Abstract
Cell cryopreservation enables maintaining cellular life at sub-zero temperatures by slowing down biochemical processes. Various cell types are routinely cryopreserved in modern reproductive, regenerative, and transfusion medicine. Current cell cryopreservation methods involve freezing (slow/rapid) or vitrifying cells in the presence of a cryoprotective agent (CPA). Although these methods are clinically utilized, cryo-injury due to ice crystals, osmotic shock, and CPA toxicity cause loss of cell viability and function. Recent approaches using minimum volume vitrification provide alternatives to the conventional cryopreservation methods. Minimum volume vitrification provides ultra-high cooling and rewarming rates that enable preserving cells without ice crystal formation. Herein, we review recent advances in cell cryopreservation technology and provide examples of techniques that are utilized in oocyte, stem cell, and red blood cell cryopreservation.
Keywords: Cryopreservation, Vitrification, Regenerative medicine, Transfusion medicine, Reproductive medicine
1 Introduction
The need to cryopreserve cells is an escalating clinical problem due to high demands of various cell types in clinical medicine including human oocytes, stem cells (SCs), and red blood cells (RBCs) [1]. In reproductive medicine, oocyte cryopreservation has emerged as a viable option to maintain female fertility [2, 3]. The ability to preserve oocytes for a long time would maintain fertility options for female patients who suffer from pathological conditions (e.g., premature ovarian failure, cysts, and tumors) or receiving anticancer therapy such as chemo/radio-therapy or other gonadotoxic therapy [2, 3]. In regenerative medicine, human SC therapy is one of the promising therapeutic approaches [4]. Particularly, hematopoietic SCs, human mesenchymal stem cells (hMSCs), human embryonic stem cells (hESCs), and umbilical cord SCs are being utilized in treating various diseases such as cardiovascular diseases, diabetes, immune-modulatory disease, and cancer [5–9]. According to a recent report from clinical trials database by National Institute of Health (http://clinicaltrials.gov/), 123 clinical trial sites have been registered for evaluating hMSC therapy throughout the world. The global SC market is forecasted to reach $63.8 billion by 2015 [10]. With an increasing use of stem cells in therapeutics and drug screening, it has become important to cryopreserve human SCs for a continuous quality-controlled supply and transportation between different sites [11]. In transfusion medicine, the demand of blood products has constantly increased during the last decade and the current blood biopreservation is approximately a $11–12 billion market in the United States alone as recently reported by US Blood & Organ Banks Market Research Report. According to the latest National Blood Collection and Utilization Survey Report compiled by U.S. Department of Health and Human Services, among 15.5 million units of blood that were collected in 2011, 13.8 million units were transfused, leaving ~1.7 million units to be discarded mainly due to short shelf-life (i.e., 42 days) [12]. This limited shelf-life storage resulted in significant blood waste (~382 million USD annually) [12]. Furthermore, millions of health complications resulted from the local blood shortages in the clinical settings [13]. According to this survey report, up to 3.3% of US hospitals have reported delays in the elective surgeries due to blood inventory shortage [12]. New technologies in RBC cryopreservation would have a significant impact on blood supply system, thus reducing frequent blood shortage, outdating of blood units, as well as decreasing the incidence of post-transfusion complications.
Cell cryopreservation is a process to maintain cellular life at extremely low temperatures. During cryopreservation, a chemical substance (cryoprotective agent, CPA) is utilized to protect the cellular structures from damage during cooling and rewarming processes. Two main groups of CPAs are used: (i) intracellular CPAs that penetrate the cell membrane such as dimethyl sulfoxide (DMSO or Me2SO), glycerol, and 1, 2-propanediol; and (ii) extracellular CPAs that do not penetrate the cell membrane such as large molecular weight polymers and sugars (e.g., hydroxyethyl starch (HES), polyvinyl pyrrolidone (PVP), and poly (ethylene glycol) (PEG))[14–17]. Although both groups have shown to be useful in protecting the cellular components during cryopreservation, controlled addition and removal of such CPAs is necessary to prevent cell lysis, differentiation or toxicity [11, 15]. Recently, synthetic anti-freeze (glyco)protein and bio-inspired cryo-agents that are isolated from extremophylic bacteria, such as ectoine and trehalose are being investigated for preserving mammalian cells [1, 18–21].
Slow and rapid freezing are the two conventional approaches utilized in clinical practice for cell cryopreservation [15]. In more commonly used slow freezing method, cells or tissues are cooled down at a rate of ~1°C/min and eventually stored at −80 °C [22]. Intracellular ice formation is reduced in slow freezing as water gets enough time to diffuse into extracellular solution and new equilibrium point state is achieved. On the other hand, in slow freezing, cells are exposed for a longer time to high CPA concentrations resulting in potentially damaging effects [23]. Further, during slow freezing, cells squeeze into channels between ice crystals. As temperature decreases, the ice crystals grow to close the channels. The growing ice crystals exert mechanical forces on squeezed cells resulting in cryo-injury [24, 25]. In contrast during rapid freezing, cells or tissues are cooled down at high freezing rates (60–120°C/min) [26]. During cooling process, ice is formed in extracellular region that removes water from solution in the form of ice. This removal of water increases the CPA concentration in the remaining solution. To achieve new equilibrium state, intracellular water diffuses to extracellular solution. At high cooling rates, water does not get enough time to diffuse to extracellular region leading to the formation of intracellular ice crystals. The intracellular ice formation results in various adverse changes, which are collectively referred as a “cryo-injury” or “cryo-damage” [27]. Cryo-injury leads to loss of cell viability or compromises cell function by damaging cell membrane, morphology, and cytoskeletal components [28–31].
Vitrification has emerged as an alternative approach to conventional freezing methods to minimize cryo-injury. Vitrification method involves ultra-fast cooling rates by submerging cells in liquid nitrogen (LN2) (−196 °C) or LN2 vapor (−165 °C) [15]. During vitrification the cell transforms rapidly into a glass-like solidification status (i.e. vitreous) where ice crystallization is avoided [15, 32]. However, the high CPA concentration that is required to achieve vitrification results in osmotic dehydration to cells. New vitrification approaches have emerged as alternative techniques, which have shown the ability to significantly reduce cryo-injury (Table 1) [33]. Utilizing these minimum volume vitrification approaches have enhanced the cooling/rewarming rates up to 700,000 °C/min [34, 35] and reduced the CPA concentration that is required to achieve vitrification [26, 33]. In this review, we describe the current methodologies in cell cryopreservation focusing on vitrification of oocytes, SCs, and RBCs as these cell types have broad and significant applications in medicine.
Table 1.
