…with tolvaptan at present not being available for clinical care, other options that lower AVP activity should be considered. An alternative might be to lower AVP concentration by increasing water intake. In a rat model for polycystic kidney disease, a 3.5 fold increased water intake reduced urinary osmolality, renal expression of the AVP V2 receptor and reduced kidney weight compared to normal water intake…
Keywords: autosomal dominant polycystic kidney disease, glomerular filtration rate, kidney volume, urine volume, vasopressin
Abstract
Background
The clinical effects of increased water intake on autosomal dominant polycystic kidney disease (ADPKD) progression are unknown.
Methods
ADPKD patients with creatinine clearance ≧50 mL/min/1.73 m2 were divided into high (H-, n = 18) and free (F-, n = 16) water-intake groups, mainly according to their preference. Prior to the study, 30 patients underwent annual evaluation of total kidney volume (TKV) and 24-h urine for an average of 33 months. During the 1-year study period, TKV and 24-h urine were analyzed at the beginning and end of the study and every 4 months, respectively.
Results
During the pre-study period, urine volume (UV) in the H-group was higher (P = 0.034), but TKV and kidney function and their slopes were not significantly different between the two groups. After the study commenced, UV further increased (P < 0.001) in the H-group but not in the F-group. During the study period, TKV and kidney function slopes were not significantly different between the two groups (primary endpoint). Plasma copeptin was lower (P = 0.024) in the H-group than in the F-group. TKV and kidney function slopes became worse (P = 0.047 and 0.011, respectively) after high water intake (H-group) but not in the F-group. High UV was associated with increased urine sodium, and urine sodium positively correlated with the % TKV slope (P = 0.014).
Conclusions
Although the main endpoint was not significant, high water intake enhanced disease progression in the H-group when compared with the pre-study period. These findings necessitate a long-term randomized study before drawing a final conclusion.
INTRODUCTION
Innumerable cysts develop in renal tubule cells, causing progressive renal enlargement and functional deterioration in autosomal dominant polycystic kidney disease (ADPKD). Tubule cell proliferation and luminal fluid secretion are stimulated by 3′-5′-cyclic adenosine monophosphate (c-AMP). Arginine vasopressin (AVP) stimulates the production of c-AMP in the distal and collecting tubules by binding to AVP-V2 receptors (V2R) [1, 2]. Blocking the effects of AVP and thereby decreasing c-AMP levels by means of high water intake [3], genetic elimination of vasopressin [4] and AVP-V2R antagonist [5, 6] attenuate renal enlargement and kidney functional deterioration in animal models. Tolvaptan, a AVP-V2R antagonist, slowed the increase in total kidney volume (TKV) and decline in kidney function in patients with ADPKD [7, 8].
Since high water ingestion in PCK rat decreased the progression of polycystic kidney disease [3], motivated patients with ADPKD have already commenced increasing daily water intake as a convenient way to reduce AVP effects. Those patients ask how much ‘water cure’ is sufficient [9]; however, we still do not know the actual effects of increased water intake and potential adverse renal consequences of water diuresis in human settings. The effects of increased urine volume on TKV and kidney function in patients with ADPKD needs to be examined.
MATERIALS AND METHODS
ADPKD patient selection
The study was conducted from April 2011 to December 2012 on 35 patients aged from 20 to 65 years. All patients fulfilled Pei's diagnostic criteria of ADPKD [10]. Patients with an estimated glomerular filtration rate (eGFR) or creatinine clearance <50 mL/min/1.73 m2 were excluded for safety reasons to avoid pulmonary edema or hyponatremia in case of allocation to the high water-intake group. Patients were considered ineligible for the study if they had diabetes mellitus, congestive heart failure or edematous status, had a history of central nervous vascular disease or cardiovascular disease, were taking anti-depressants or diuretics medicine or were unable to undergo magnetic resonance imaging (MRI) due to a metallic foreign body or pregnancy.
After screening, patients were divided into high (H-) and free (F-) water-intake groups, mostly depending on their own preference, since several patients had already been informed that high water intake might ameliorate disease progression and were motivated to drink a large quantity of fluid [9, 11]. For such patients, randomization to the F-group was practically difficult. After inquiring about their preference, those who selected a particular group were allocated to that group, and those who did not have a preference were randomized. Patients allocated to the H-group were asked to drink ∼50 mL water/kg body weight/day (2.5–3.0 L of water/day) or more. They were also allowed to reduce the amount of water depending on their tolerability. Patients allocated to the F-group were allowed to drink as much water as they liked daily without any special direction. Caffeine-containing fluid was listed and both groups were recommended to avoid it. More detailed instructions on how to drink water are described in the Supplementary data.
This study was performed in adherence to the Declaration of Helsinki. The protocol was approved by an institutional review board (No. 441) and registered in ClinicalTrials.gov (NCT 01348035). All participants gave written informed consent.
Measurements
Blood pressure was assessed with an automatic device at the outpatient clinic. Patients collected 24-h urine at the base-line and every 4 months (total of four times) during the water study. The baseline urine collection and TKV measurement were obtained within 1 month after starting high water intake in the H-group. Urine volume, protein, osmolality, creatinine, electrolytes (Na, Cl and K), urea and c-AMP were measured in 24-h urine samples. Simultaneously with urine collection, blood samples were drawn for determination of osmolality, creatinine, Na, Cl, K, cystatin C, AVP and copeptin. Serum creatinine was measured enzymatically. Protein intake was estimated by Maroni's equation [12].
Cystatin C was measured by the latex agglutination method. Copeptin is a stable glycopeptide comprising the C-terminal of the AVP prohormone and was measured using a modified immunoluminometric assay [13]. AVP was measured by a double-antibody radioimmunoassay [14].
Kidney function was estimated with creatinine clearance using 24-h urine and the eGFR by two equations. eGFR(Eqcr) was calculated using the Japanese coefficient of the modified isotope dilution mass spectrometry (IDMS)-Modification of Diet in Renal Disease (MDRD) study [15]. eGFR(Eqcr-cys) is a Japanese modification of MDRD with cystatin C [16, 17].
