Abstract
Duchenne muscular dystrophy (DMD) is a progressive, lethal neuromuscular disorder caused by the absence of dystrophin protein due to mutations of the dystrophin gene. Drisapersen is a 2′-O-methyl-phosphorothioate oligonucleotide designed to skip exon 51 in dystrophin pre-mRNA to restore the reading frame of the mRNA. This study assessed safety, tolerability, and pharmacokinetics of drisapersen after a single subcutaneous administration in non-ambulatory subjects. Eligible subjects were non-ambulant boys aged ≥9 years, in wheelchairs for ≥1 to ≤4 years, with a diagnosis of DMD resulting from a mutation correctable by drisapersen treatment. Four dose cohorts were planned (3, 6, 9 and 12 mg/kg), but study objectives were met with the 9 mg/kg dose. Less than proportional increase in exposure was demonstrated over the 3–9 mg/kg dose range, though post hoc analysis showed dose proportionality was more feasible over the 3–6 mg/kg range. Single doses of drisapersen at 3 and 6 mg/kg did not result in significant safety or tolerability concerns; however, at the 9 mg/kg dose, pyrexia and transient elevations in inflammatory parameters were seen. The maximum tolerated dose of 6 mg/kg drisapersen was identified for further characterization in multiple dose studies in the non-ambulant DMD population.
Keywords: Drisapersen; Duchenne muscular dystrophy; DMD; Dystrophin; Exon 51; Non-ambulant; Oligonucleotide, Pharmacokinetics; Safety
1. Introduction
Duchenne muscular dystrophy (DMD) is an inheritable, X chromosome-linked lethal childhood disease with an incidence of approximately 1 in 3500 newborn boys [1]. DMD is caused by mutations in the gene coding for the protein dystrophin resulting in little or no dystrophin being produced. Dystrophin is essential for the integrityand functioning of muscle fibers [2,3]. Absence of dystrophin leads to progressive muscle weakness, with DMD patients typically wheelchair-bound before the age of 12. As the disease progresses, respiratory and cardiac muscles are affected, orthopedic complications occur, and, in the absence of intervention, death occurs at approximately age 19 [4]. Current supportive treatments include physiotherapy, mechanical supports, orthopedic surgery, assisted ventilation, disease management (e.g., for respiratory infections, cardiomyopathy) and glucocorticosteroids. This multidisciplinary approach to treatment has prolonged life expectancy, with some patients surviving into the fourth decade and beyond [4,5].
In DMD, genetic mutations, such as a deletion of one or more exons, result in an out-of-frame transcription product and subsequent disrupted dystrophin protein synthesis [6]. The aim of oligonucleotide-based therapy is to manipulate the post-transcriptional splicing process of the pre-mRNA in such a way that the reading frame of the resulting mRNA is restored. This would result in the production of a shortened yet partially functional dystrophin protein, analogous to that seen in the less severe Becker muscular dystrophy (BMD). Such treatment could potentially delay disease progression and improve function in the remaining muscle [7].
Drisapersen (formerly GSK2402968 and PRO051) is a 20mer chemically-modified (2′-O-methyl-phosphorothioate) oligonucleotide with a sequence optimized to skip exon 51 in the human dystrophin pre-mRNA. Mutations thought to be correctable by skipping exon 51 account for approximately 13% of all DMD patients [8]. Drisapersen has been shown to induce novel dystrophin production after both local injection [9] and systemic administration [10], and weekly subcutaneous drisapersen treatment (6 mg/kg/week) has shown encouraging results on functional endpoints, including the 6 Minute Walk Distance (6MWD) test [10].
The efficacy and safety of drisapersen is currently being studied in ambulant boys with DMD in several clinical trials worldwide [11–15]; however, there has been limited experience in non-ambulant boys with DMD. Since muscle potentially accounts for a large proportion of uptake of drisapersen, there is the potential that the pharmacokinetics of drisapersen may be different in the non-ambulant DMD population due to reduced lean muscle mass relative to ambulant boys. The purpose of the current study (DMD114118; ClinicalTrials.gov Identifier: NCT01128855) was to assess the safety, tolerability, and pharmacokinetics of drisapersen after a single subcutaneous administration at different dose levels in non-ambulant subjects with DMD.
