Abstract
Background
3T3-L1 cells have been widely used as a model for adipogenesis. However, despite its popularity, differentiation of this cell line has been reported to be inconsistent with low efficiency.
Objective
To investigate the effect of media height during adipocyte differentiation on lipid accumulation and adipokine secretion in mature adipocytes.
Methods
Three cell lines (3T3-L1, OP9, and ChubS7) were used to test the influence of media volume on adipogenesis. Total lipid content and lipid droplet size and number were quantified. Adipocyte related gene expressions were quantified during the course of differentiation. Secretion of leptin and adiponectin from mature adipocytes were measured using Enzyme-Linked Immunosorbent Assays. The influence of oxygen partial pressure on adipogenesis was investigated using three oxygen percentages: 5%, 21%, and 30%. Insulin sensitivity was measured by insulin inhibition of isoproterenol-induced lipolysis and phosphorylation of Insulin Receptor Substrate-1 (IRS-1).
Results
A lower media height during adipogenesis increased total lipid accumulation, NEFA release and leptin and adiponectin secretion in mature adipocytes. Insulin sensitivity was not affected by media height during differentiation.
Conclusion
Media height during adipogenesis was inversely correlated with lipid content in mature adipocytes. To achieve a high lipid content and greater adipokine secretion, it is best to use a low media volume during differentiation.
Keywords: 3T3-L1, OP9, adipogenesis, lipid, hypoxia, media volume
Introduction
Adipogenesis and adipocyte physiology have been widely studied in vitro using two cell culture models: 3T3-L1 and OP9. 3T3-L1 cells were originally isolated from clones of Swiss 3T3 fibroblasts1. OP9 cells were established from the calvaria of newborn mice genetically deficient in functional macrophage colony-stimulating factor (M-CSF)2.Both cell lines can be induced to differentiate into adipocytes following a method utilizing an adipogenic hormone cocktail (AHC), first described in 1980 by Reed et al.3. The most commonly used protocol involves treating the confluent cell monolayer with AHC containing isobutylmethylxanthine (IBMX), dexamethasone, and insulin for 48 hours, then removing IBMX and dexamethasone while further treating with insulin for 48 hours. Both 3T3-L1 and OP9 cells differentiate into mature adipocytes 7 days post AHC treatment. However, there have been reports on low differentiation yields and variations in differentiation efficiency4–6. ChubS7 is an immortalized human pre-adipocyte cell line.
We have observed inconsistency in the degree of lipid accumulation (Oil Red O staining and visualization of percent of differentiated cells) using this protocol in both 3T3-L1 and OP9 cells, particularly when cells are differentiated in different tissue culture plates and flasks. One potential difference between tissue culture containers is the variation in media height above the cell layer, when media volume is not adjusted accordingly. This could be due to change in oxygen content at the cell layer, since oxygen content decreases rapidly with increased medium height7. Since hypoxia is known to inhibit adipogenesis8, we hypothesized that one of the contributors to poor yield of adipocyte differentiation is the height of the media above the cell layer. The present study was designed to determine the impact of media height on adipocyte differentiation using three adipocyte cell lines: 3T3-L1, OP9, and ChubS7.
Materials and methods
Cell lines and differentiation material
3T3-L1 (murine embryonic fibroblast) and OP9 (murine bone marrow mesenchymal fibroblast) cells were purchased from American Type Culture Collection (ATCC). 3T3-L1 cells were cultured in DMEM high glucose (Invitrogen) supplemented with 10% fetal bovine serum (FBS), sodium pyruvate (1mM), Glutamax (2mM), and gentamicin (10μg/mL). OP9 cells were cultured in MEM-α (Invitrogen) supplemented with 20% FBS, sodium pyruvate (1mM), Glutamax (2mM), and gentamicin (10μg/mL). Both cell lines were differentiated into adipocytes using the same protocol. Cells were seeded on day 0 and grown to confluence in poly-D-Lysine coated wells for 72 hours. On day 3, the media were changed to Diff media (DMEM high glucose with aforementioned supplements with 15% FBS, 20mM HEPES), plus the AHC (0.5mM IBMX (Sigma), 150nM porcine insulin (Sigma), and 250nM dexamethasone (Sigma)). On day 5, the media were refreshed plus insulin only. On day 7, the media were refreshed again. Mature adipocytes were observed on day 10.