Summary of vitrification systems used in RBCs, SCs and Oocytes.
| Cell Type |
Vitrification techniques |
CPA | CPA concentration (%)/CPA level (M) |
Freezing rate °C/min |
Carrier-free method |
Clinically in Use |
Viability | Sample Volume/Size |
Ref. |
|---|---|---|---|---|---|---|---|---|---|
| RBCs | Minimum Volume Vitrification | Glycerol | 23% (2.5M) | NA | NA | No | NA | 200μL/min per ejector | [26, 103] |
| Stem Cells | Open pull Straw | DMSO/Ethylene glycol | 10–20%/10–20% | 16,700 | No | No | > 70% | 1–20μL | [104] |
| Bulk Method | DMSO/Ethylene glycol | 10–20%/10–20% | NA | Yes | No | > 94% | NA | [81] | |
| Quartz Microcapillary | 1,2-Propanediol/Trehalose | 2M/0.5M | 250,000 | No | No | > 70% | ~3 mm (inner diameter) | [32, 33] | |
| Surface-based method | DMSO/Ethylene glycol | 10–20%/10–20% | NA | Yes | No | > 89% | NA | [80] | |
| Oocyte | Conventional Straw | DMSO/Acetamide/Propylene glycol/Polyethylene glycol | 20.5%/15.5%/10%/6% | 2500 | No | Yes | > 80% | 0.25 mL | [105] |
| Cryoloop | DMSO/Ethylene glycol | 10–20%/10–20% | 700,000 | No | Yes | > 90% | 0.5–0.7 mm (inner diameter) | [106] | |
| Cryotop | DMSO/Ethylene glycol | 15%/15% | 40,000 | No | Yes | < 90% | <1 μL | [34, 107] | |
| Nanoliter Vitrification | DMSO/Ethylene glycol | 4–8%/4–8% | N/A | Yes | No | > 89% | ~ 1 nL | [54] |
2 Oocyte Cryopreservation and Reproductive Medicine
Currently oocyte cryopreservation has been achieved by slow freezing and vitrification methods. [36]. When oocytes are cryopreserved using the slow freezing method for in vitro fertilization, low pregnancy rates are reported (13%) [31, 37–41]. This outcome has been attributed to the permanent damage to cryopreserved oocytes such as misalignment of chromosomes and hardening of the zona during slow freezing [28–30]. To minimize oocyte damage during cyropreservation, minimum volume vitrification techniques such as closed-pulled straw, cryoloop, and cryotop have been developed (Figure 1). These methods have efficiently improved the pregnancy rates (36–61%) [41–46].
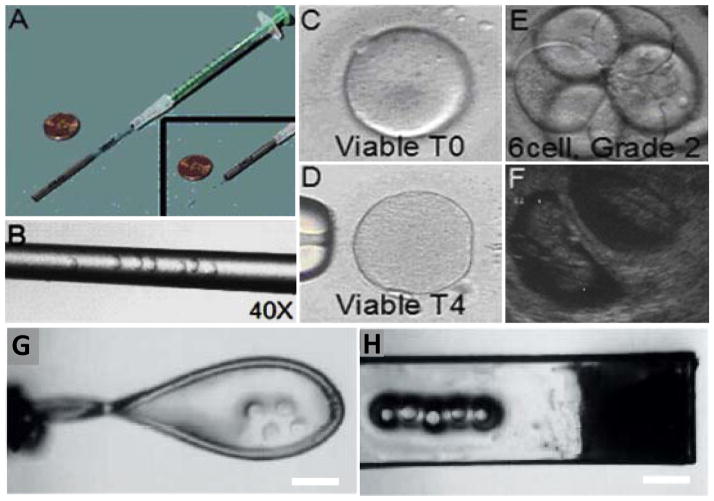
Figure 1.
Minimum volume vitrification methods. (A) An image of closed-pull straw system. (B) Magnified image of straw with oocytes loaded inside. The straw diameter is 200 μm. (C) Image of a viable oocyte just after warming (T1 stage). (D) Image of a viable oocyte 4 hours after warming (T4 stage), just before intracytoplasmic sperm injection. (E) Image of a Day 3 human embryo at 6 cell grade 2 stage. (F) Sonogram showing a ten week clinical pregnancy following a transfer of 3 embryos. A–F are reprinted by copyright permissions from [41]. (G) Cryoloop carrier loaded 4 human oocytes. (H) Cryotop carrier loaded with 5 oocytes. Scale bars indicate 400 μm. G and H are reprinted by copyright permissions from [108].
Closed-pulled straw is one of the oocyte vitrification techniques that utilize minimum volume approach to enhance the cooling and rewarming rates. About 4 to 6 oocytes are pulled into a straw using a syringe and placed into LN2. Air segments before and after oocyte solution inside a straw protect oocytes form direct contact LN2 [47]. According to a recent clinical study, oocytes vitrified using closed-pulled straw method showed 81% survival after rewarming [41]. These oocytes were further used in intracytoplasmic sperm injection and showed successful embryo development and clinical pregnancy rates up to 38% [41] (Figure 1A–F). The cryoloop technique is another method that has provided a uniform and rapid exchange of heat during the cooling process due to the reduced sample volume (<1 μl) (Figure 1G). It has demonstrated the ability to relatively increase oocyte survivability compared to the straw-based approach [48]. However, the cleavage rate and blastocyst development after in vitro fertilization was significantly reduced. On the other hand, cryotop is currently used as one of the most common carrier-based vitrification methods. This approach is based on coating a polyethylene strip with small sample volume (<1 μl) that is sufficient to cover the intended cell to cryopreserve (Figure 1H). Following submerging in LN2 the strip is enclosed with plastic cover to protect the cryopreserved oocyte during cryo-storage. Due to the minimum sample volume and the thin strip, this approach has provided high cooling and rewarming rates up to 40,000 C°/min [34]. This is a major advantage particularly during the rewarming process as this could prevent crystallization reducing the chance of cryo-injury. Recent reports have highlighted that during the cryopreservation, rewarming rate is more critical than cooling rate, as cryo-injury is more likely to occur during the former process [49–52]. Recently, four clinical randomized trails demonstrated high cell survival rate after rewarming (90–97%) when this approach was used [43–46]. This approach has demonstrated a significant increase in pregnancy rates (36–61%) compared to slow freezing methods [43–46].
In addition to these carrier-based systems that require manual oocyte handling and skilled operators, carrier-free droplet generation methods are being developed that increase cooling and rewarming rates due to the absence of a bulky carrier [33, 53]. In a recent report, the ejector-based droplet vitrification platform has been developed to cryopreserve mouse oocytes in nanoliter droplets [54]. High oocyte survival (89.9%) and cleavage rates (97%) were reported after rewarming compared to the fresh oocytes [54]. The reported results are based on mouse oocyte vitrification; further evaluation with human oocytes is required.
Overall, minimum volume vitrification technologies have shown high oocyte survival rates and pregnancy outputs in clinical settings. These results are promising for establishing universal oocyte banking in the future, which would lower the cost and eliminate the waiting period for matching donors. As minimum volume vitrification methods are recently developed, a thorough evaluation will be needed to analyze the long term potential effects of vitrification on children born using cryopreserved oocytes.