TKV was determined at baseline and at 1 year using a magnetic resonance imaging (MRI), which was performed by two experienced MR technologists (K.K. and I.M.). The non-contrast MRI acquisition protocol was according to that of the Consortium for Radiological Imaging Studies of Polycystic Kidney Disease (CRISP cohort) [18] and that of Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes (TEMPO 3-4 study) [7, 8]. Kidney volume acquisition includes coronal T2-weighted single-shot fast spin echo/half-Fourier acquired single turbo spin echo (SSFSE/HASTE) images with fat saturation. Coronal images covering the entire kidney were acquired in one breath-hold with slice thickness of 4 mm. All images were transferred to a 3D-work station (ZAIOSTATION System1000, Ziosoft Inc., Tokyo, Japan). Using the signal intensity threshold difference of renal parenchyma and the background, the kidney border was delineated and the 3-D kidney image was extracted automatically. When the border of the kidney was obscure, delineation was assisted manually. The correlation coefficient was 0.999 for 10 different single kidney volume measurements at different times when two readers were blinded to the first measurement. The mean of the % difference between two measurements was 0.29 ± 3.28 standard deviation (SD) % [19].
TKV was adjusted for height (Ht-TKV, mL/m) [20]. The slopes of TKV, adjusted TKV parameters and kidney function parameters were calculated using linear regression analysis for each patient. The %TKV slope was calculated with baseline TKV as 100%. The slope of TKV during the water study (1 year) is expressed as the change over 1 year (slope) by adjusting for small variances in the measurement interval.
Questionnaires on voiding frequencies and voiding-related quality of life were obtained every 4 months at the time of 24-h urine collection.
Pre-study data
Annual measurements of TKV and collection of 24-h urine had been repeated as routine evaluations of ADPKD since April, 2007. Annual data collected for >1 year were available on 31 participants at enrollment and were used as pre-study data. Pre-study measurements did not include plasma cystatin C, plasma and urine osmolality, urine c-AMP, plasma AVP and copeptin. Pre-study data were used for comparisons in the slopes of TKV and kidney function before and during the water study.
Statistical analyses
Analyses were performed with SAS 10 and StatMate 4 for Windows. Parametric variables are expressed as the mean ± SD. Variables between two groups were compared by the Welch t-test or z test. Individually related data pre- and during the water study were examined by the paired t-test. Correlations between two variables were examined by linear regression analysis, and Pearson correlation coefficient (r) was expressed. Two-sided P < 0.05 was considered to indicate statistical significance.
RESULTS
Patients
Most patients assigned to the H-group actively selected this group. Ten patients preferred free water intake and six patients without a preference were assigned to the F-group. One patient in the H-group withdrew from the study because of pregnancy and her data were deleted from the analyses.
The characteristics of participating patients are depicted in Table 1. Pre-study urine volume was higher in the H-group than in the F-group. However, there was no significant difference in pre-study parameters relating to volume enlargement and function deterioration between the two groups. Baseline 0-month data were not statistically different between the two groups (see also Supplementary data Table S1).
Table 1.
Baseline data of the pre- and present study
| High water-intake group | Free water-intake group | P value | |
|---|---|---|---|
| Baseline data of pre-study period | |||
| Patient number | 17 | 13 | |
| Observation period (months) | 33.8 ± 9.1 | 32.7 ± 10.5 | 0.78 |
| Frequency of TKV measurements | 3.7 ± 0.5 | 3.7 ± 0.7 | 1.0 |
| TKV slope (mL/year) | 72 ± 91 | 92 ± 105 | 0.60 |
| % TKV slope (%/year) | 3.8 ± 5.7 | 5.3 ± 8.3 | 0.56 |
| Ccr slope (mL/min/1.73 m2/year) | 0.35 ± 6.61 | −0.52 ± 7.94 | 0.76 |
| eGFR(Eqcr) slope (mL/min/1.73 m2/year) | −0.30 ± 3.00 | −1.51 ± 4.06 | 0.35 |
| Urine volume (mL/day) | 2037 ± 661 | 1519 ± 588 | 0.034 |
| Baseline 0-month data of present study | |||
| Patient number | 18 | 16 | |
| (Male/female) | (6/12) | (7/9) | 0.53 |
| Age (year) | 42.9 ± 10.0 | 42.1 ± 11.0 | 0.79 |
| Height (cm) | 163.3 ± 7.3 | 165.1 ± 11.3 | 0.60 |
| Body weight (kg) | 63.3 ± 12.7 | 58.9 ± 12.2 | 0.32 |
| Body surface area (m2) | 1.68 ± 0.17 | 1.64 ± 0.22 | 0.62 |
| Total kidney volume (mL) | 1743 ± 797 | 1607 ± 1130 | 0.71 |
| Creatinine clearance (Ccr) (mL/min/1.73 m2) | 121 ± 35 | 98 ± 32 | 0.065 |
| eGFR(Eqcr-cys) (mL/min/1.73 m2) | 85 ± 26 | 79 ± 27 | 0.48 |
| eGFR(Eqcr) (mL/min/1.73 m2) | 79 ± 25 | 72 ± 25 | 0.46 |
| Systolic blood pressure (mmHg) | 125 ± 12 | 122 ± 7 | 0.37 |
| Diastolic blood pressure (mmHg) | 81 ± 11 | 77 ± 7 | 0.31 |
| Hypertension treatment yes versus no | 13 versus 5 | 9 versus 7 | 0.33 |
Pre-study data were available for 17/18 and 13/16 patients in high and free water-intake groups, respectively. TKV, total kidney volume; GFR, glomerular filtration rate; eGFR(Eqcr-cys), estimated GFR using the Japanese modification of MDRD with cystatin C incorporated; eGFR(Eqcr-cys) = 92 × SCystC−0.575 × SCr−0.670 × 0.995Age × 0.784 (if female); eGFR(Eqcr), estimated GFR using the Japanese modification of IDMS-MDRD, eGFR(Eqcr) = 194 × Cr−1.094 × Age−0.287 × 0.739 (if female); MDRD, modification of diet in renal disease; IDMS, isotope dilution mass spectrometry.