ChubS7 (immortalized human pre-adipose cell line) were purchased from Nestec LTD. The cells were cultured in DMEM/F12 (Invitrogen) with 10% FBS, sodium pyruvate, Glutamax, and antibiotics. To differentiate ChubS7 cells into adipocytes, cells were seeded in their culture media in poly-D-Lysine coated wells for 72 hours to be grown to confluence. On day 3, media were changed to serum-free DMEM/F12 with sodium pyruvate, Glutamax, and antibiotics plus 17μM D-pantothenic acid, 15mM HEPES, 33μM biotin, 1nM triiodothyronine, 10μg/mL transferrin, 850nM porcine insulin, 500μg/mL fetuin, 1μM dexamethasone, 15.6μM rosiglitazone, and 0.5μM isobutylmethylxanthine (IBMX). On day 5, media were refreshed to serum-free DMEM/F12 with all of the above supplements except IBMX. Media were refreshed every two to three days from day 5 to day 24. Mature adipocytes were observed on day 24. All three cell lines were maintained in culture in an incubator with 95% air and 5% CO2 at 37°C and 100% humidity.
Cell culture and differentiation systems
When differentiating 3T3-L1 and OP9 cells in a 24-well plate, 50 000 cells were plated per well 72 hours prior to initiation of differentiation. Media volumes used in a 24-well plate were 1.5mL, 650uL, and 325uL. For ChubS7 cells, 65 000 cells were plated per well and the same media volumes were used. When differentiating 3T3-L1 cells in a 6-well plate, 350 000 cells were plated per well 72 hours prior to initiation of differentiation, and the media volumes were 5mL, 3.2mL, and 1.6mL. When using T150 tissue culture flasks, cells were plated at 4 000 000 cells per flask, and 20mL media volume was used for differentiation. For the tilting flask experiment, 4 000 000 cells were allowed to adhere to the horizontally placed flask for 72 hours. On day 0, the flask was propped to be at a 5° angle and 35mL media volume was used. Cells that were not covered by media towards the cap of the flask were scraped off. Differentiating plates and flasks were kept in an incubator with 5% CO2 and either 21% O2 (normoxia), 5% O2 (hypoxia), or 30% O2 (hyperoxia) at 37°C and 100% humidity.
Gene expression analysis
3T3-L1 cells were plated in 6-well plates and differentiated with the three volumes according to the protocol described. Cells were collected on days 0, 1, 3, 4, 7, 11, and 14 and resuspended in RNAProtect (Qiagen), then cells were lysed with QIAzol (Qiagen) and RNA were extracted and purified with RNEasy Mini kits (Qiagen). The quantity and quality of extracted RNA were measured by NanoDrop (Thermo Scientific). Two thousand nanograms of RNA from each condition were reverse transcribed to cDNA with a High Capacity 1st Strand Synthesis kit (Applied Biosystems). The expressions of adiponectin, leptin, and pparγ were quantified with rtPCR using 25ng of cDNA, Power SYBR Green PCR Master Mix (Applied Biosystems), and 200nM primers generated using National Center for Biotechnology Information Primer-BLAST. Murine adiponectin was amplified using primers 5′-GTGCAGGTTGGATGGCAGGCA-3′ (forward) and 5′-CAGTGACGCGGGTCTCCAGC-3′ (reverse). Murine PPARγ was amplified using primers 5′-GCCTTCGCTGATGCACTGCC-3′ (forward) and 5′-CAGCAACCATTGGGTCAGCTCT-3′ (reverse). Murine leptin was amplified using primers 5′-CCCTGTGGAGGTGAGCGGGA-3′ (forward) and 5′-CCAGCCACCACGAGCCTTCG-3′ (reverse). Beta-actin was used as house-keeping gene (5′-TTGCTGACAGGATGCAGAAG-3′ (forward) and 5′-AAGGGTGTAAAACGCAGCTC-3′ (reverse)). Gene expression levels were quantified using the ABI 7900HT Sequence Detection System with the following thermal profile: 10 minutes at 95°C followed by 49 repeats of 95°C for 15 seconds, 60 degrees for 1 minute, and a final dissociation stage of 95°C for 15 seconds, 60°C for 15 seconds, and 95°C for 15 seconds. Transcript levels were normalized to beta-actin.