2. Patients and methods
Eligible subjects were non-ambulant boys aged ≥9 years, in wheelchair for at least one year but no more than 4 years, and with a diagnosis of DMD resulting from a mutation correctable by treatment with drisapersen. Patients with additional mutations not correctable by drisapersen, with a history of renal or hepatic disease, or with symptomatic cardiomyopathy were excluded. Treatment with glucocorticosteroids was allowed, but these had to be started at least 6 months and have been dosed with a stable regimen for at least 3 months before the anticipated first administration of study medication. Use of anticoagulants, antithrombotics or antiplatelet agents, previous treatment with investigational drugs within 6 months of the first administration of study medication, and use of idebenone or other forms of Coenzyme Q10 within 1 month of study medication was prohibited.
Following a screening period of up to 2 weeks, subjects were randomized to receive a single subcutaneous dose of drisapersen or dose-matched placebo. Subjects were assigned to study treatment in accordance with a central randomization schedule. In each cohort, subjects were randomized to a single dose level of drisapersen or dose-matched placebo, in a ratio of 3:1. Drisapersen was supplied as a solution for subcutaneous injection, 200 mg/mL. The precise amount administered was titrated against body weight in accordance with the protocol instructions. The study was designed to enroll up to 32 subjects within 4 cohorts, with the final 2 cohorts being divided into 2 sub-groups (1, 2, 3a/3b, 4a/ 4b). Each planned cohort was comprised of 8 subjects (6 active and 2 placebo) not to exceed the following levels:
Cohort 1: 3 mg/kg drisapersen or placebo;
Cohort 2: 6 mg/kg drisapersen or placebo;
Cohort 3: 9 mg/kg drisapersen or placebo;
Cohort 4: 12 mg/kg drisapersen or placebo
Cohorts 3 and 4 were divided into sub-groups 3a/3b and 4a/4b, with 4 subjects in each sub-group. All subjects in Cohort 3 (3a and 3b) were planned to receive up to 9 mg/kg, and all subjects in Cohort 4 (4a and 4b) were planned to receive up to 12 mg/kg. There was planned to be at least 14 days between treating the last subject of sub-group a and treating the first subject in sub-group b, with safety parameters reviewed prior to moving to subgroup b.
The study allowed dose levels in subsequent cohorts to be modified downwards or repeated at the same dose of the previous cohort following review of data by a Safety Review Team. Subjects had a 1 month active study period and a 5 month post-dose follow-up period.
The primary pharmacokinetic (PK) endpoints included: area under the plasma concentration–time curve (AUC) from time 0 to 24 h post-dose (AUC0–24h), and time 0 to 7 days post-dose (AUC0–7d); the observed maximum plasma concentration post-dose (Cmax); and the time of maximum plasma concentration post-dose (Tmax). The primary safety and tolerability endpoints included: adverse event (AE) monitoring; 12-lead ECG; vital signs; laboratory safety tests (biochemistry, hematology, urinalysis and coagulation parameters); and local tolerability. All safety laboratory values were assayed at a single central laboratory.
3. Results
A total of 21 subjects were screened, of whom 20 were randomized into the study at 2 study sites, one in the US (n = 16 subjects), and one in France (n = 4 subjects). All randomized subjects completed the study. It was concluded that the study objectives had been met following analysis of cohort 3a and a decision was taken after completion of the first 9 mg/kg cohort not to proceed to the next 9 mg/kg cohort (see discussion below). Therefore, only the results for these completed cohorts are reported.
Table 1 shows the baseline characteristics of the study population, including concomitant medications. No major differences were observed across treatment groups in demographic characteristics with two exceptions: the 6 mg/kg drisapersen treatment group on average weighed more than the other 3 treatment groups and the 9 mg/kg drisapersen treatment group were younger on average than the other 3 treatment groups. The duration of time since first DMD symptoms, time since first diagnosis and time since loss of ambulation were similar across groups with the exception of the 9 mg/kg drisapersen treated group which had a shorter duration for all of these characteristics. The majority of subjects were treated with glucocorticosteroids.