3 Stem Cell Preservation and Regenerative Medicine
The ability of SCs to differentiate into various cell types have found applications in many areas including drug screening, regenerative medicine, and tissue engineering [5, 7, 55–58]. These applications require a continuous supply of SCs that can be achieved by using cryopreservation technology. Current SC cryopreservation protocols involve slow-freezing using DMSO alone or combined with other CPAs such as glycerol and proline, along with animal/human serum [59–63]. In the case of hMSC cryopreservation, DMSO based slow freezing protocols provide cell recovery up to 90% after cryopreservation [64]. The current research focus in hMSC cryopreservation is on clinical safety by avoiding or reducing the DMSO concentration and eliminating the use of animal/human serum in cell cryopreservation protocols. There is a wide range of DMSO concentrations used without any clear guidelines and scientific rationale as revealed by a study of 444 European Group for Blood and Marrow Transplantation centers [65]. The potential side effects related to indiscriminate transfusion of cryopreserved cells along with DMSO intended for transplant therapy include cardiovascular failure and respiratory distress such as bradycardia, hypotension, respiratory arrest and fatal arrhythmias [65]. In addition, many reports have shown neural lineage differentiation when DMSO is used to cryopreserve SCs [66–68]. Only 1% DMSO has been reported to induce differentiation in ESCs to mesendoderm [69, 70]. Clinically employed protocols such as the New York Blood Bank Protocol washed out the DMSO and serum before infusion [71, 72]. Washing out DMSO and serum from the cells significantly decreases infusion-related toxicity, however, some adverse reactions have also been reported after using these washing protocols [65, 73, 74]. Utilizing alternative CPAs in combination with minimal amount of DMSO, or alone, are being investigated [75, 76]. To reduce the DMSO concentration, PVP and HES have been used in combination with DMSO for cryopreservation [75, 76]. Recently, the ability to protect the hMSCs from cryo-damage has been reported using a bio-inspired ectoin solution [18]. This study reported the use of ectoin with serum-free cryo-medium to preserve hMSCs by following a slow freezing approach. Although the viability of cryopreserved cells was 72% post-thawing, developing a DMSO-free and serum-free cryopreservation approache for SCs would make it more suitable for clinical therapy. In another effort to eliminate animal serum, a serum-free CPA (7.5% DMSO, 2.5% PEG), 2% serum albumin) has been developed where fetal bovine serum is replaced with serum albumin [64]. The hMSC cells showed 82.9% post-thaw viability which was comparable to when animal serum was used with 10% DMSO.
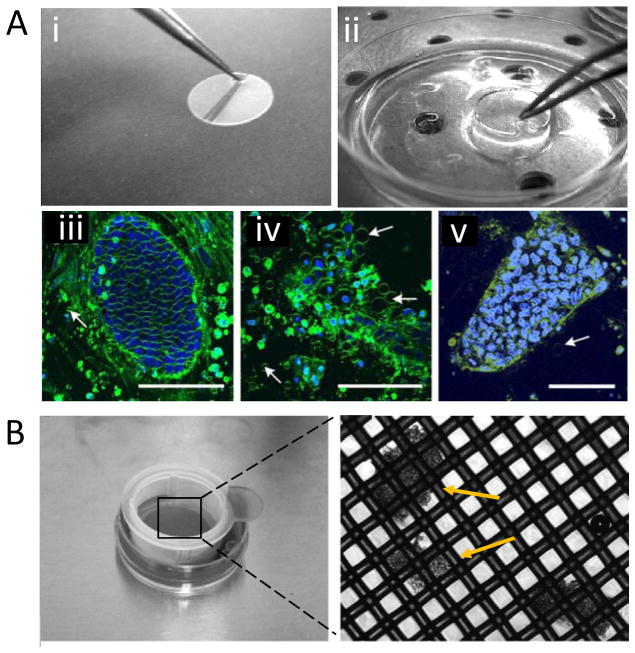
Besides the use of non-toxic CPAs in SC cryopreservation, vitrification has become an attractive approach due to its higher cell survival rate compared to slow freezing especially for adherent SCs such as hESC [77, 78]. Vitrification of mouse ESCs has been demonstrated using Cryotip and Quartz microcapillary approaches [32, 66, 79]. However, these two methods suffer from low throughput. The efficiency of these techniques has to be further investigated in hESCs. Alternatively, a surface-based vitrification technique using Thermanox© coverslip has been developed (Figure 2A) [80]. In this method a bulk quantity of adherent hESC can be preserved efficiently, exhibiting a higher survival rate after rewarming (89%) compared to slow frozen colonies (51%). This approach allows a precise handling and storage of SCs. The cryopreserved hESC cells were also tested for their ability to differentiate using staining antibodies against pluripotent markers such as Oct-4 and Tra-1-81. Approximately 80% (±12%) of the cryopreserved cells were positive for pluripotency compared with 81% (±13%) in unfrozen control colonies [80]. In another study, a bulk vitrification method based on cell strainer was utilized to cryopreserve large quantities of hESC clumps (Figure 2B) [81]. In these two methods, the cells have to be in contact with LN2. Although this is potentially considered to increase the possibility of contamination, LN2 can be sterilized by utilizing ultra-violet radiation or sterile filters, which would minimize such contamination risks [82–84]. In summary, various CPA formulations and vitrification methods have been investigated for SC cryopreservation that showed good cell recovery after rewarming. As new batch of serum-free and DMSO-free CPA formulations are being developed, there is a need to standardize the SC cryopreservation methods in terms of CPA choice and used concentrations.
Figure 2.
(A) Surface-based vitrification method. (i) A modification of Thermanox cultivation disc with a small tip to handle with tweezer. (ii) Disc incubation in CPA. Multi photon laser scanning micrographs of hESC-colonies (iii) Control colony, (iv) Cryopreserved colony using slow rate freezing, and (v) Cryopreserved colony using surface based vitrification. Fewer membrane vesicles (arrows) were observed in vitrified sample. Scale bars indicate 100 μm. Reprinted by copyright permissions from [80]. (B) Bulk vitrification method. hESC cell clumps were loaded on the nylon mesh of a cell strainer and incubated in CPA. The inset (right) shows the magnified view of nylon mesh with cell clumps indicated by arrows. Reprinted by copyright permissions from [81].
4 Red Blood Cell Preservation and Transfusion Medicine
Recent studies have underscored questions about the clinical effectiveness of blood units biopreserved using refrigeration [85, 86]. For instance, increased mortality and morbidity rates were reported in compromised patients after cardiac surgery [87]. These complications were correlated with longer storage of the blood units (> 14 days), which suggests the responsibility of progressive adverse changes seen in stored RBCs [87]. Cryopreservation of RBCs would offer an alternative approach to address the current challenges in the field of transfusion medicine by providing the extended means of RBC preservation and reducing storage damage. Currently, there are two methods used clinically for RBC cryopreservation: high glycerol-slow freezing and low glycerol-rapid freezing methods [88, 89]. The high glycerol/slow freezing technique (common in the USA and Canada) utilizes 40% (w/v) glycerol in conjunction with a freezing rate of ~1°C/min and storing at −80 °C. The low glycerol/rapid freezing method (routinely used in Europe) involves the application of 10–20% glycerol and rapid freezing rates (60–120°C/min) by submersing the CPA containers in LN2 or LN2 vapor (−196 °C or −165 °C, respectively). Glycerol is a penetrating CPA that efficiently prevents the formation of ice crystals during RBC cryopreservation [90]. For glycerol based freezing methods, it is important to remove the intracellular glycerol after thawing to minimize the RBC hemolysis following transfusion [15, 91, 92]. This deglycerolization process requires multiple washing steps where about 15% cells are hemolysed [93, 94]. Recently published results affirm the adverse changes in RBC morphology after cryopreservation and deglycerolization [94]. Such changes negatively affect RBC function by reducing its deformability, which allows cells to flow through ultra-thin capillaries to oxygenate target tissues [95]. Despite these limitations, glycerol based cryopreservation is being used effectively in clinics. To overcome the challenges associated with glycerol-based cryopreservation, alternative materials including anti-freeze proteins and their synthetic mimics such as poly(vinyl alcohol) (PVA) are being investigated for their potential use in transfusion medicine. PVA is appealing because of its properties to inhibit ice recrystallization [96]. PVA is known for its minimal toxicity and is already approved by Food and Drug Administration for dietary use [97]. Recently, PVA is used at significantly low concentrations (0.1% wt) compared to glycerol (rapid freezing (20% wt) or slow freezing (40% wt)) for blood cryopreservation and enabled cell recovery of >40% after rewarming [1]. Although >40% is a low cell recovery, cryopreservation of RBCs at extremely low PVA concentrations is achieved due to its ability to inhibit ice crystallization without dynamic ice shaping [1]. Alternative CPAs such as Ectoine have shown good results with other cell types and are being explored in transfusion medicine to limit washing steps. Ectoine is expensive as compared to glycerol but it is a biocompatible [98, 99] low molecular weight solute which is currently used in low concentrations (0.1%–1%) for protein and cell freezing [68, 100], hence, multiple washing steps may not be required.