Cell staining, microscopy and lipid quantification
On day 12 for 3T3-L1 and OP9 and day 26 for ChubS7, adipocytes were stained with Oil Red O and Hematoxylin (Sigma) following manufacturer’s protocol. Upon staining, adipocytes were viewed under a Zeiss Axio Observer microscope and pictures were taken at 200X using AxioVision software. Each volume condition was done in quadruplicate wells, and a blinded observer took pictures of at least five randomly chosen fields per well. ImageJ software (NIH) was used to quantify the number of red pixels in each picture, which correlated to the total lipid content in a given field.
Cytokine production quantification with ELISA
On day 10 for 3T3-L1 and OP9 cells, conditioned media experiments were carried out in 24-well plates with 1mL of media per well. After 48 hours of conditioning, media were collected, passed through a 0.22μM filter (Millipore) and stored in −80°C. Adiponectin and leptin concentrations were measured using ELISA kits (Millipore).
Insulin inhibition of lipolysis
Adipocytes were serum-starved in serum-free DMEM with 0.2% Bovine Serum Albumin (BSA) for 2hrs. Following starvation, cells were washed twice with Krebs Ringer Phosphate (KRP) buffer and incubated for 3 hrs in KRP buffer with 4% Free-Fatty Acid (FFA)-Free BSA alone or containing 1μM Isoproterenol and/or 100nM Insulin. Media FFA content was measured with the NEFA-HR(2) kit (Wako Pure Chemicals Industries, Ltd., Osaka, Japan).
Western blot
3T3-L1 adipocytes were harvested in lysis buffer [50mM Tris HCl at pH 8, 150mM NaCl, 0.1% SDS, 1% Igapal, 0.5% sodium deoxycholate, 100 μg/mL phenylmethylsulfonyl fluoride (PMSF), Phosphatase Inhibitor Cocktail Set II (Calbiochem, Temecula, CA, USA), and Halt Protease Inhibitor Cocktail (Fisher Scientific, Pittsburgh, PA, USA)] and sonicated. Lysates were centrifuged at 15 000 g for 20 min at 4°C and the supernatant was retained. Protein concentration was quantified by bicinchoninic acid assay (Pierce Biotechnology, Rockford, IL, USA). Samples containing 15 μg of protein was run on a 4–12% Bis-Tris gel (Life Technologies) and transferred to a 0.45 μM pore size nitrocellulose membrane (Life Technologies). The membrane was probed with primary antibodies specific for IRS-1 (Cell Signaling, Danvers, MA, USA, #2382), pIRS-1 Y895 (Cell Signaling, #3070) and glyceraldehyde-3-phosphate dehydrogenase (Cell Signaling, #2118), followed by an incubation with HRP-conjugated anti-rabbit secondary antibody (Cell Signaling, #7074). Bands were detected using HyGLO-HRP detection kit (Denville Scientific, South Plainfield, NJ, USA) and developed with HyBLOT-CL Autoradiography Film (Denville Scientific). Densitometric band analysis was performed using Image J (National Institute of Health, Betheseda, MD, USA).
Calculations and statistical analysis
Lipid content quantification was done using ImageJ, by generating a histogram of results of a picture with red pixels only. The summation of pixel numbers of greater than and equal to bin=50 were calculated and defined as the total number of red pixels in a picture. MetaMorph® Microscopy Automation & Image Analysis Software (Sunnyvale, CA) was used to quantify the number of lipid droplets and the size of each droplet. Repeated measures linear regression was utilized to examine the results of all lipid quantification experiments. Analysis of total lipid content in three pre-adipocyte cell lines was done treating media volume as a continuous predictor. Volume and region, in the tilting flask experiment, were treated as a continuous variable, while oxygen level was treated as a categorical variable. The log10 transformation was utilized to lessen the effects of outliers due to the highly skewed distribution of lipid content. Additionally, the analysis is performed on the rank of the observations to confirm the results of the data with extreme outliers. The mean droplet size and the total droplet count of each image were analyzed separately. The log10 transformed qPCR data was analyzed separately for each gene, but presented in raw scale. ELISA data was log10 transformed and analyzed separately for each cell line and cytokine. The media volume was treated as both a continuous and categorical variable. All analyses were performed using Stata (version 11) (StataCorp, TX).