Adherence to protocol
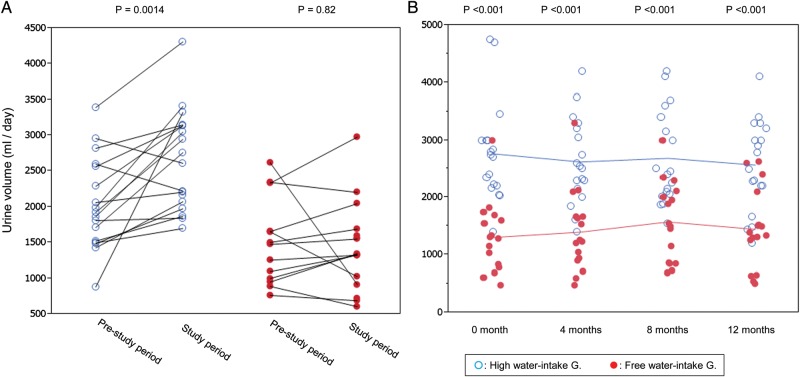
After starting the water study, urine volume further increased in the H-group, but not in the F-group. High urine volume was sustained for 1 year in the H-group (Figure 1, P < 0.001).
FIGURE 1:
Changes in urine volume from the pre-study period to water-study period (A), and during the water study period (B). Urine volume in the pre-study period is the mean of 24-h urine collected yearly for 2–3 years and that during the study period is the mean of four 24-h urine samples collected during the water study. Connected line represents the same patient and unconnected single points correspond to the participants without pre-study data. Single data were input to draw the figure but only paired data were used for statistical calculation. (A). In the pre-study period, urine volume was larger in the high water-intake group and further increased significantly from 2048 ± 648 to 2691 ± 710 mL/day (mean of paired samples). Higher urine volume was sustained for 1 year in the high water-intake group (B).
Pertinent data during the water study in Table 2 are the means of four measurements. Compared with the F-group, urine volume, urine sodium and urine solute excretion were higher and urine osmolality, plasma osmolality and plasma sodium were lower in the H-group. Kidney function parameters were not statistically different between the two groups (Table 2 and Supplementary data Table S1).
Table 2.
Pertinent data related to water-drinking study
| High water-intake group | Free water-intake group | P value | |
|---|---|---|---|
| Urine volume (mL/day) | 2662 ± 700 | 1430 ± 616 | <0.001 |
| Urine osmolality (mOsm/kgH2O) | 329 ± 90 | 523 ± 161 | <0.001 |
| Urine Na excretion (mEq/day) | 189 ± 48 | 144 ± 43 | 0.0055 |
| Urine K excretion (mEq/day) | 44 ± 13 | 44 ± 17 | 0.91 |
| Urine urea excretion (g/day) | 8.6 ± 2.0 | 7.3 ± 1.4 | 0.046 |
| Urine solute excretion (mOsm/day) | 819 ± 170 | 655 ± 148 | <0.001 |
| Free water clearance (mL/day) | −201 ± 675 | −840 ± 438 | 0.0029 |
| Urine cyclic AMP excretion (μmol/day) | 3.5 ± 1.0 | 3.0 ± 0.7 | 0.12 |
| Estimated protein intake (g/day) | 66 ± 14 | 57 ± 11 | 0.053 |
| Plasma Na (mEq/L) | 139.3 ± 1.3 | 140.7 ± 1.9 | 0.027 |
| Plasma osmolality (mOsm/kgH2O) | 286.0 ± 3.5 | 289.0 ± 3.0 | <0.001 |
| Creatinine clearance (mL/min/1.73 m2) | 110 ± 90 | 95 ± 28 | 0.16 |
| eGFR (Eqcr-cys) (mL/min/1.73 m2) | 81 ± 25 | 78 ± 29 | 0.75 |
| eGFR (Eqcr) (mL/min/1.73 m2) | 73 ± 22 | 71 ± 26 | 0.71 |
| Systolic blood pressure (mmHg) | 124 ± 8 | 124 ± 7 | 0.87 |
| Diastolic blood pressure (mmHg) | 81 ± 6 | 76 ± 6 | 0.047 |
Data are the mean of four measurements for each participant during 1-year study. c-AMP, 3′-5′-cyclic adenosine monophosphate; protein intake was estimated by Maroni's equation [12]; protein intake (g/day) = (urine urea excretion (mg/day) +31 × body weight (kg)) × 0.00625; eGFR(Eqcr-cys), estimated GFR using the Japanese modification of MDRD with cystatin C incorporated, eGFR(Eqcr-cys) = 92 × SCystC−0.575 × SCr−0.670 × 0.995Age × 0.784 (if female); eGFR(Eqcr), estimated GFR using the Japanese modification of IDMS-MDRD, eGFR(Eqcr) = 194 × Cr−1.094 × Age−0.287 × 0.739 (if female). Data of each measurement are shown in Supplementary data Table S1.
Primary and secondary endpoints
The primary endpoint of this study, a comparison of TKV changes between two groups was not statistically different. However, the increase rate of kidney volume was slightly larger in than H-group than in the F-group (Table 3).
Table 3.