Vitrification has not been well investigated for transfusion medicine because of throughput challenges. Recently, a scalable method for blood cryopreservation has been reported where cells are cryopreserved in microdroplets generated by an ejector at low glycerol concentrations (~ 23%) [26]. Each ejector processes 0.2 mL of blood in a minute, but making arrays of ejectors would enable high throughput cryopreservation of RBCs.
During RBC cryopreservation, blood needs to be stored at −80 °C or even lower temperatures, which makes the whole process economically less feasible. There is also a high cost associated with the complex processing requiring trained personnel. For these reasons, currently, blood is cryopreserved in limited amount only for natural calamities, rare blood groups, and military settings [91, 101].
5 Future prospects and conclusions
The integration of nano- and micro-scale technologies with bio-inspired and synthetic materials has brought innovative ideas targeting the existing challenges in cryobiology [102]. For instance, a recent finding that PVA at extremely low concentrations works as an effective inhibitor for ice recrystallization, can be adapted to cryopreservation field to address the toxicity issues of conventional CPAs. Utilizing biocompatible materials at low CPA concentrations simplifies cryopreservation protocols by reducing multiple CPA unloading steps.
Manipulating cells in minimal volume droplets of CPA increase cooling and warming rates, which would potentially reduce cell damage. This minimal volume approach would potentially eliminate the requirements for high CPA concentrations to achieve cell vitrification and minimize osmotic shock and toxicity. Minimal volume vitrification techniques are successfully being applied for oocyte and SC cryopreservation. Although some of the reported vitrification techniques have automation potential, alternative methods should be investigated to meet the high throughput requirements for commercial applications. To reduce the cost associated with storing the vitrified cells at extremely low temperatures, there is a need to investigate cell lyophilization or freeze-drying technologies for cell preservation at room temperature. Technologies to store cells in dry form are especially needed in transfusion medicine where blood is stored in large volumes. Overall, these emerging technologies open new avenues to address today’s challenges in cell cryopreservation enabling new applications in tissue engineering, regenerative medicine, personalized medicine, drug screening, and bio-banking.
Acknowledgments
We would like to thank Sinan Guven and Irep Gozen for their comments that helped us improve the manuscript. We would also like to thank Mudit Tandon from Belmont Hill School and Hassan Sakhta, a high school student under the Student Success Job Program at Brigham and Women’s Hospital, Harvard Medical School for contributing to this review. This work was partially supported by NIH R21-HL095960 and NIH R01-EB015776.
List of abbreviations
- CPA
Cryoprotective agent
- SCs
Stem cells
- RBCs
Red blood cells
- hMSCs
Human mesenchymal stem cells
- hESCs
Human embryonic stem cells
- DMSO or Me2SO
dimethyl sulfoxide
- HES
Hydroxyethyl starch
- PVP
Polyvinyl pyrrolidone
- LN2
Liquid nitrogen
- PEG
Poly (ethylene glycol)
- PVA
Poly (vinyl alcohol)
Biographies
 Waseem Asghar is a Postdoctoral Scholar at Canary Center for Cancer Early Detection, Department of Radiology, Stanford University School of Medicine, Palo Alto, CA. Before moving to Stanford, he was a Postdoctoral Research Fellow in Medicine at Harvard Medical School (HMS), and Brigham and Women’s Hospital, Boston, MA. He received his Ph.D. from University of Texas at Arlington, Texas, USA in Spring 2012. His current research interests are in the field of disease diagnosis and prognosis, point-of-care diagnostics, and biomaterials. He has published more than 35 peer reviewed journal and conference papers, and 2 book chapters. His research achievements have been recognized by numerous awards and fellowships: Nanofab Best Graduate Student Award, IEngage Mentoring Fellowship, and Science, Technology, Engineering or Mathematics (STEM) Fellowship.
Waseem Asghar is a Postdoctoral Scholar at Canary Center for Cancer Early Detection, Department of Radiology, Stanford University School of Medicine, Palo Alto, CA. Before moving to Stanford, he was a Postdoctoral Research Fellow in Medicine at Harvard Medical School (HMS), and Brigham and Women’s Hospital, Boston, MA. He received his Ph.D. from University of Texas at Arlington, Texas, USA in Spring 2012. His current research interests are in the field of disease diagnosis and prognosis, point-of-care diagnostics, and biomaterials. He has published more than 35 peer reviewed journal and conference papers, and 2 book chapters. His research achievements have been recognized by numerous awards and fellowships: Nanofab Best Graduate Student Award, IEngage Mentoring Fellowship, and Science, Technology, Engineering or Mathematics (STEM) Fellowship.
 Rami El Assal is a Postdoctoral Scholar at Canary Center for Cancer Early Detection, Department of Radiology, Stanford University School of Medicine, Palo Alto, CA. Before moving to Stanford, he was a Postdoctoral Research Fellow in Medicine at Harvard Medical School (HMS), and Brigham and Women’s Hospital, Boston, MA. Dr. El Assal received his Doctor of Dental Surgery (D.D.S.) degree from Ajman University of Science and Technology, Ajman, UAE in 2007. His research interests revolved around the applications of nanotechnology and bio-inspired materials in medicine, including regenerative and transfusion medicine. He has published more than 11 peer reviewed journal and conference papers. His research achievements have been recognized by the Center of Nanoscale Systems (CNS) at Harvard University.
Rami El Assal is a Postdoctoral Scholar at Canary Center for Cancer Early Detection, Department of Radiology, Stanford University School of Medicine, Palo Alto, CA. Before moving to Stanford, he was a Postdoctoral Research Fellow in Medicine at Harvard Medical School (HMS), and Brigham and Women’s Hospital, Boston, MA. Dr. El Assal received his Doctor of Dental Surgery (D.D.S.) degree from Ajman University of Science and Technology, Ajman, UAE in 2007. His research interests revolved around the applications of nanotechnology and bio-inspired materials in medicine, including regenerative and transfusion medicine. He has published more than 11 peer reviewed journal and conference papers. His research achievements have been recognized by the Center of Nanoscale Systems (CNS) at Harvard University.