Oxygen partial pressures at different depths in 5%, 21% and 30% oxygen were calculated using the formula below:
where UO2*t is the amount of oxygen consumed per minute by 200,000 3T3-L1 cells, h is the media height above the cell layer, D is the oxygen diffusion constant, α is the solubility coefficient of oxygen, and A is the surface area at the air-media interface9,10.
For all statistical analyses, p<0.05 was defined as significant. All bar graphs were plotted with mean±SEM, unless otherwise indicated.
Results
Lipid accumulation in mature adipocytes is directly proportional to media height
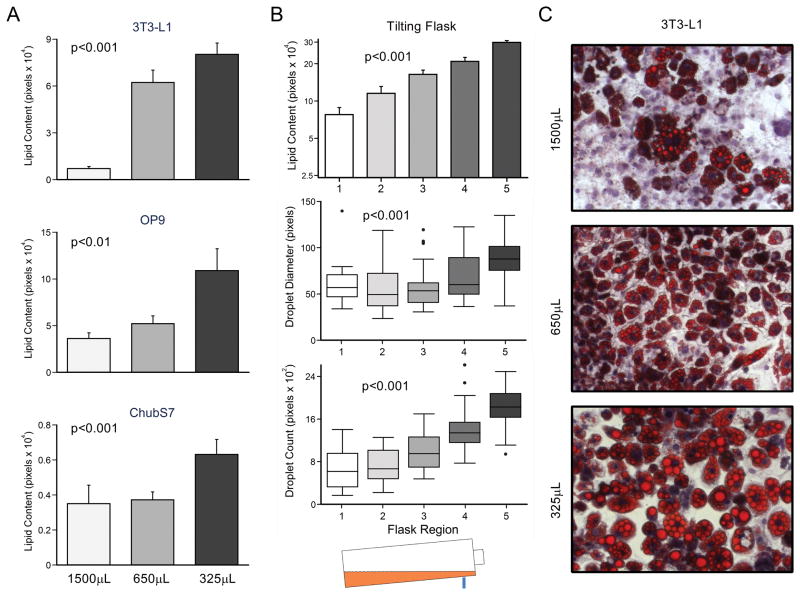
To study the direct effect of media height on adipogenesis, 3T3-L1, OP9, and ChubS7 cells were differentiated into mature adipocytes in 24-well plates using three media volumes: 1500, 650, and 325μL, corresponding to media heights of 0.75, 0.325, and 0.1625cm. As shown in figure 1A, there was a significant increase in total lipid content associated with decreased media volume (p<0.01). These three cell lines differed intrinsically in their differentiation capacity, where 3T3-L1 cells were the most sensitive to volume changes, with the greatest relative increase in lipid content with a decrease in media volume.
Figure 1.
Lipid accumulation in mature adipocytes with respect to media height. (A) Lipid content in three pre-adipocyte cell lines after differentiation in 24-well plates using three media volumes. (B) 3T3-L1 cells differentiated in a tilting flask accumulate lipids differently at different depths. (C) Representative pictures of mature 3T3-L1 adipocytes differentiated using three volumes. Cells stained with oil red O (lipid droplet) and hematoxylin (nucleus), shown at 200X magnification.
A reason why a lower volume leads to greater lipid accumulation could be the concentration of secreted molecules participating in autocrine signaling. We developed a tilting flask model in which 3T3L1 cells were differentiated in the same media, though at different depths. The flask was arbitrarily divided into five equal regions, where region 1 had the greatest height of media (~12 mm) and region 5 had the least (~2 mm). As in the 24 well plates, media height was inversely associated with lipid accumulation (p<0.001) (Figure 1B). This effect was due to both an increase in the mean droplet size (p<0.001) and total droplet number (p<0.001) with decreasing media height (Figure 1B and C).