Effect of high water intake on primary and secondary end points
| High water-intake group | Free water-intake group | P value | |
|---|---|---|---|
| Primary end point (kidney volume slope) | |||
| TKV slope (mL/year) | 163 ± 124 | 99 ± 118 | 0.13 |
| % TKV slope (%/year) | 9.68 ± 6.64 | 5.28 ± 7.70 | 0.083 |
| Ht-TKV slope (mL/m/year) | 99.5 ± 75.1 | 61.4 ± 76.2 | 0.15 |
| Secondary end point (kidney function slope) | |||
| Ccr slope (mL/min/1.73 m2/year) | −15.4 ± 34.0 | −2.6 ± 19.2 | 0.19 |
| eGFR (Eqcr-cys) slope (mL/min/1.73 m2/year) | −5.6 ± 6.5 | −1.1 ± 7.0 | 0.059 |
| eGFR (Eqcr) slope (mL/min/1.73 m2/year) | −7.1 ± 8.6 | −2.7 ± 7.3 | 0.12 |
| Secondary end point (AVP and copeptin) | |||
| Plasma AVP (pg/mL) | 2.7 ± 2.4 | 5.2 ± 3.6 | 0.024 |
| Plasma copeptin (pmol/L) | 7.6 ± 4.0 | 14.4 ± 10.5 | 0.016 |
| Secondary end point (QOL) | |||
| How many times did you void during daytime? | 8.6 ± 1.6 | 6.5 ± 1.3 | <0.001 |
| How many times did you void at night? | 0.8 ± 0.6 | 0.5 ± 0.4 | 0.10 |
| How problematic is daytime frequency?a | 2.5 ± 0.9 | 1.8 ± 1.1 | 0.069 |
| How problematic is nocturnal frequency?a | 2.3 ± 1.0 | 1.6 ± 1.0 | 0.070 |
eGFR (Eqcr-cys) = 92 × SCystC−0.575 × SCr−0.670 × 0.995Age × 0.784 (if female). eGFR (Eqcr) = 194 × Cr−1.094 × Age−0.287 × 0.739 (if female). AVP, arginine vasopressin; TKV, total kidney volume; Ht-TKV, height adjusted TKV; Ccr, creatinine clearance; eGFR, estimated glomerular filtration rate.
aQOL score (0—delighted, 1—pleased, 2—mostly satisfied, 3—mixed, 4—mostly dissatisfied, 5—unhappy, 6—terrible) is derived from the QOL score due to the urinary symptom score of the International Prostate Symptom Score (I-PSS).
Secondary endpoints, including eGFR changes, plasma AVP (copeptine) level and quality of life (QOL) score, are shown in Table 3. Although there was no significant difference in eGFR slope, a similar beneficial tendency in the slope of kidney functional parameters was identified in the F-group. Plasma AVP and copeptin decreased in the H-group (data also shown in Supplementary data Table S1). Diurnal urinary frequency increased in the H-group, but the impact of the increased problematic score was modest for patients in H-group.
Correlation between laboratory parameters
Changes in plasma osmolality and plasma copeptin and those in plasma sodium concentration and urine sodium excretion during the study are illustrated in Figures 2 and 3, respectively.
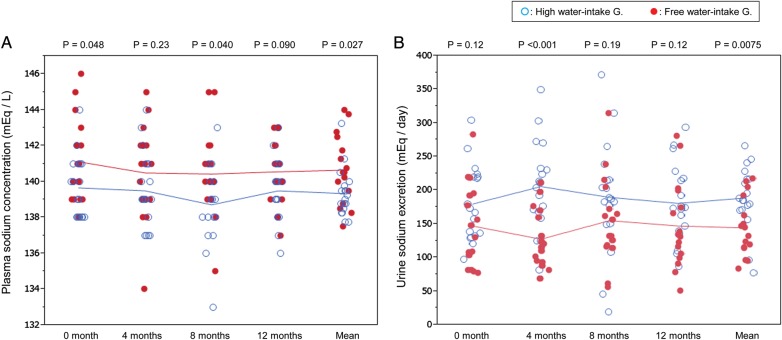
FIGURE 2:
Changes of plasma osmolality (A) and plasma copeptin (B) during the water study. The right-most column represents the mean of four measurements. High water intake accompanied with low plasma osmolality and low plasma vasopressin (copeptin) level.
FIGURE 3:
Changes of plasma sodium concentration (A) and urinary sodium excretion (B) during the water study. The right-most column represents the mean of four measurements. High water intake induced high urinary sodium excretion, which resulted in low plasma concentration.
The correlation coefficients between urine volume and the main endpoint parameters are shown in Table 4. Although urine volume did not significantly correlate with most variables, it tended to correlate positively with the kidney volume slope and negatively with the function slope. The negative correlation between urine volume and the slope of eGFR(Eqcr-cys) was significant (P = 0.043). The tendencies of relationships of urine volume and the slopes of kidney volume and function are entirely opposite to pre-study expectations. There was a significant correlation between urine volume and urine sodium (Figure 4). Urinary sodium excretion was correlated with % increase in TKV (Figure 5A). Plasma copeptin levels were weakly correlated with the TKV slope (Figure 5B). The correlation coefficients (r) of urine volume with various parameters are summarized in Table 5. Generally, urine volume was correlated positively with urine constituents and negatively with plasma osmolality and plasma AVP and copeptin concentrations.
Table 4.
Pearson's correlation coefficient (r) between urine volume and slopes of kidney volume and function
| r | P value of r | |
|---|---|---|
| TKV slope (mL/year) | 0.23 | 0.19 |
| Ht-TKV slope (mL/m/year) | 0.22 | 0.22 |
| % TKV slope (%/year) | 0.24 | 0.17 |
| Ccr slope (mL/min/1.73 m2/year) | −0.25 | 0.15 |
| eGFR (Eqcr-cys) slope (mL/min/1.73 m2/year) | −0.35 | 0.043 |
| eGFR (Eqcr) slope (mL/min/1.73 m2/year) | −0.23 | 0.19 |
TKV, total kidney volume; Ht-TKV, height adjusted TKV; Ccr, creatinine clearance; eGFR, estimated glomerular filtration rate. eGFR(Eqcr-cys) = 92 × SCystC−0.575 × SCr−0.670 × 0.995Age × 0.784 (if female). eGFR(Eqcr) = 194 × Cr−1.094 × Age−0.287 × 0.739 (if female).