 Utkan Demirci is an Associate Professor at Canary Center for Cancer Early Detection, Department of Radiology, Stanford University School of Medicine, Palo Alto, CA. Before moving to Stanford, he was as an Associate Professor of Medicine and Health Sciences and Technology at the Harvard Medical School (HMS), and Massachusetts Institute of Technology (MIT). He received his bachelor’s degree from the University of Michigan, Ann Arbor, his master’s degrees in Electrical Engineering in 2001 and in Management Science and Engineering in 2005, and his doctorate in Electrical Engineering in 2005 all from Stanford University. Dr. Demirci received IEEE EMBS Early Career Award; IEEE EMBS Translational Science Award; NSF Career Award; Coulter Foundation Early Career Award; HMS-Young Investigator Award; and Chinese International Young Scientist Award. In 2006, he was selected to TR-35 as one of the world’s top 35 young innovators under the age of 35 by the MIT Technology Review.
Utkan Demirci is an Associate Professor at Canary Center for Cancer Early Detection, Department of Radiology, Stanford University School of Medicine, Palo Alto, CA. Before moving to Stanford, he was as an Associate Professor of Medicine and Health Sciences and Technology at the Harvard Medical School (HMS), and Massachusetts Institute of Technology (MIT). He received his bachelor’s degree from the University of Michigan, Ann Arbor, his master’s degrees in Electrical Engineering in 2001 and in Management Science and Engineering in 2005, and his doctorate in Electrical Engineering in 2005 all from Stanford University. Dr. Demirci received IEEE EMBS Early Career Award; IEEE EMBS Translational Science Award; NSF Career Award; Coulter Foundation Early Career Award; HMS-Young Investigator Award; and Chinese International Young Scientist Award. In 2006, he was selected to TR-35 as one of the world’s top 35 young innovators under the age of 35 by the MIT Technology Review.
Footnotes
Conflict-of-interest statement
Dr. Utkan Demirci is a founder of, and has an equity interest in: (i) DxNow Inc., a company that is developing microfluidic and imaging technologies for point-of-care diagnostic solutions, and (ii) Koek Biotech, a company that is developing microfluidic IVF technologies for clinical solutions. Dr. Utkan Demirci’s interests were viewed and managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies.
References
- 1.Deller RC, Vatish M, Mitchell DA, Gibson MI. Synthetic polymers enable non-vitreous cellular cryopreservation by reducing ice crystal growth during thawing. Nature communications. 2014;5:3244. doi: 10.1038/ncomms4244. [DOI] [PubMed] [Google Scholar]
- 2.Tao T, Del Valle A. Human oocyte and ovarian tissue cryopreservation and its application. Journal of assisted reproduction and genetics. 2008;25:287–296. doi: 10.1007/s10815-008-9236-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agca Y. Cryopreservation of oocyte and ovarian tissue. ILAR J. 2000;41:207–220. doi: 10.1093/ilar.41.4.207. [DOI] [PubMed] [Google Scholar]
- 4.Hanna J, Hubel A. Preservation of stem cells. Organogenesis. 2009;5:134–137. doi: 10.4161/org.5.3.9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malgieri A, Kantzari E, Patrizi MP, Gambardella S. Bone marrow and umbilical cord blood human mesenchymal stem cells: state of the art. Int J Clin Exp Med. 2010;3:248–269. [PMC free article] [PubMed] [Google Scholar]
- 6.Stockschlader M, Hassan HT, Krog C, Kruger W, et al. Long-term follow-up of leukaemia patients after related cryopreserved allogeneic bone marrow transplantation. British journal of haematology. 1997;96:382–386. doi: 10.1046/j.1365-2141.1997.d01-2032.x. [DOI] [PubMed] [Google Scholar]
- 7.Heng BC, Kuleshova LL, Bested SM, Liu H, Cao T. The cryopreservation of human embryonic stem cells. Biotechnology and applied biochemistry. 2005;41:97–104. doi: 10.1042/BA20040161. [DOI] [PubMed] [Google Scholar]
- 8.Gurkan U, Sung Y, El Assal R, Xu F, et al. Journal of Tissue Engineering and Regenerative Medicine. 2012:366–366. [Google Scholar]
- 9.Geckil H, Xu F, Zhang X, Moon S, Demirci U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine (Lond) 2010;5:469–484. doi: 10.2217/nnm.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stem Cells Market And Drug Discovery Applications, 2009–2015. Axis Research Mind; 2010. [Google Scholar]
- 11.Coopman K. Large-scale compatible methods for the preservation of human embryonic stem cells: Current perspectives. Biotechnology progress. 2011;27:1511–1521. doi: 10.1002/btpr.680. [DOI] [PubMed] [Google Scholar]
- 12.Whitaker B, Hinkins S. The 2011 Nationwide Blood Collection and Utilization Survey Report. Department of Health and Human Services; U. S. A: 2011. [Google Scholar]
- 13.Shander A, Goodnough LT. Why an alternative to blood transfusion? Critical care clinics. 2009;25:261–277. doi: 10.1016/j.ccc.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Karlsson JOM, Toner M, editors. Cryopreservation. Academic Press; San Diego: 2000. [Google Scholar]
- 15.Meryman HT. Cryopreservation of living cells: principles and practice. Transfusion. 2007;47:935–945. doi: 10.1111/j.1537-2995.2007.01212.x. [DOI] [PubMed] [Google Scholar]
- 16.Hubel A. Parameters of cell freezing: implications for the cryopreservation of stem cells. Transfusion medicine reviews. 1997;11:224–233. doi: 10.1053/tmrv.1997.0110224. [DOI] [PubMed] [Google Scholar]
- 17.Pegg DE. Cryopreservation and Freeze-Drying Protocols. Springer; 2007. Principles of cryopreservation; pp. 39–57. [Google Scholar]
- 18.Grein TA, Freimark D, Weber C, Hudel K, et al. Alternatives to dimethylsulfoxide for serum-free cryopreservation of human mesenchymal stem cells. The International journal of artificial organs. 2010;33:370–380. [PubMed] [Google Scholar]
- 19.Sasnoor LM, Kale VP, Limaye LS. Supplementation of conventional freezing medium with a combination of catalase and trehalose results in better protection of surface molecules and functionality of hematopoietic cells. Journal of hematotherapy & stem cell research. 2003;12:553–564. doi: 10.1089/152581603322448268. [DOI] [PubMed] [Google Scholar]
- 20.Eroglu A, Russo MJ, Bieganski R, Fowler A, et al. Intracellular trehalose improves the survival of cryopreserved mammalian cells. Nature Biotechnology. 2000;18:163–167. doi: 10.1038/72608. [DOI] [PubMed] [Google Scholar]