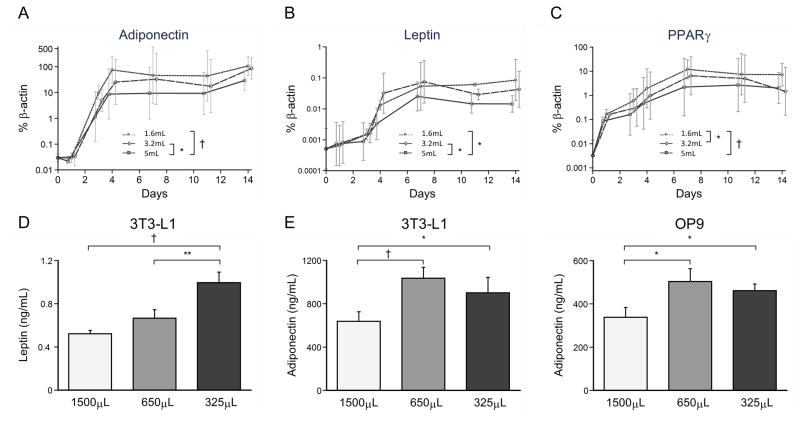
Lower media height increases adipocyte related gene expressions and leptin secretion
To investigate the effect of media volume on adipocyte differentiation on a molecular level, we measured adiponectin, leptin, and PPARγ expression by qPCR in 3T3-L1 cells differentiated with 5, 3.2, and 1.6mL in a 6-well plate. These volumes yielded media heights comparable to those we used in the 24-well plates. Adiponectin and leptin expression were not different between the 1.6 and 3.2mL media volumes, but were both lowest in the highest media volume (Figures 2A & B). PPARγ expression was highest in the lowest media volume compared to other two volumes (Figure 2C).
Figure 2.
Expression of adipocyte-specific markers in relation to media heights during differentiation. (A – C) Gene expression of adipocyte-specific markers over time during 3T3-L1 differentiation process. (D) Leptin secretion over 48 hours from 3T3-L1 cells differentiated in different volumes. (E) Adiponectin secretion from both 3T3-L1 and OP9 adipocytes differentiated in different volumes (1500, 650, and 325μL). *p<0.05; **p<0.01; †p<0.001
Similar to gene expression patterns, both adiponectin and leptin secretion increased with decreasing volume of media during differentiation (Figure 2D and E). Interestingly, there were no detectable amounts of leptin in OP9 conditioned media, though it is possible that levels were below the limit of detection for our assay (0.2ng/mL; data not shown).
Hypoxia inhibits adipogenesis in 3T3-L1 cells
To investigate whether differences in adipogenesis were caused by differing oxygen concentrations at the cell layers, we differentiated 3T3-L1 preadipocytes in 5%, 21%, and 30% O2. Two differentiation volumes were used in each oxygen percentage in 24-well plates: 1500μL and 325μL. The partial pressures of oxygen at the cell layers were calculated for each condition (see formula in methods, Table 1). Three sets of hypoxia experiments and two sets of hyperoxia experiments were performed, each including a simultaneous normoxia control.
Table 1.
PO2 (mmHg) at the cell layer with different ambient oxygen percentages and media volumes in a 24-well plate.
| 5% | 21% | 30% | |
|---|---|---|---|
| 1500μL | 0 | 115.41 | 183.81 |
| 650μL | 18.85 | 140.45 | 208.85 |
| 325μL | 28.57 | 150.02 | 218.57 |
In all three oxygen percentages, the lower media volume was associated with a significant increase in total lipid content (p<0.001), lipid droplet size (p<0.001), and droplet number (p<0.001) (Figures 3A–C). Hypoxia significantly inhibited lipid accumulation in mature 3T3-L1 cells (p<0.001). Lipid droplet number (grouping both volumes of differentiation) increased significantly with hyperoxia (p<0.05, Figure 3C), though there was no significant effect on overall lipid content (p=0.16, Figure 3A). Interestingly, in the 325μL condition, lipid droplet number decreased while the droplet diameter increased in 30% oxygen. As a result, the difference in total lipid content between 21% and 30% were not statistically significant.