FIGURE 4:
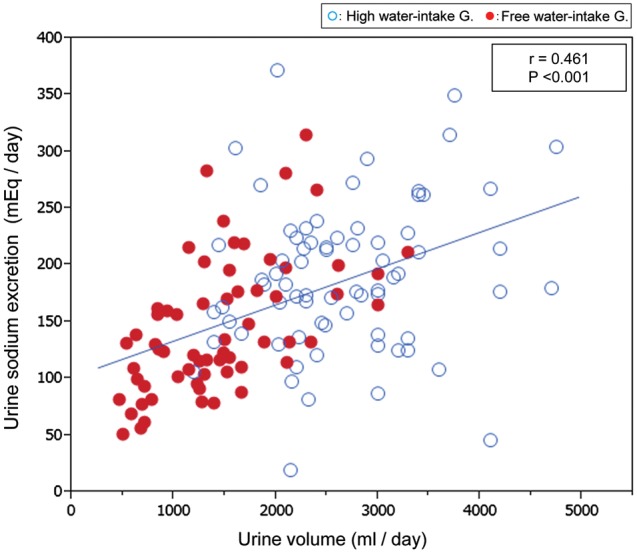
The relationships between urine volume and urine sodium excretion. Urine volume correlated positively with urine sodium excretion with a highly significant correlation coefficient. Although patients were directed to drink solute-free water, urine sodium increased concomitantly with increased urine volume.
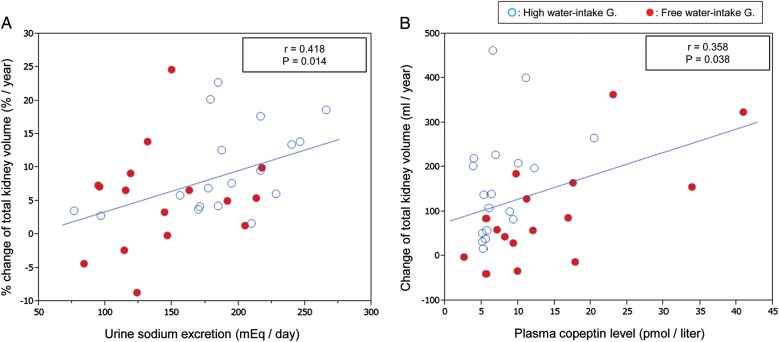
FIGURE 5:
The relationships between urine sodium and % change of TKV (A) and between plasma copeptin level and TKV slope (B). Urine sodium had a positive correlation with % increase in TKV (A). Plasma copeptin had a positive but weak correlation with the increased rate of TKV (B), which is consistent with previous experimental findings.
Table 5.
Pearson's correlation coefficient (r) between urine volume and various parameters
| r | P value of r | |
|---|---|---|
| Urine osmolality (mOsm/kgH2O) | −0.80 | P < 0.001 |
| Urine Na excretion (mEq/day) | 0.46 | P < 0.001 |
| Urine K excretion (mEq/day) | 0.39 | P < 0.001 |
| Urine solute excretion (mOsm/day) | 0.54 | P < 0.001 |
| Urine c-AMP excretion (μmol/day) | 0.29 | P < 0.001 |
| Estimated protein intake (g/day) | 0.52 | P < 0.001 |
| Plasma osmolality (mOsm/kgH2O) | −0.37 | P < 0.001 |
| Plasma AVP (pg/mL) | −0.26 | 0.0020 |
| Plasma copeptin (pmol/L) | −0.332 | P < 0.001 |
c-AMP, 3′-5′-cyclic adenosine monophosphate; protein intake was estimated by Maroni's equation [12]; protein intake (g/day) = (urine urea excretion (mg/day) + 31 × body weight (kg)) × 0.00625. AVP, arginine vasopressin.
Changes in kidney volume and eGFR slope pre- and during water study
Basic data for the pre-study period are shown in Table 1. Average observation period was not different between the two groups. Last TKV data for the pre-study period were the same as baseline 0-month data in the present study, except for a lack of pre-study data for four participants.
The changes of eGFR(Eqcr) slope, TKV slope and %TKV slope pre- and during the water study are illustrated in Figure 6. The slope of eGFR(Eqcr) and TKV slope were significantly accelerated in the H-group but not in the F-group during high water intake.
FIGURE 6:

Changes in eGFR(Eqcr) slope (A), TKV slope (B) and %TKV slope (C) between pre-study and during water study periods. The slopes in the pre-study period were calculated from 2 to 3 years of observation and those during the water study from 1-year observation. The lines connect individual data and unconnected single points correspond to the participants without pre-study data. Single data were input to draw the figure but only paired data were used for statistical calculation. Horizontal bar represents the mean. After increasing water intake in the high water-intake group, eGFR(Eqcr) slope (A) became more negative (from −0.3 ± 3.0 to −7.1 ± 8.6) and TKV slope (B) became more positive (from 72 ± 89 to 149 ± 113). The %TKV slope (C) also increased (from 3.8 ± 5.7 to 9.1 ± 6.3) in the high water-intake group, but the changes did not reach statistical significance. (The data in parentheses are the means ± SD of paired data.)
DISCUSSION
The present study shows that lower plasma AVP (copeptin) in the H-group might contribute to the slower TKV increase. However, increased urine sodium and/or unknown factors concurrent with increased urine volume might accelerate disease progression more strongly than the beneficial effects brought about by the decreased plasma AVP level.
The effect of increased urine volume on kidney function in patients with chronic kidney disease (CKD) including ADPKD is controversial [11, 21]. A long-term interventional study to examine the effect of water intake was carried out in patients with calcium nephrolithiasis [22], but only short-term pilot studies have been reported in ADPKD [23, 24]. In an observational study examining the effect of fluid intake on kidney function [21], it was difficult to differentiate cause and consequence [11], since urine volume increases as urine-concentrating ability deteriorates due to CKD.
