhAntag on radiation sensitivity of 4 NSCLC cell lines in vitro (Fig. 1). At concentrations of 50 nmol and 0.5 μmol, HhAntag exhibited no radio-sensitization in any of the 4 cell lines tested. HhAntag at a suprapharmacologic concentration of 5 μmol was able to partially inhibit clonogenic survival of radiated A549 cells, with an enhancement ratio of 1.4 to 1.8 (Fig. 1A). H2170, H520, and H460 showed no radiosensitization with HhAntag treatment (Fig. 1B–D). HhAntag alone had no significant effect on clonogenic survival at any dose tested in any of the cell lines. These data suggest that on-target inhibition of Hedgehog signaling within the cancer cell itself had minimal effect on NSCLC radiosensitivity.
Fig. 1.
HhAntag radiosensitizes the A549 NSCLC cell line in vitro at high doses. (A) A549 cells were incubated ± HhAntag for 24 hours before RT, and clonogenic survival was assessed with enhancement ratio = 1.4–1.8 at 5 μmol but no effect at lower concentrations (P<.05). (B–D) Clonogenic survival curves for 3 other cell lines with no radiosensitization at any of the tested HhAntag concentrations (highest concentration = 5 μmol). (B) H520 cells. (C) H2170 cells. (D) H460 cells.
HhAntag and radiation induced synergistic tumor growth delay in vivo
A549 cells were implanted in the flanks of nude mice to assess the effects of HhAntag and irradiation in vivo. As shown in Figure 2A, B, tumors in untreated mice quadrupled in size in an average of 4 days. Quadrupling time was delayed in each of the treatment arms: 5.8 days with HhAntag only, 8 days with radiation only, and 11.2 days with combined HhAntag and radiation (P<.001 between the arms by 1-way analysis of variance; P<.05 between individual arms by unpaired t test). The combination of HhAntag and radiation delayed tumor growth in vivo in a supraadditive manner.
Fig. 2.
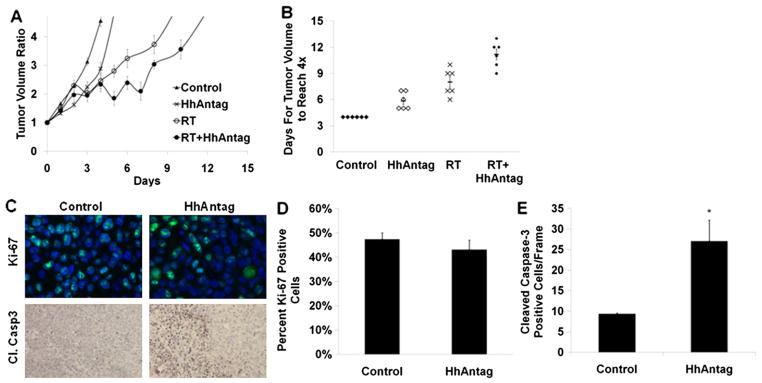
HhAntag radiosensitizes lung cancer xenografts in vivo. (A) A549 hind-flank xenograft model shows HhAntag + radiation leading to longer tumor growth delay than either therapy alone, n=6 mice per arm. Error bars represent SEM. (B) Average time to reach 4× tumor volume per treatment group is plotted with SEM (P<.05). For immunofluorescence and immunohistochemistry on tumors, nude mice bearing A549 xenografts were treated with HhAntag for 3 days, then tumor was removed and fixed. (C) Above, sample immunofluorescence images (60×) from xenografts showing Ki-67 staining. Below, sample immunohistochemistry image (40×) from xenografts showing increased cleaved caspase-3 staining by HhAntag treatment. (D) Quantifying the Ki-67 staining shows that it is unchanged by HhAntag treatment. (E) Quantifying cleaved caspase-3 staining shows that it is significantly higher in the tumors treated by HhAntag (P<.001) (n=2 mice per arm).
To examine whether HhAntag altered tumor cell proliferation or apoptotic fraction in vivo, we examined Ki-67 and cleaved caspase-3 by expression. Although no evident effect was seen in Ki-67 staining, we did observe an increase in cleaved caspase-3 (Fig. 2C–E).