Figure 3.
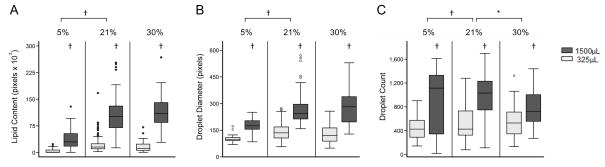
Lipid accumulation in 3T3-L1 cells differentiated under different oxygen contents. (A) Lipid content, (B) droplet diameter, and (C) droplet count in 3T3-L1 adipocytes differentiation in two different media volumes (1500 and 325μL) and three different oxygen contents (5%, 21%, and 30%). *p<0.05; †p<0.001
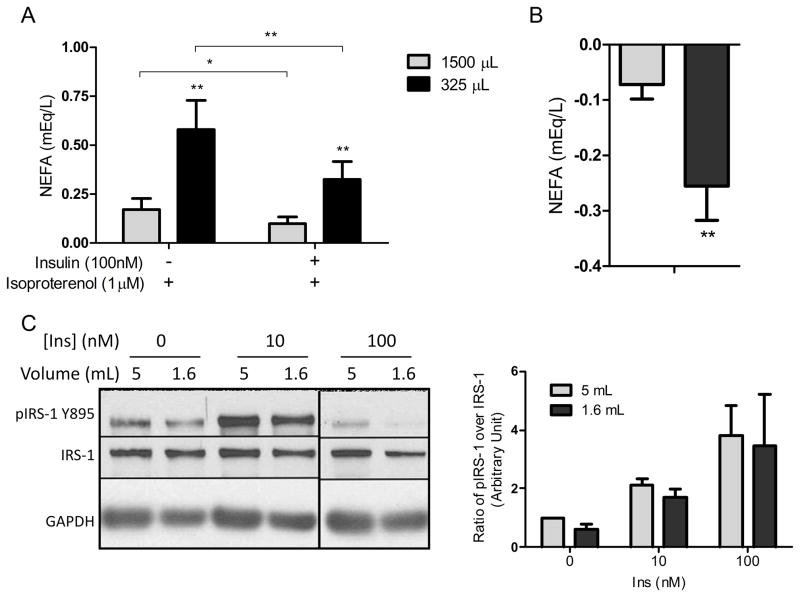
Insulin sensitivity is similar in 3T3-L1 adipocytes differentiated with different volumes
To investigate insulin sensitivity in 3T3-L1 adipocytes caused by a difference in differentiation volume, we performed lipolysis assays and Western blotting of IRS-1 and phosphoIRS-1. Non-esterified fatty acid (NEFA) release in media was significantly greater from adipocytes differentiated with the lower volume following treatment with 1μM isoproterenol (Figure 4A). Upon addition of insulin, NEFA release was significantly suppressed, by just under 50%, from both conditions. However, the absolute amount of suppression was approximately 3 times greater in adipocytes differentiated in 325 μL (Figure 4B). Interestingly, both basal and stimulated phosphorylation of IRS-1 Y895 was lower in adipocytes differentiated in the lower volume. However, insulin stimulation of pIRS-1 measured as fold-change over basal phosphorylation was similar between volumes (Figure 4C).
Figure 4.
Insulin sensitivity in 3T3-L1 cells differentiated under different volumes. Experiments in panels A and B were conducted in adipocytes differentiated in 24-well plates with either 1500μL or 325μL. In panel C, adipocytes were differentiated in 6-well plates with either 5mL or 1.6mL. (A) Adipocyte NEFA secretion plus 1μM isoproterenol with and without insulin (100nM). (B) Suppression of NEFA release by 100nM insulin in the two volumes of differentiation. (C) Representative Western blot of phosphoIRS-1 Y895, total IRS-1 and GAPDH of adipocytes stimulated with different concentrations of insulin, and densitometric quantification of n=3 blots. *p<0.05; **p<0.01
Discussion
Since the establishment of 3T3-L1 as a model of adipocytes in vitro, protocols have been established to differentiate 3T3-L1 cells into adipocytes. The most widely used protocols now are modified versions of the differentiation cocktail method first described by Reed et al. in 1980, in which they described using 3.5mL medium in a 6-cm dish11, which we calculate would yield a media height of 0.124cm. However, in an earlier publication in 1976, the same group described using 16mL medium in a 10-cm dish to differentiate the 3T3-L1 cells12, which is 0.204cm of media. Articles since then utilizing similar protocols to induce adipogenesis have reported results of differentiation to be inconsistent4. We have also noticed inconsistency in the degree of differentiation (Oil Red O staining and visualization of percent of differentiated cells) in 3T3-L1 cells, which prompted us to investigate the possible causes of this problem.