The subjects assigned to the H-group already had higher urine volume during pre-study period than the subjects assigned to the F-group (Table 1), mainly because they were motivated to drink a large amount of water for cure [9, 11]. The participants assigned to the H-group further increased mean urine volume up to ∼1200 mL/day higher than the F-group for 1 year (Figure 1 and Table 2). High urine volume persisting for 1 year (Figure 1B, Supplementary data Table S1) confirms the compliance of patients in the H-group with high water intake. Their water intake above the thirst requirement was indirectly suggested by a fall of plasma osmolality and sodium concentration (Figures 2 and 3) [25]. Pre-study slopes of TKV and kidney function were not significantly different in the face of mean urine volume difference of ∼500 mL/day between the two groups. This urine volume difference might not have been large enough to affect the slopes of kidney volume and function in either direction. Therefore, the two groups actually commenced the water study at a comparable level of kidney function, kidney volume and their slopes (Table 1).
As TKV change can be detected within 6 months [26], a 1-year study was assumed to be sufficient if water intake affected renal volume change. The effect of tolvaptan was observed at 1 year in a relatively small number of 17 Japanese patients [7]. In addition, eGFR(Eqcr-cys) was shown to be in good agreement with GFR in patients with ADPKD [17]. The use of the eGFR(Eqcr-cys) equation was supposed to enhance the sensitivity to detect kidney functional change. These reports and experiences allowed us to design a protocol for this duration and patient number.
Vasopressin directly stimulates cyst growth and water ingestion ameliorates disease progression in polycystic animal models [4, 5]. The detrimental effects of vasopressin have been reported in ADPKD patients [27, 28]. Since ADPKD patients have impaired urine-concentrating capacity even in an early CKD stage, they tend to lose water and became dehydrated. As a result, vasopressin concentration increases to maintain fluid balance [29]. The plasma copeptin concentration was associated with kidney function decline [27, 30] and disease severity [24]. It is quite natural to postulate that the same analogy as in animal models will work in human clinical settings by drinking a large amount of water [9, 11, 24].
As expected from the clinical and experimental studies mentioned above, plasma osmolality and plasma copeptin level decreased by high water intake (Figure 2), and a positive correlation between plasma copeptin and TKV slope was observed in the present study (Figure 5B). Increased urine volume by drinking relatively solute-free water was supposed to ameliorate cyst growth via decreased AVP. However, the actual effects of increased urine volume on TKV and kidney function slope were entirely opposite to the expectations.
Slightly higher (P = 0.065) baseline creatinine clearance in the H-group (Tables 1 and 2) might affect slope variance. However, the difference in eGFR(Eqcr-cys) between the two groups was much smaller (Table 1, P = 0.48). Better kidney function is generally associated with a slow deterioration rate of kidney function and volume [19, 31]. Therefore, the difference in kidney function hardly explains the slope variance.
Increased urine volume was associated with increased total solute excretion into urine (Tables 2 and 5). This difference in urine solute excretion is accounted for by a significant increase in urinary sodium chloride and urea excretion. A highly correlated relation between urine volume and urine sodium is recognized in Figure 4. The participants were asked to drink different amounts of water, but no guidance on sodium intake was given.
The study in healthy volunteers suggested a direct association of the level of hydration with urinary sodium excretion, i.e. high hydration increased natriuresis [32]. In patients with polydipsia-related hyponatremia, the causes were equally solute loss into urine and water retention [33, 34].
Several actions of AVP on V2R contribute to the urinary concentrating process: (i) insertion of aquaporin 2 water channels into the apical cell membrane of the collecting duct (CD); (ii) activation of the urea transporters in the inner medullary CD; (iii) stimulation of the epithelial sodium channel ENaC to increase sodium reabsorption by cortical and outer medullary CD; (iv) stimulation of sodium reabsorption and increased Na–K–2Cl cotransporter expression in the thick ascending limb of Henle [11]. These actions of AVP are decreased during water load, and sodium reabsorption is suppressed via the mechanisms of (iii) and (iv). These physiological findings [11] along with clinical observations [32–34] support the view that large water intake induces sodium loss into urine.
The urine-diluting capacity remains not well understood in ADPKD patients, despite well-proven impaired urine-concentrating capacity [29, 35, 36]. Although not adequately studied in ADPKD patients, urine-diluting capacity was suggested to be impaired [36]. Large solute delivery to the diluting nephron segment by high water intake may result in loss of sodium into urine even in a normal kidney [32–34], and can be reasonably anticipated in ADPKD. These factors (water load and impaired diluting capacity) might contribute to high urine sodium and low plasma sodium concentration in the H group (Figure 3).
High urine sodium in the H-group could be argued to be a consequence of primary high salt intake. Plasma sodium might have decreased further in the H-group if they had not increased sodium intake [33, 34]. The increased urinary sodium was a result of high sodium intake. Whether high sodium intake was primary or secondary to urine sodium loss is a point raised in the present study because of the non-randomized method of dividing the patients into the two study groups. The chronic effects of high water intake on sodium homeostasis remain to be elucidated.
A reversible reduction in GFR was observed during 1-week administration of tolvaptan to patients with ADPKD [37]. A similar acute and reversible effect of high hydration to decrease GFR was reported in normal volunteers [32]. High water intake might have lowered eGFR in the H-group. The baseline and final eGFR were measured during high water intake in the H-group. If eGFR was influenced by high water intake, both baseline and final eGFR were influenced in the same direction. Therefore, this acute and reversible effect of high water intake on the eGFR slope during the water study period might be abolished.
Recently, it was reported that higher urine sodium excretion at baseline was associated with greater log-TKV growth and greater GFR decline [38, 39]. The present study also confirmed the association between higher urine sodium and faster %TKV growth (Figure 5A).
The detrimental effects of salt intake on hypertension, cardiovascular disease and CKD have been investigated widely for a long time but little attention has been focused on ADPKD [40]. The present results and those of previous studies [38, 39] suggest that high sodium intake may activate renal enlargement and functional deterioration in ADPKD.
Disturbed sodium balance increases endogenous cardiotonic steroids (ECS) [41, 42] and transforming growth factor-β (TGF-β) [43]. Endogenous low-level concentration of ouabain (ECS) stimulates fluid secretion and cell proliferation in ADPKD cells [44, 45]. Nitric oxide serves as a negative regulator of TGF-β production, and impaired endogenous production of nitric oxide has been demonstrated in ADPKD patients [46]. These findings may explain the association of high urine sodium, stimulated TKV enlargement and enhanced eGFR(Eqcr-cys) decline.