Table 1.
Subject baseline characteristics, including concomitant medications.
| Number of Subjects | Placebo | 3mg/kg Drisapersen | 6mg/kg Drisapersen | 9mg/kg Drisapersen |
|---|---|---|---|---|
| Number of subjects planned, N: | 5 | 6 | 6 | 3 |
| Number of subjects randomized, N: | 5 | 6 | 6 | 3 |
| Number of subjects withdrawn, n (%): | 0 | 0 | 0 | 0 |
| Age in years | ||||
| Mean (SD) | 12.2 (0.84) | 13.8 (1.72) | 13.3 (1.21) | 10.3 (1.53) |
| Median | 12.0 | 13.0 | 13.5 | 10.0 |
| Min, Max | 11, 13 | 12, 16 | 12, 15 | 9, 12 |
| Weight (kg) | ||||
| Mean (SD) | 49.10 (13.99) | 50.45 (13.52) | 59.08 (12.09) | 49.73 (8.62) |
| Median | 51.20 | 49.85 | 57.50 | 50.40 |
| Min, Max | 28.6, 64.4 | 31.9, 73.2 | 46.0, 75.1 | 40.8, 58.0 |
| Height (cm) | ||||
| Mean (SD) | 147.6 (23.88) | 149.5 (10.15) | 143.5 (7.26) | 147.3 (7.51) |
| Median | 135.0 | 149.0 | 143.5 | 147.0 |
| Min, Max | 122, 176 | 137, 165 | 132, 152 | 140, 155 |
| Time since first symptoms (months) | ||||
| Mean (SD) | 123.4 (15.09) | 132.4 (26.45) | 114.5 (21.64) | 96.2 (7.62) |
| Median | 124.4 | 131.4 | 115.3 | 92.7 |
| Time since diagnosis (months) | ||||
| Mean (SD) | 110.2 (16.56) | 116.0 (15.91) | 107.7 (24.92) | 87.9 (4.39) |
| Median | 111.7 | 120.1 | 114.8 | 85.8 |
| Time since loss of ambulation (months) | ||||
| Mean (SD) | 25.3 (11.22) | 36.5 (10.66) | 29.5 (12.18) | 17.3 (7.47) |
| Median | 21.8 | 38.6 | 30.8 | 13.6 |
| Any medication, n (%) | ||||
| Glucocorticosteroids | 3 (60) | 3 (50) | 5 (83) | 2 (67) |
| ACE Inhibitors | 4 (80) | 3 (50) | 3 (50) | 2 (67) |
| Beta blockers | 1 (20) | 2 (33) | 0 | 0 |
3.1. Pharmacokinetic results
The pharmacokinetics of single subcutaneous injections of drisapersen in non-ambulant subjects with DMD were assessed in this study at doses of 3, 6 and 9 mg/kg (Table 2). When analyzing all data from all doses for dose proportionality, dose proportionality was not demonstrated over the range of 3–9 mg/kg, although a post hoc analysis showed that the proportionality is more feasible over the range of 3–6 mg/kg than the 3–9 mg/kg range (Table 3).
Table 2.
Summary of selected drisapersen pharmacokinetic parameters.
| Parameter | n | 3mg/kg Drisapersen | n | 6mg/kg Drisapersen | n | 9mg/kg Drisapersen |
|---|---|---|---|---|---|---|
| Cmax (ng/mL)a | 6 | 4990 (36.0) | 6 | 8140 (25.9) | 3 | 8940 (20.8) |
| AUC(0–24)(ng.hr/mL)a | 6 | 44,600 (18.5) | 6 | 87,800 (33.4) | 3 | 97,800 (10.7) |
| AUC(0–168) (ng.hr/mL)a | 4 | 45,500 (16.0) | 3 | 87,300 (18.7) | 2 | 112000 (0.6) |
| Tmax (hr)b | 6 | 3.01 (2.97–5.78) | 6 | 3.00 (2.98–6.00) | 3 | 6.00 (3.00–6.00) |
Geometric mean (CV%).