In this study, we have shown that adipocyte differentiation is very sensitive to media volume. Lower media volume during adipogenesis results in 1) a higher amount of lipid accumulation, due to increase in both lipid droplet size and number; 2) up-regulation of genes involved in adipogenesis; and 3) higher adiponectin secretion in mature 3T3-L1 and OP9 adipocytes, and 4) higher leptin secretion in mature 3T3-L1 adipocytes. We further show that adipocyte lipid accumulation is sensitive to oxygen, where hypoxia inhibits lipid accumulation. Interestingly, we have also noticed using a low media volume during differentiation, 3T3-L1 cells were able to retain their differentiation efficiency with increased passage number (data not shown). Our data clearly demonstrated the importance of keeping the media height consistent during differentiation, especially when using cell culture dishes and plates of various surface areas.
The tilting flask experiment showed a clear linear correlation between total lipid accumulation and media height above the cell layer in mature adipocytes. Since the cells in the flask were exposed to the same media, the effect is not likely to be caused by differences in concentrations of secreted molecules by the differentiating cells. Since media height is directly correlated with the partial pressure of oxygen at the cell layer, we investigated into the effects of oxygen on adipogenesis.
Hypoxia is known to inhibit certain types of differentiation in stem cells13–15. Our findings that higher media volume and lower oxygen percentage inhibit 3T3-L1 adipogenesis may be related to this. However, it is interesting that the differentiation of mesenchymal stem cells and preadipocytes into adipocytes often occurs in relatively hypoxic environments, such as the bone marrow16 and adipose tissue17, which could have oxygen level of 15.2mmHg (2%) in obese mice18. Further investigation is needed to determine the molecular mechanism linking oxygen tension and lipid accumulation.
Although adipocytes differentiated in a lower volume exhibited amplified lipid accumulation, increased adipogenic gene expression and greater basal lipolysis, these effects did not coincide with altered IRS-1 phosphorylation at tyrosine 895. Because phosphorylation of IRS-1 at various serine and tyrosine residues determines its activity, further experiments are needed to confirm the effect of differentiation volume on IRS-1 signaling. In any case, researchers measuring insulin signaling or sensitivity in adipocytes should pay close attention to media volume during differentiation.
In conclusion, our study demonstrated that a small change in media volume during adipogenesis could lead to dramatic changes in the lipid content and differentiation of mature adipocytes. A lower media volume (media height of 0.16cm) leads to more complete adipogenesis in 3T3-L1, OP9, and ChubS7 pre-adipocyte cell lines. This finding may be valuable to researchers using pre-adipocyte cell lines for obtaining better, more consistent results in adipogenesis.
Acknowledgments
Funding: The present work was supported by the National Cancer Institute (CA139060). XS was supported by a pre-doctoral award from The Saban Research Institute.