- 21.Guven SUD. Integrating nanoscale technologies with cryogenics: a step towards improved biopreservation. Nanomedicine (Lond) 2012;7:1787–1789. doi: 10.2217/nnm.12.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Santis L, Coticchio G. Theoretical and experimental basis of slow freezing. Reproductive biomedicine online. 2011;22:125–132. doi: 10.1016/j.rbmo.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Lovelock J. The haemolysis of human red blood-cells by freezing and thawing. Biochimica et biophysica acta. 1953;10:414–426. doi: 10.1016/0006-3002(53)90273-x. [DOI] [PubMed] [Google Scholar]
- 24.Hubel A, Cravalho E, Nunner B, Körber C. Survival of directionally solidified B-lymphoblasts under various crystal growth conditions. Cryobiology. 1992;29:183–198. doi: 10.1016/0011-2240(92)90019-x. [DOI] [PubMed] [Google Scholar]
- 25.Mazur P. Freezing of living cells: mechanisms and implications. American Journal of Physiology-Cell Physiology. 1984;247:C125–C142. doi: 10.1152/ajpcell.1984.247.3.C125. [DOI] [PubMed] [Google Scholar]
- 26.Samot J, Moon S, Shao L, Zhang X, et al. Blood banking in living droplets. PloS one. 2011;6:e17530. doi: 10.1371/journal.pone.0017530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fowler A, Toner M. Cryo-Injury and Biopreservation. Annals of the New York Academy of Sciences. 2006;1066:119–135. doi: 10.1196/annals.1363.010. [DOI] [PubMed] [Google Scholar]
- 28.Lucena E, Bernal DP, Lucena C, Rojas A, et al. Successful ongoing pregnancies after vitrification of oocytes. Fertility and sterility. 2006;85:108–111. doi: 10.1016/j.fertnstert.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Leibo S, Pool TB. The principal variables of cryopreservation: solutions, temperatures, and rate changes. Fertility and sterility. 2011;96:269–276. doi: 10.1016/j.fertnstert.2011.06.065. [DOI] [PubMed] [Google Scholar]
- 30.Desrosiers P, Légaré C, Leclerc P, Sullivan R. Membranous and structural damage that occur during cryopreservation of human sperm may be time-related events. Fertility and sterility. 2006;85:1744–1752. doi: 10.1016/j.fertnstert.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 31.Chen SU, Yang YS. Slow freezing or vitrification of oocytes: their effects on survival and meiotic spindles, and the time schedule for clinical practice. Taiwanese journal of obstetrics & gynecology. 2009;48:15. doi: 10.1016/S1028-4559(09)60030-9. [DOI] [PubMed] [Google Scholar]
- 32.He X, Park EY, Fowler A, Yarmush ML, Toner M. Vitrification by ultra-fast cooling at a low concentration of cryoprotectants in a quartz micro-capillary: a study using murine embryonic stem cells. Cryobiology. 2008;56:223–232. doi: 10.1016/j.cryobiol.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X, Catalano PN, Gurkan UA, Khimji I, Demirci U. Emerging technologies in medical applications of minimum volume vitrification. Nanomedicine (Lond) 2011;6:1115–1129. doi: 10.2217/nnm.11.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology. 2007;67:73–80. doi: 10.1016/j.theriogenology.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 35.Isachenko E, Isachenko V, Katkov II, Dessole S, Nawroth F. Vitrification of mammalian spermatozoa in the absence of cryoprotectants: from past practical difficulties to present success. Reproductive biomedicine online. 2003;6:191–200. doi: 10.1016/s1472-6483(10)61710-5. [DOI] [PubMed] [Google Scholar]
- 36.Liebermann J. Current frontiers in cryobiology. Rijeka: InTech; 2012. Vitrification of oocytes and embryos; pp. 169–184. [Google Scholar]
- 37.Oktay K, Cil AP, Bang H. Efficiency of oocyte cryopreservation: a meta-analysis. Fertil Steril. 2006;86:70–80. doi: 10.1016/j.fertnstert.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 38.Saragusty J, Arav A. Current progress in oocyte and embryo cryopreservation by slow freezing and vitrification. Reproduction. 2011;141:1–19. doi: 10.1530/REP-10-0236. [DOI] [PubMed] [Google Scholar]
- 39.Campos JR, Rosa-e-Silva ACJ. Cryopreservation and fertility: current and prospective possibilities for female cancer patients. ISRN obstetrics and gynecology 2011. 2011 doi: 10.5402/2011/350813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keskintepe L, Sher G, Machnicka A, Tortoriello D, et al. Vitrification of human embryos subjected to blastomere biopsy for pre-implantation genetic screening produces higher survival and pregnancy rates than slow freezing. Journal of assisted reproduction and genetics. 2009;26:629–635. doi: 10.1007/s10815-009-9369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith GD, Serafini PC, Fioravanti J, Yadid I, et al. Prospective randomized comparison of human oocyte cryopreservation with slow-rate freezing or vitrification. Fertility and sterility. 2010;94:2088–2095. doi: 10.1016/j.fertnstert.2009.12.065. [DOI] [PubMed] [Google Scholar]
- 42.The Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99:37–43. doi: 10.1016/j.fertnstert.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 43.Cobo A, Kuwayama M, Pérez S, Ruiz A, et al. Comparison of concomitant outcome achieved with fresh and cryopreserved donor oocytes vitrified by the Cryotop method. Fertility and Sterility. 2008;89:1657–1664. doi: 10.1016/j.fertnstert.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 44.Cobo A, Meseguer M, Remohí J, Pellicer A. Use of cryo-banked oocytes in an ovum donation programme: a prospective, randomized, controlled, clinical trial. Human Reproduction. 2010;25:2239–2246. doi: 10.1093/humrep/deq146. [DOI] [PubMed] [Google Scholar]
- 45.Parmegiani L, Cognigni G, Bernardi S, Cuomo S, et al. Efficiency of aseptic open vitrification and hermetical cryostorage of human oocytes. Reproductive biomedicine online. 2011;23:505–512. doi: 10.1016/j.rbmo.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Rienzi L, Romano S, Albricci L, Maggiulli R, et al. Embryo development of fresh ‘versus’ vitrified metaphase II oocytes after ICSI: a prospective randomized sibling-oocyte study. Human Reproduction. 2010;25:66–73. doi: 10.1093/humrep/dep346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen SU, Lien YR, Cheng YY, Chen HF, et al. Vitrification of mouse oocytes using closed pulled straws (CPS) achieves a high survival and preserves good patterns of meiotic spindles, compared with conventional straws, open pulled straws (OPS) and grids. Hum Reprod. 2001;16:2350–2356. doi: 10.1093/humrep/16.11.2350. [DOI] [PubMed] [Google Scholar]
- 48.Lane M, Schoolcraft WB, Gardner DK. Vitrification of mouse and human blastocysts using a novel cryoloop container-less technique. Fertil Steril. 1999;72:1073–1078. doi: 10.1016/s0015-0282(99)00418-5. [DOI] [PubMed] [Google Scholar]