The fact that patients were not randomized, but were divided over the two study groups according to their own preference, is a major limitation. This limitation, however, is partly compensated for by comparing the TKV and eGFR changes between pre-study and study periods in the same patient. Other limitations are the low number of patients and short observation period. GFR was estimated by creatinine clearance and equations. Four of 34 participants lacked pre-water study data.
The present data do not verify the advice to increase water intake to ameliorate disease progression in ADPKD patients. Given the observational form of our data, a randomized, long-term study is needed to reach a final conclusion.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
None declared. (See related article by Meijer and Casteleijn. Riding the waves: evidence for a beneficial effect of increased water intake in autosomal dominant polycystic kidney disease patients? Nephrol Dial Transplant 2014; 29: 1615–1617.)
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported in part by a Grant-in-Aid for Progressive Renal Diseases Research from the Ministry of Health, Labor and Welfare of Japan.
REFERENCES
- 1.Hanaoka K, Guggino WB. cAMP regulates cell proliferation and cyst formation in autosomal polycystic kidney disease cells. J Am Soc Nephrol. 2000;11:1179–1187. doi: 10.1681/ASN.V1171179. [DOI] [PubMed] [Google Scholar]
- 2.Yamaguchi T, Pelling JC, Ramaswamy NT. cAMP stimulates the in vitro proliferation of renal cyst epithelial cells by activating the extracellular signal-regulated kinase pathway. Kidney Int. 2000;57:1460–1471. doi: 10.1046/j.1523-1755.2000.00991.x. [DOI] [PubMed] [Google Scholar]
- 3.Nagao S, Nishii K, Katsuyama M. Increased water intake decreases progression of polycystic kidney disease in the PCK rat. J Am Soc Nephrol. 2006;17:2220–2227. doi: 10.1681/ASN.2006030251. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Wu Y, Ward CJ, et al. Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol. 2008;19:102–108. doi: 10.1681/ASN.2007060688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gattone VH, Wang X, Harris PC, et al. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med. 2003;9:1323–1326. doi: 10.1038/nm935. [DOI] [PubMed] [Google Scholar]
- 6.Torres VE, Wang X, Qian Q, et al. Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med. 2004;10:363–364. doi: 10.1038/nm1004. [DOI] [PubMed] [Google Scholar]
- 7.Higashihara E, Torres VE, Chapman AB. Tolvaptan in autosomal dominant polycystic kidney disease: three years’ experience. Clin J Am Soc Nephrol. 2011;6:2499–2507. doi: 10.2215/CJN.03530411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torres VE, Chapman AB, Devuyst O. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grantham JJ. Therapy for polycystic kidney disease? It's water, stupid! J Am Soc Nephrol. 2008;19:1–2. doi: 10.1681/ASN.2007101100. [DOI] [PubMed] [Google Scholar]
- 10.Pei Y, Obaji J, Dupuis A. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol. 2009;20:205–212. doi: 10.1681/ASN.2008050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torres VE, Bankir L, Grantham JJ. A case for water in the treatment of polycystic kidney disease. Clin J Am Soc Nephrol. 2009;4:1140–1150. doi: 10.2215/CJN.00790209. [DOI] [PubMed] [Google Scholar]
- 12.Maroni BJ, Steinman TI, Mitch WE. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int. 1985;27:58–65. doi: 10.1038/ki.1985.10. [DOI] [PubMed] [Google Scholar]
- 13.Morgenthaler NG, Struck J, Alonso C, et al. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52:112–119. doi: 10.1373/clinchem.2005.060038. [DOI] [PubMed] [Google Scholar]
- 14.Jaffe BM, Behrmann HR. Methods of Hormone Radioimmunoassays. 2nd edn. New York, NY: Academic Press; 1979. [Google Scholar]
- 15.Matsuo S, Imai E, Horio M. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 16.Horio M, Imai E, Yasuda Y, et al. Collaborators Developing the Japanese Equation for Estimated GFR. GFR estimation using standardized serum cystatin C in Japan. Am J Kidney Dis. 2013;61:197–203. doi: 10.1053/j.ajkd.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Orskov B, Borresen ML, Feldt-Rasmussen B, et al. Estimating glomerular filtration rate using the new CKD- EPI equation and other equations in patients with autosomal dominant polycystic kidney disease. Am J Nephrol. 2010;31:53–57. doi: 10.1159/000256657. [DOI] [PubMed] [Google Scholar]
- 18.Chapman AB, Guay-Woodford LM, Grantham JJ. Renal structure in early autosomal dominant polycystic kidney disease (ADPKD): The consortium for radiologic imaging studies of polycystic kidney disease (CRISP) cohort. Kidney Int. 2003;64:1035–1045. doi: 10.1046/j.1523-1755.2003.00185.x. [DOI] [PubMed] [Google Scholar]
- 19.Higashihara E, Nutahara K, Okegawa T. Kidney volume and function in autosomal dominant polycystic kidney disease. Clin Exp Nephrol. 2014;18:157–165. doi: 10.1007/s10157-013-0834-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapman AB, Bost JE, Torres VE. Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2012;7:479–486. doi: 10.2215/CJN.09500911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hebert LA, Greene T, Levey A, et al. High urine volume and low urine osmolality are risk factors for faster progression of renal disease. Am J Kidney Dis. 2003;41:962–971. doi: 10.1016/s0272-6386(03)00193-8. [DOI] [PubMed] [Google Scholar]
- 22.Borghi L, Meschi T, Amato F, et al. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: a 5-year randomized prospective study. J Urol. 1996;155:839–843. [PubMed] [Google Scholar]