This work was supported by an NCI RO1 Award and funds from The Saban Research Institute. We would like to thank Dr. Esteban Fernandez at the Imaging Core at CHLA for helping with microscopy and image analyses, and Dr. Richard Sposto for assistance with statistical analysis.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Green H, Kehinde O. Sublines of mouse 3T3 cells that accumulate lipid. Cell. 1974;1:113–116. [Google Scholar]
- 2.Nakano T, Kodama H, Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 1994;265:1098–1101. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- 3.Reed BC, Moss J, Fishman PH, Lane MD. Loss of choleragen receptors and ganglioside upon differentiation of 3T3-L1 preadipocytes. J Biol Chem. 1980;255:1711–1715. [PubMed] [Google Scholar]
- 4.Mehra A, Macdonald I, Pillay TS. Variability in 3T3-L1 adipocyte differentiation depending on cell culture dish. Analytical Biochemistry. 2007;362:281–283. doi: 10.1016/j.ab.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Wolins NE, Quaynor BK, Skinner JR, Tzekov A, Park C, Choi K, et al. OP9 mouse stromal cells rapidly differentiate into adipocytes: characterization of a useful new model of adipogenesis. J Lipid Res. 2006;47:450–460. doi: 10.1194/jlr.D500037-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Zebisch K, Voigt V, Wabitsch M, Brandsch M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Analytical Biochemistry. 2012;425:88–90. doi: 10.1016/j.ab.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Pettersen EO, Larsen LH, Ramsing NB, Ebbesen P. Pericellular oxygen depletion during ordinary tissue culturing, measured with oxygen microsensors. Cell Proliferation. 2005;38:257–267. doi: 10.1111/j.1365-2184.2005.00345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yun Z, Maecker HL, Johnson RS, Giaccia AJ. Inhibition of PPARγ2 Gene Expression by the HIF-1-Regulated Gene DEC1/Stra13: A Mechanism for Regulation of Adipogenesis by Hypoxia. Developmental Cell. 2002;2:331–341. doi: 10.1016/s1534-5807(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 9.Metzen E, Wolff M, Fandrey J, Jelkmann W. Pericellular PO2 and O2 consumption in monolayer cell cultures. Respiration Physiology. 1995;100:101–106. doi: 10.1016/0034-5687(94)00125-j. [DOI] [PubMed] [Google Scholar]
- 10.Heimburg D, von Hemmrich K, Zachariah S, Staiger H, Pallua N. Oxygen consumption in undifferentiated versus differentiated adipogenic mesenchymal precursor cells. Respiratory Physiology & Neurobiology. 2005;146:107–116. doi: 10.1016/j.resp.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Reed BC, Lane MD. Insulin receptor synthesis and turnover in differentiating 3T3-L1 preadipocytes. Proc Natl Acad Sci U S A. 1980;77:285–289. doi: 10.1073/pnas.77.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackall JC, Student AK, Polakis SE, Lane MD. Induction of lipogenesis during differentiation in a ‘preadipocyte’ cell line. J Biol Chem. 1976;251:6462–6464. [PubMed] [Google Scholar]
- 13.Lee JH, Kemp DM. Human adipose-derived stem cells display myogenic potential and perturbed function in hypoxic conditions. Biochemical and Biophysical Research Communications. 2006;341:882–888. doi: 10.1016/j.bbrc.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 14.Basciano L, Nemos C, Foliquet B, de Isla N, de Carvalho M, Tran N, et al. Long term culture of mesenchymal stem cells in hypoxia promotes a genetic program maintaining their undifferentiated and multipotent status. BMC Cell Biol. 2011;12:12. doi: 10.1186/1471-2121-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holzwarth C, Vaegler M, Gieseke F, Pfister SM, Handgretinger R, Kerst G, et al. Low physiologic oxygen tensions reduce proliferation and differentiation of human multipotent mesenchymal stromal cells. BMC Cell Biol. 2010;11:11. doi: 10.1186/1471-2121-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fink T, Abildtrup L, Fogd K, Abdallah BM, Kassem M, Ebbesen P, et al. Induction of Adipocyte-Like Phenotype in Human Mesenchymal Stem Cells by Hypoxia. STEM CELLS. 2004;22:1346–1355. doi: 10.1634/stemcells.2004-0038. [DOI] [PubMed] [Google Scholar]
- 17.Trayhurn P, Wang B, Wood IS. Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? British Journal of Nutrition. 2008;100:227–235. doi: 10.1017/S0007114508971282. [DOI] [PubMed] [Google Scholar]
- 18.Ye J, Gao Z, Yin J, He Q. Hypoxia Is a Potential Risk Factor for Chronic Inflammation and Adiponectin Reduction in Adipose Tissue of Ob/Ob and Dietary Obese Mice. Am J Physiol Endocrinol Metab. 2007;293:E1118–E1128. doi: 10.1152/ajpendo.00435.2007. [DOI] [PubMed] [Google Scholar]