- 49.Seki S, Mazur P. The dominance of warming rate over cooling rate in the survival of mouse oocytes subjected to a vitrification procedure. Cryobiology. 2009;59:75–82. doi: 10.1016/j.cryobiol.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seki S, Mazur P. Effect of warming rate on the survival of vitrified mouse oocytes and on the recrystallization of intracellular ice. Biology of reproduction. 2008;79:727–737. doi: 10.1095/biolreprod.108.069401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seki S, Mazur P. Ultra-rapid warming yields high survival of mouse oocytes cooled to −196 C in dilutions of a standard vitrification solution. PloS one. 2012;7:e36058. doi: 10.1371/journal.pone.0036058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mazur P, Seki S. Survival of mouse oocytes after being cooled in a vitrification solution to −196 C at 95 to 70,000 C/min and warmed at 610 to 118,000 C/min: A new paradigm for cryopreservation by vitrification. Cryobiology. 2011;62:1–7. doi: 10.1016/j.cryobiol.2010.10.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ceyhan E, Xu F, Gurkan UA, Emre AE, et al. Prediction and control of number of cells in microdroplets by stochastic modeling. Lab Chip. 2012;12:4884–4893. doi: 10.1039/c2lc40523g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang X, Khimji I, Shao L, Safaee H, et al. Nanoliter droplet vitrification for oocyte cryopreservation. Nanomedicine (Lond) 2012;7:553–564. doi: 10.2217/nnm.11.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gurkan UA, El Assal R, Yildiz SE, Sung Y, et al. Engineering anisotropic biomimetic fibrocartilage microenvironment by bioprinting mesenchymal stem cells in nanoliter gel droplets. Molecular pharmaceutics. 2014 doi: 10.1021/mp400573g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Asghar W, Kim YT, Ilyas A, Sankaran J, et al. Synthesis of nano-textured biocompatible scaffolds from chicken eggshells. Nanotechnology. 2012;23:475601. doi: 10.1088/0957-4484/23/47/475601. [DOI] [PubMed] [Google Scholar]
- 57.Asghar W, Islam M, Wadajkar AS, Wan Y, et al. PLGA micro-and nanoparticles loaded into gelatin scaffold for controlled drug release. Nanotechnology, IEEE Transactions on. 2012;11:546–553. [Google Scholar]
- 58.Ilyas A, Islam M, Asghar W, Menon J, et al. Salt-leaching Synthesized Porous PLGA Nanoparticles Show Enhanced Drug Release. IEEE Transactions on Nanotechnology. 2013;12 [Google Scholar]
- 59.Fleming KK, Hubel A. Cryopreservation of hematopoietic and non-hematopoietic stem cells. Transfus Apher Sci. 2006;34:309–315. doi: 10.1016/j.transci.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 60.Nie Y, Bergendahl V, Hei DJ, Jones JM, Palecek SP. Scalable culture and cryopreservation of human embryonic stem cells on microcarriers. Biotechnol Prog. 2009;25:20–31. doi: 10.1002/btpr.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao J, Hao HN, Thomas RL, Lyman WD. An efficient method for the cryopreservation of fetal human liver hematopoeitic progenitor cells. Stem Cells. 2001;19:212–218. doi: 10.1634/stemcells.19-3-212. [DOI] [PubMed] [Google Scholar]
- 62.Yang H, Zhao H, Acker JP, Liu JZ, et al. Effect of dimethyl sulfoxide on post-thaw viability assessment of CD45+ and CD34+ cells of umbilical cord blood and mobilized peripheral blood. Cryobiology. 2005;51:165–175. doi: 10.1016/j.cryobiol.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 63.Yeh HJ, Yao CL, Chen HI, Cheng HC, Hwang SM. Cryopreservation of human limbal stem cells ex vivo expanded on amniotic membrane. Cornea. 2008;27:327–333. doi: 10.1097/ICO.0b013e31815dcfaf. [DOI] [PubMed] [Google Scholar]
- 64.Liu Y, Xu X, Ma X, Martin-Rendon E, et al. Cryopreservation of human bone marrow-derived mesenchymal stem cells with reduced dimethylsulfoxide and well-defined freezing solutions. Biotechnology progress. 2010;26:1635–1643. doi: 10.1002/btpr.464. [DOI] [PubMed] [Google Scholar]
- 65.Windrum P, Morris TC, Drake MB, Niederwieser D, Ruutu T. Variation in dimethyl sulfoxide use in stem cell transplantation: a survey of EBMT centres. Bone Marrow Transplant. 2005;36:601–603. doi: 10.1038/sj.bmt.1705100. [DOI] [PubMed] [Google Scholar]
- 66.Ji L, de Pablo JJ, Palecek SP. Cryopreservation of adherent human embryonic stem cells. Biotechnology and bioengineering. 2004;88:299–312. doi: 10.1002/bit.20243. [DOI] [PubMed] [Google Scholar]
- 67.Hegner B, Weber M, Dragun D, Schulze-Lohoff E. Differential regulation of smooth muscle markers in human bone marrow-derived mesenchymal stem cells. Journal of hypertension. 2005;23:1191–1202. doi: 10.1097/01.hjh.0000170382.31085.5d. [DOI] [PubMed] [Google Scholar]
- 68.Grein TA, Freimark D, Weber C, Hudel K, et al. Alternatives to dimethylsulfoxide for serum-free cryopreservation of human mesenchymal stem cells. International Journal of Artificial Organs. 2010;33:370–380. [PubMed] [Google Scholar]
- 69.Nishigaki T, Teramura Y, Nasu A, Takada K, et al. Highly efficient cryopreservation of human induced pluripotent stem cells using a dimethyl sulfoxide-free solution. Int J Dev Biol. 2011;55:305–311. doi: 10.1387/ijdb.103145tn. [DOI] [PubMed] [Google Scholar]
- 70.Rambhatla L, Chiu CP, Kundu P, Peng Y, Carpenter MK. Generation of hepatocyte-like cells from human embryonic stem cells. Cell transplantation. 2003;12:1–11. doi: 10.3727/000000003783985179. [DOI] [PubMed] [Google Scholar]
- 71.Rubinstein P, Dobrila L, Rosenfield RE, Adamson JW, et al. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proceedings of the National Academy of Sciences. 1995;92:10119–10122. doi: 10.1073/pnas.92.22.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berz D, McCormack EM, Winer ES, Colvin GA, Quesenberry PJ. Cryopreservation of hematopoietic stem cells. American journal of hematology. 2007;82:463–472. doi: 10.1002/ajh.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Y, Ma T. Bioprocessing of Cryopreservation for Large-Scale Banking of Human Pluripotent Stem Cells. BioResearch open access. 2012;1:205–214. doi: 10.1089/biores.2012.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thirumala S, Goebel WS, Woods EJ. Clinical grade adult stem cell banking. Organogenesis. 2009;5:143–154. doi: 10.4161/org.5.3.9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thirumala S, Wu X, Gimble JM, Devireddy RV. Evaluation of polyvinylpyrrolidone as a cryoprotectant for adipose tissue-derived adult stem cells. Tissue engineering Part C, Methods. 2010;16:783–792. doi: 10.1089/ten.TEC.2009.0552. [DOI] [PubMed] [Google Scholar]
- 76.Stiff PJ, Murgo AJ, Zaroulis CG, DeRisi MF, Clarkson BD. Unfractionated human marrow cell cryopreservation using dimethylsulfoxide and hydroxyethyl starch. Cryobiology. 1983;20:17–24. doi: 10.1016/0011-2240(83)90054-8. [DOI] [PubMed] [Google Scholar]
- 77.Fahy GM, MacFarlane DR, Angell CA, Meryman HT. Vitrification as an approach to cryopreservation. Cryobiology. 1984;21:407–426. doi: 10.1016/0011-2240(84)90079-8. [DOI] [PubMed] [Google Scholar]