HhAntag radiosensitized in an autochthonous lung cancer model
To further explore in vivo effects, we next turned to an autochthonous lung adenocarcinoma model based on inducible concomitant transgenic expression of Twist1 and KrasG12D in type II pneumocytes. Tumor moribund mice were assigned to 4 treatment arms: (1) control/mock treatment; (2) HhAntag (7 consecutive days); (3) radiation therapy (RT) (15 Gy in 1 fraction); and (4) RT + HhAntag. Tumor response was quantified weekly by serial micro-CT (Fig. 3). Figure 3A shows sample mouse lungs on micro-CT (2 weeks after treatment) and hematoxylin and eosin stain (4 weeks after treatment), with significantly larger tumors in single-therapy arms (HhAntag or RT) compared with combined therapy (RT + HhAntag). As shown in Figure 3B, HhAntag alone did not have an effect on tumor growth compared with control mice. At 1 week after radiation, control and HhAntag-treated tumors grew by 12%, whereas tumors treated with radiation 15 Gy in 1 fraction decreased by 17%, and tumors treated with radiation plus HhAntag decreased 45% in volume (P<.05). At 3 weeks after radiation, control and HhAntag-treated tumors both grew by approximately 40%, and radiation-treated tumors grew by 6%, whereas tumors treated with radiation plus HhAntag were still 13% smaller than baseline (P<.05). These effects were also seen in fractionated RT (Fig. E1). After 3 Gy in 10 fractions, tumors treated with RT/HhAntag were 14% smaller, whereas tumors in the RT-only group had grown by 6% (P<.05).
Fig. 3.
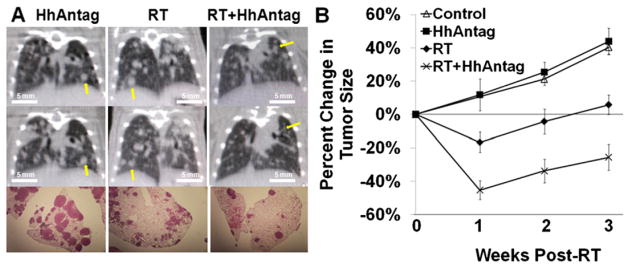
HhAntag radiosensitizes in an autochthonous lung tumor model. Mice were treated with HhAntag for 7 days, and radiation 15 Gy in 1 fraction was given to whole lung after third dose of HhAntag. (A) Above, tumor burden immediately before treatment. Middle, same animals 2 weeks after radiation. Below, hematoxylin and eosin staining of lungs from mice at week 4. Arrowheads indicate tumors at week 0 and week 2 of incubation. Photomicrographs taken at 1× magnification. (B) Graph depicting reduction of lung tumor volume, quantified using Pinnacle (n=3–5 mice per arm, ≥3 index tumors per mouse; error bars represent SEM).
These results further support the observations made from the use of human NSCLC lines and A549 subcutaneous xenografts: Hedgehog pathway suppression alone had minimal efficacy against this model but significantly augmented the efficacy of concurrent radiation.