- 23.Barash I, Ponda MP, Goldfarb DS, et al. A pilot clinical study to evaluate changes in urine osmolality and urine cAMP in response to acute and chronic water loading in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2010;5:693–697. doi: 10.2215/CJN.04180609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang CJ, Creed C, Winklhofer FT, et al. Water prescription in autosomal dominant polycystic kidney disease: a pilot study. Clin J Am Soc Nephrol. 2011;6:192–197. doi: 10.2215/CJN.03950510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halperin ML, Kamel KS, Goldstein MB. , 4th edn. Philadelphia, PA: Saunders; 2010. Fluid, electrolyte, and acid-base physiology, pp. 246–313. [Google Scholar]
- 26.Kistler AD, Poster D, Krauer F. Increases in kidney volume in autosomal dominant polycystic kidney disease can be detected within 6 months. Kidney Int. 2009;75:235–241. doi: 10.1038/ki.2008.558. [DOI] [PubMed] [Google Scholar]
- 27.Meijer E, Bakker SJ, van der Jagt EJ, et al. Copeptin, a surrogate marker of vasopressin, is associated with disease severity in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2011;6:361–368. doi: 10.2215/CJN.04560510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meijer E, Boertien WE, Zietse R, et al. Potential deleterious effects of vasopressin in chronic kidney disease and particularly autosomal dominant polycystic kidney disease. Kidney Blood Press Res. 2011;34:235–244. doi: 10.1159/000326902. [DOI] [PubMed] [Google Scholar]
- 29.Zittema D, Boertien WE, van Beek AP. Vasopressin, copeptin, and renal concentrating capacity in patients with autosomal dominant polycystic kidney disease without renal impairment. Clin J Am Soc Nephrol. 2012;7:906–913. doi: 10.2215/CJN.11311111. [DOI] [PubMed] [Google Scholar]
- 30.Boertien WE, Meijer E, Zittema D. Copeptin, a surrogate marker for vasopressin, is associated with kidney function decline in subjects with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2012;27:4131–4137. doi: 10.1093/ndt/gfs070. [DOI] [PubMed] [Google Scholar]
- 31.Grantham JJ, Chapman AB, Torres VE. Volume progression in autosomal dominant polycystic kidney disease: the major factor determining clinical outcomes. Clin J Am Soc Nephrol. 2006;1:148–157. doi: 10.2215/CJN.00330705. [DOI] [PubMed] [Google Scholar]
- 32.Anastasio P, Cirillo M, Spitali L, et al. Level of hydration and renal function in healthy humans. Kidney Int. 2001;60:748–756. doi: 10.1046/j.1523-1755.2001.060002748.x. [DOI] [PubMed] [Google Scholar]
- 33.Berl T. Impact of solute intake on urine flow and water excretion. J Am Soc Nephrol. 2008;19:1076–1078. doi: 10.1681/ASN.2007091042. [DOI] [PubMed] [Google Scholar]
- 34.Musch W, Xhaet O, Decaux G. Solute loss plays a major role in polydipsia-related hyponatraemia of both water drinkers and beer drinkers. QJM. 2003;96:421–426. doi: 10.1093/qjmed/hcg078. [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Maldonado M, Yium JJ, Eknoyan G, et al. Adult polycystic kidney disease: studies of the defect in urine concentration. Kidney Int. 1972;2:107–113. doi: 10.1038/ki.1972.78. [DOI] [PubMed] [Google Scholar]
- 36.Higashihara E, Nutahara K, Tago K, et al. Renal function studies in cystic renal diseases. Jpn J Nephrol. 1981;23:1021–1026. [PubMed] [Google Scholar]
- 37.Irazabal MV, Torres VE, Hogan MC. Short-term effects of tolvaptan on renal function and volume in patients with autosomal dominant polycystic kidney disease. Kidney Int. 2011;80:295–301. doi: 10.1038/ki.2011.119. [DOI] [PubMed] [Google Scholar]
- 38.Torres VE, Grantham JJ, Chapman AB. Potentially modifiable factors affecting the progression of autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2011;6:640–647. doi: 10.2215/CJN.03250410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torres VE, King BF, Chapman AB. Magnetic resonance measurements of renal blood flow and disease progression in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2007;2:112–120. doi: 10.2215/CJN.00910306. [DOI] [PubMed] [Google Scholar]
- 40.Klahr S, Breyer JA, Beck GJ. Dietary protein restriction, blood pressure control, and the progression of polycystic kidney disease. Modification of diet in renal disease study group. J Am Soc Nephrol. 1995;5:2037–2047. doi: 10.1681/ASN.V5122037. [DOI] [PubMed] [Google Scholar]
- 41.Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacol Rev. 2009;61:9–38. doi: 10.1124/pr.108.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manunta P, Hamilton BP, Hamlyn JM. Salt intake and depletion increase circulating levels of endogenous ouabain in normal men. Am J Physiol Regul Integr Comp Physiol. 2006;290:R553–R559. doi: 10.1152/ajpregu.00648.2005. [DOI] [PubMed] [Google Scholar]
- 43.Sanders PW. Vascular consequences of dietary salt intake. Am J Physiol Renal Physiol. 2009;297:F237–F243. doi: 10.1152/ajprenal.00027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen AN, Wallace DP, Blanco G. Ouabain binds with high affinity to the Na, K-ATPase in human polycystic kidney cells and induces extracellular signal-regulated kinase activation and cell proliferation. J Am Soc Nephrol. 2007;18:46–57. doi: 10.1681/ASN.2006010086. [DOI] [PubMed] [Google Scholar]
- 45.Jansson K, Nguyen AN, Magenheimer BS. Endogenous concentrations of ouabain act as a cofactor to stimulate fluid secretion and cyst growth of in vitro ADPKD models via cAMP and EGFR-Src-MEK pathways. Am J Physiol Renal Physiol. 2012;303:F982–F990. doi: 10.1152/ajprenal.00677.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang D, Iversen J, Wilcox CS, et al. Endothelial dysfunction and reduced nitric oxide in resistance arteries in autosomal-dominant polycystic kidney disease. Kidney Int. 2003;64:1381–1388. doi: 10.1046/j.1523-1755.2003.00236.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.