- 78.Reubinoff B, Pera M, Vajta G, Trounson A. Effective cryopreservation of human embryonic stem cells by the open pulled straw vitrification method. Human Reproduction. 2001;16:2187–2194. doi: 10.1093/humrep/16.10.2187. [DOI] [PubMed] [Google Scholar]
- 79.Kader A, Agarwal A, Sharma R, Falcone T. Vitrification of isolated mice blastomeres using a closed loading device. Reproductive Biology and Endocrinology. 2009;7:17. doi: 10.1186/1477-7827-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beier A, Schulz J, Dörr D, Katsen-Globa A, et al. Effective surface-based cryopreservation of human embryonic stem cells by vitrification. Cryobiology. 2011;63:175–185. doi: 10.1016/j.cryobiol.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 81.Li T, Zhou C, Liu C, Mai Q, Zhuang G. Bulk vitrification of human embryonic stem cells. Human Reproduction. 2008;23:358–364. doi: 10.1093/humrep/dem386. [DOI] [PubMed] [Google Scholar]
- 82.McBurnie L, Bardo B. Validation of sterile filtration of liquid nitrogen. Pharmaceutical technology. 2002;26:74–83. [Google Scholar]
- 83.Parmegiani L, Cognigni GE, Filicori M. Ultra-violet sterilization of liquid nitrogen prior to vitrification. Human Reproduction. 2009 doi: 10.1093/humrep/dep329. [DOI] [PubMed] [Google Scholar]
- 84.Bielanski A, Vajta G. Risk of contamination of germplasm during cryopreservation and cryobanking in IVF units. Hum Reprod. 2009;24:2457–2467. doi: 10.1093/humrep/dep117. [DOI] [PubMed] [Google Scholar]
- 85.Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, et al. Evolution of adverse changes in stored RBCs. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17063–17068. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, et al. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116:2544–2552. doi: 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
- 87.Koch CG, Li L, Sessler DI, Figueroa P, et al. Duration of red-cell storage and complications after cardiac surgery. The New England journal of medicine. 2008;358:1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 88.Meryman HT, Hornblower M. A method for freezing and washing red blood cells using a high glycerol concentration. Transfusion. 1972;12:145–156. doi: 10.1111/j.1537-2995.1972.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 89.Rowe AW, Eyster E, Kellner A. Liquid nitrogen preservation of red blood cells for transfusion; a low glycerol-rapid freeze procedure. Cryobiology. 1968;5:119–128. doi: 10.1016/s0011-2240(68)80154-3. [DOI] [PubMed] [Google Scholar]
- 90.Chaudhari C. Frozen red blood cells in transfusion. Medical Journal Armed Forces India. 2009;65:55–58. doi: 10.1016/S0377-1237(09)80057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hess JR. Red cell freezing and its impact on the supply chain. Transfus Med. 2004;14:1–8. doi: 10.1111/j.0958-7578.2004.00472.x. [DOI] [PubMed] [Google Scholar]
- 92.Scott KL, Lecak J, Acker JP. Biopreservation of red blood cells: past, present, and future. Transfusion medicine reviews. 2005;19:127–142. doi: 10.1016/j.tmrv.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 93.SLF . Standards for blood banks and transfusion services. American Association of Blood Banks; 2008. [Google Scholar]
- 94.Pallotta V, D’Amici GM, D’Alessandro A, Rossetti R, Zolla L. Red blood cell processing for cryopreservation: from fresh blood to deglycerolization. Blood Cells, Molecules, and Diseases. 2012;48:226–232. doi: 10.1016/j.bcmd.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 95.Tinmouth AFD, Yee IC, Hébert PC. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46:2014–2027. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 96.Congdon T, Notman R, Gibson MI. Antifreeze (Glyco) protein mimetic behavior of poly (vinyl alcohol): detailed structure ice recrystallization inhibition activity study. Biomacromolecules. 2013;14:1578–1586. doi: 10.1021/bm400217j. [DOI] [PubMed] [Google Scholar]
- 97.DeMerlis C, Schoneker D. Review of the oral toxicity of polyvinyl alcohol (PVA) Food and Chemical Toxicology. 2003;41:319–326. doi: 10.1016/s0278-6915(02)00258-2. [DOI] [PubMed] [Google Scholar]
- 98.Lippert K, Galinski EA. Enzyme stabilization be ectoine-type compatible solutes: protection against heating, freezing and drying. Applied microbiology and biotechnology. 1992;37:61–65. [Google Scholar]
- 99.Pastor JM, Salvador M, Argandoña M, Bernal V, et al. Ectoines in cell stress protection: uses and biotechnological production. Biotechnology advances. 2010;28:782–801. doi: 10.1016/j.biotechadv.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 100.Lentzen G, Schwarz T. Extremolytes: natural compounds from extremophiles for versatile applications. Applied microbiology and biotechnology. 2006;72:623–634. doi: 10.1007/s00253-006-0553-9. [DOI] [PubMed] [Google Scholar]
- 101.Lusianti RE, Benson JD, Acker JP, Higgins AZ. Rapid removal of glycerol from frozen-thawed red blood cells. Biotechnology progress. 2013 doi: 10.1002/btpr.1710. [DOI] [PubMed] [Google Scholar]
- 102.Tasoglu S, Gurkan UA, Wang S, Demirci U. Manipulating biological agents and cells in micro-scale volumes for applications in medicine. Chemical Society Reviews. 2013 doi: 10.1039/c3cs60042d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Meryman HT, Kafig E. Rapid freezing and thawing of whole blood. Experimental Biology and Medicine. 1955;90:587–589. doi: 10.3181/00379727-90-22106. [DOI] [PubMed] [Google Scholar]
- 104.Vajta G, Holm P, Kuwayama M, Booth PJ, et al. Open Pulled Straw (OPS) vitrification: a new way to reduce cryoinjuries of bovine ova and embryos. Mol Reprod Dev. 1998;51:53–58. doi: 10.1002/(SICI)1098-2795(199809)51:1<53::AID-MRD6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 105.Rall W, Fahy G. Ice-free cryopreservation of mouse embryos at −196 C by vitrification. Nature. 1985:573–575. doi: 10.1038/313573a0. [DOI] [PubMed] [Google Scholar]
- 106.Lane M, Schoolcraft WB, Gardner DK, Phil D. Vitrification of mouse and human blastocysts using a novel cryoloop container-less technique. Fertility and sterility. 1999;72:1073–1078. doi: 10.1016/s0015-0282(99)00418-5. [DOI] [PubMed] [Google Scholar]
- 107.Kuwayama M, Vajta G, Kato O, Leibo SP. Highly efficient vitrification method for cryopreservation of human oocytes. Reproductive biomedicine online. 2005;11:300–308. doi: 10.1016/s1472-6483(10)60837-1. [DOI] [PubMed] [Google Scholar]
- 108.Hochi S, Terao T, Kamei M, Kato M, et al. Successful vitrification of pronuclear-stage rabbit zygotes by minimum volume cooling procedure. Theriogenology. 2004;61:267–275. doi: 10.1016/s0093-691x(03)00232-2. [DOI] [PubMed] [Google Scholar]