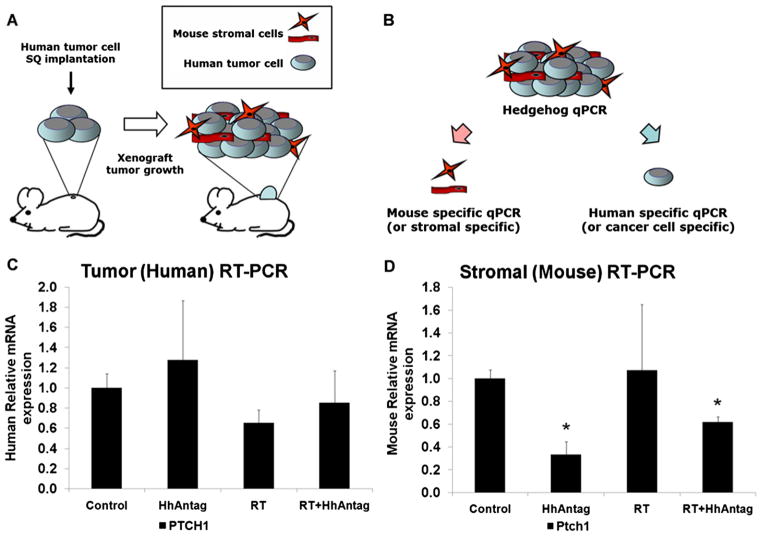
HhAntag downregulated the Hedgehog pathway in stromal cells but not cancer cells in vivo
The observed dichotomy between the minimal evidence of radiosensitization of cell lines in vitro and the consistent substantial radiosensitization in vivo is suggestive of the effect on tumor-associated stroma. Conversely, these observations may result from differential Hedgehog pathway activity of tumor cells in vitro and in vivo. To distinguish between these models, we assessed genes in mice with subcutaneous flank A549 tumors that were mock-treated or given 3 days of HhAntag. Hedgehog pathway target genes were assessed by the use of qPCR in tumor cells versus tumor stromal components (Fig. 4A, B). We verified the specificity of our primers for human and mouse genes against cDNA standards (Fig. E2). Using this approach (Fig. 4A, B), we did not observe significant downregulation of the Hedgehog pathway genes after HhAntag therapy (Fig. E3). The Human Hedgehog Signaling Pathway PCR Array profiles the expression of 84 genes, none of which showed a significant change between tumors from mock-treated and HhAntag-treated mice (defined as P<.05 with at least 2-fold change in expression level). A canonical Hedgehog pathway-responsive gene, PTCH1, is shown for illustrative purposes in Figure 4C, D. Using mouse-specific primers for PTCH1, HhAntag consistently resulted in an approximately 3 times downregulation of PTCH1 in the mouse stromal components of these tumors (Fig. 4D) (P<.05). Radiation alone did not have a significant impact on PTCH1 expression in the mouse stromal cells. Our results confirm previously published data. Inhibition of Smoothened with HhAntag prevents the activation of GLI transcription factors and decreases transcription and mRNA levels of Hedgehog signaling molecules (15).
Fig. 4.
HhAntag downregulates Hedgehog pathway key gene expression in mouse stromal cells but not human A549 tumor cells in a xenograft model. (A) Nude mice bearing A549 hind-flank xenografts were treated with HhAntag for 3 days, and tumors were removed. (B) mRNA was isolated from xenografts, and species-specific primers were used to differentiate between human A549 tumor cells and mouse stromal cells. (C) Relative PTCH1 mRNA expression in xenografts using human primers. (D) Relative PTCH1 mRNA expression in xenografts using mouse primers. All genes normalized to mouse β-actin (n=3 mice per arm; error bars represent SEM). Asterisks represents a statistically significant difference (P<.05).
To further characterize the stromal effects of HhAntag, CD 31 staining was assessed as a function of HhAntag treatment (Fig. 5). CD 31 expression was reduced in tumors treated with HhAntag (P=.003) compared with control tumors and the CD31 foci and further reduced in tumors treated with HhAntag + radiation (P<.001).
Fig. 5.
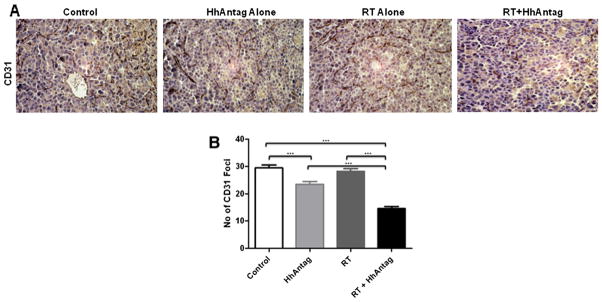
HhAntag reduces vasculature in an autochthonous lung tumor model. Mice were treated with daily HhAntag for 7 days, and radiation 15 Gy in 1 fraction was given to the whole lung after the third dose of HhAntag. Lungs were removed, fixed in buffered formalin, and paraffin embedded for sectioning. (A) Representative images of lung tumor sections stained for CD31. Images are taken at 40× objective magnification. (B) Quantifying CD31 staining showing that it is significantly lower in the tumors treated by HhAntag (P=.003) compared with control tumors and the CD31 foci and further reduced in tumors treated with HhAntag + radiation (P<.001). Bar graph depicting the reduction in CD31 foci in the lung tumors (n=3 mice per treatment group with 10 fields per animal).
Discussion
The role of Hedgehog signaling in cancers not characterized by pathway mutations has been an area of controversy. In particular, the relative importance of tumor cell autonomous signaling versus paracrine signaling between cancer cell and tumor-associated stroma has not been clearly defined. These roles are not mutually exclusive; both may be operant, and their contributions to tumor growth and therapeutic efficacy may vary considerably among different tumor types. The data presented here define a novel role for stromal Hedgehog signaling in NSCLC and support the importance of this recently defined pathway in therapeutic responses to radiation.
The interactions between radiation and Hedgehog signaling have not been extensively explored, and in vitro analyses have led to disparate conclusions. In vitro stimulation of Hedgehog signaling by exogenous ligand was recently reported to reduce radiation sensitivity in hepatocellular carcinoma through an autocrine mechanism (19). Treatment of an esophageal cancer cell line with a Hedgehog inhibitor increased radiation sensitivity in vitro (13). Even in NSCLC cell lines that express Hedgehog and have high levels of GLI transcription factors, Hedgehog antagonist insensitivity has been shown (10). These cell lines may bypass sensitivity by functioning downstream of the Hedgehog antagonist target. By contrast, comparison of wild-type versus Hedgehog pathway-activated mouse embryonic fibroblasts (with genetic disruption of PTCH1) suggests that active Hedgehog signaling can enhance radioresistance in vitro (20).
We are unaware of any prior reports combining radiation with Hedgehog pathway inhibition invivo. In this study, we evaluated the efficacy of radiation and targeted Hedgehog pathway inhibition in 2 in vivo models of NSCLC: a human tumor xenograft model and an inducible genetically engineered immunocompetent murine model. Despite minimal evidence of any direct effect on radiosensitivity of NSCLC cell lines in vitro, both in vivo models demonstrated robust enhancement of radiosensitivity in vivo. Our data suggest that radiosensitization in vivo may involve paracrine stromal signaling. Analysis of Hedgehog pathway activity in tumor-associated murine stroma in the human tumor xenograft model fully supports this proposed mechanism. These data both offer further evidence of the paracrine Hedgehog signaling mechanism and demonstrate clear therapeutic implications of this paracrine mechanism for the treatment of NSCLC. The stromal mediated effects of HhAntag and RT suggest that clinical activity may not be dependent on the molecular profile of an individual tumor but rather have generalized application in locally advanced NSCLC.
Thoracic radiation is an integral part of treatment in patients with locally advanced NSCLC. However, the risk of local recurrence remains high. Our data provide preclinical evidence of improved radiation efficacy through the sensitizing effects of Hedgehog pathway signaling inhibition. Taken together, our data support early-phase clinical investigation of Hedgehog pathway inhibition with thoracic radiation to explore the safety, tolerability, and clinical efficacy of this combination.
Supplementary Material
Summary.
Hedgehog pathway has been implicated in tumor growth and survival. Whether Hedgehog inhibition can impact radiation efficacy in vivo has not been reported. We evaluated effects of a Hedgehog inhibitor (HhAntag) and radiation on in vitro and in vivo models of non-small cell lung cancer. Although HhAntag showed little radiosensitization in vitro, in human xenograft and murine inducible transgenic models, HhAntag enhanced radiation efficacy. This effect seems to be stromal rather than cancer cell specific.
Acknowledgments
Supported by NCI SPORE grant P50CA058184.
Footnotes
Conflict of interest: Charles M. Rudin has consulted for Genentech and Novartis in the past. The authors report no other conflict of interest.
Supplementary material for this article can be found at www.redjournal.org
References
- 1.Ettinger DS, Akerley W, Bepler G, et al. Non-small cell lung cancer. J Natl Compr Canc Netw. 2010;8:740–801. doi: 10.6004/jnccn.2010.0056. [DOI] [PubMed] [Google Scholar]
- 2.Le Chevalier T, Arriagada R, Tarayre M, et al. Significant effect of adjuvant chemotherapy on survival in locally advanced non-small-cell lung carcinoma. J Natl Cancer Inst. 1992;84:58. doi: 10.1093/jnci/84.1.58. [DOI] [PubMed] [Google Scholar]
- 3.Ingham PW, McMahon AP. Hedgehog signaling in animal development: Paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 4.Yang L, Xie G, Fan Q, et al. Activation of the hedgehog-signaling pathway in human cancer and the clinical implications. Oncogene. 2010;29:469–481. doi: 10.1038/onc.2009.392. [DOI] [PubMed] [Google Scholar]
- 5.Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164–1172. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 6.Rudin CM, Hann CL, Laterra J, et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361:1173–1178. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merchant AA, Matsui W. Targeting hedgehog—a cancer stem cell pathway. Clin Cancer Res. 2010;16:3130–3140. doi: 10.1158/1078-0432.CCR-09-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yauch RL, Gould SE, Scales SJ, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 9.Singh S, Wang Z, Liang Fei D, et al. Hedgehog-producing cancer cells respond to and require autocrine hedgehog activity. Cancer Res. 2011;71:4454–4463. doi: 10.1158/0008-5472.CAN-10-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan Z, Goetz JA, Singh S, et al. Frequent requirement of hedgehog signaling in non-small cell lung carcinoma. Oncogene. 2007;26:1046–1055. doi: 10.1038/sj.onc.1209860. [DOI] [PubMed] [Google Scholar]
- 11.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shafaee Z, Schmidt H, Du W, et al. Cyclopamine increases the cytotoxic effects of paclitaxel and radiation but not cisplatin and gemcitabine in hedgehog expressing pancreatic cancer cells. Cancer Chemother Pharmacol. 2006;58:765–770. doi: 10.1007/s00280-006-0227-4. [DOI] [PubMed] [Google Scholar]
- 13.Sims-Mourtada J, Izzo JG, Apisarnthanarax S, et al. Hedgehog: An attribute to tumor regrowth after chemoradiotherapy and a target to improve radiation response. Clin Cancer Res. 2006;12:6565–6572. doi: 10.1158/1078-0432.CCR-06-0176. [DOI] [PubMed] [Google Scholar]
- 14.Tran P, Shroff E, Burns T, et al. Twist1 suppresses senescence programs and thereby accelerates and maintains mutant kras-induced lung tumorigenesis. PLoS Genetics. 2012;8:e1002650. doi: 10.1371/journal.pgen.1002650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romer JT, Kimura H, Magdaleno S, et al. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in ptc1(+/−)p53(−/−) mice. Cancer Cell. 2004;6:229–240. doi: 10.1016/j.ccr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Wong J, Armour E, Kazanzides P, et al. High-resolution, small animal radiation research platform with x-ray tomographic guidance capabilities. Int J Radiat Oncol Biol Phys. 2008;71:1591–1599. doi: 10.1016/j.ijrobp.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fertil B, Dertinger H, Courdi A, et al. Mean inactivation dose: A useful concept for intercomparison of human cell survival curves. Radiat Res. 1984;99:73–84. [PubMed] [Google Scholar]
- 18.Morgan MA, Parsels LA, Kollar LE, et al. The combination of epidermal growth factor receptor inhibitors with gemcitabine and radiation in pancreatic cancer. Clin Cancer Res. 2008;14:5142–5149. doi: 10.1158/1078-0432.CCR-07-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen YJ, Lin CP, Hsu ML, et al. Sonic hedgehog signaling protects human hepatocellular carcinoma cells against ionizing radiation in an autocrine manner. Int J Radiat Oncol Biol Phys. 2011;80:851–859. doi: 10.1016/j.ijrobp.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Leonard JM, Ye H, Wetmore C, et al. Sonic hedgehog signaling impairs ionizing radiation-induced checkpoint activation and induces genomic instability. J Cell Biol. 2008;183:385–391. doi: 10.1083/jcb.200804042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.