Median (range).
Table 3.
Summary of results of statistical analysis to estimate dose proportionality after single doses of drisapersen.
| Parameter | n | Adjusted mean slope | Standard error | 90% Confidence interval for slopea, b |
|---|---|---|---|---|
| Drisapersen 3–9 mg/kg | ||||
| Cmax | 15 | 0.581 | 0.1712 | (0.278–0.884) |
| AUC(0–24) | 15 | 0.791 | 0.1505 | (0.524–1.057) |
| AUC(0–168) | 9 | 0.848 | 0.1107 | (0.638–1.058) |
| Drisapersen 3–6 mg/kg only (Post hoc analysis) | ||||
| Cmax | 12 | 0.705 | 0.2547 | (0.243–1.167) |
| AUC(0–24) | 12 | 0.977 | 0.2201 | (0.578–1.376) |
| AUC(0–168) | 7 | 0.939 | 0.1871 | (0.562–1.316) |
For single doses of drisapersen 3–9 mg/kg, dose proportional range was 0.797–1.203 for all parameters.
For single doses of drisapersen 3–6 mg/kg only, dose proportional range was 0.678–1.322 for all parameters.
Since antisense oligonucleotides like drisapersen are known to distribute into adipose tissue, and non-ambulant boys with DMD generally have a high percentage body fat as compared to healthy or ambulant boys with DMD, the relation between body fat and exposure was explored. Plotting Cmaxand AUC(0–24)versus the percentage body fat showed no relationship between these PK parameters over the range of body fat percentages tested (Fig. 1).
Fig. 1.
Pharmacokinetic parameters versus body fat.
3.2. Safety results
No deaths, serious adverse events (SAEs), or withdrawals were reported on the study. Adverse events (AEs) for this study were classified into treatment emergent AEs and follow-up AEs. Treatment emergent AEs were defined as AEs occurring from the start of study treatment up to and including Day 28. Follow-up AEs were defined as those AEs beginning from Day 29 up to and including the final follow-up contact (5 months after administration of study treatment). AEs of special interest were defined as injection site reactions, hepatic toxicity, renal toxicity, thrombocytopenia, inflammatory reactions, and coagulation abnormalities. These were defined based on hazards identified in clinical and preclinical studies of drisapersen. The summary of all AEs is shown in Table 4. All subjects receiving drisapersen experienced at least 1 treatment emergent AE, the majority of which were considered drug related, compared to 2 subjects (40%) receiving placebo, 1 of whom reported an AE considered to be drug related. The incidence of follow-up phase AEs was variable across the treatment groups and was less frequent than in the treatment phase. The only AE occurring in ≥2 subjects in any group that was not an AE of special interest was headache, which occurred in 1 subject in each of the placebo, 3 mg/kg and 6 mg/kg active groups and in 2 subjects in the 9 mg/kg active group. A summary of all AEs of special interest is shown in Table 5. A higher percentage of injection site reactions were reported in the drisapersen treated groups compared to placebo, with increasing proportions with increasing dose. Renal AEs (α1-microglobulin increased) were seen only in the 9 mg/kg treated group. Inflammatory AEs were seen in both the 6 mg/kg (1 subject, 17%) and 9 mg/kg treatment groups (3 subjects, 100%). No hepatic toxicity, thrombocytopenia, or coagulation abnormalities were reported.
Table 4.
Summary of all adverse events by treatment.
| Number (%) of subjects | |||||
|---|---|---|---|---|---|
| Placebo (N = 5) |
3 mg/kg Drisapersen (N = 6) |
6 mg/kg Drisapersen (N = 6) |
9 mg/kg Drisapersen (N = 3) |
Total (N = 20) |
|
| Any AE by phase | |||||
| Treatment emergent | 2 (40) | 6 (100) | 6 (100) | 3 (100) | 17 (85) |
| Follow-up | 2 (40) | 3 (50) | 4 (67) | 0 | 9 (45) |
| Drug related AEs (per investigator judgment) | 1 (20) | 5 (83) | 5 (83) | 3 (100) | 14 (70) |
| AEs of special interest | |||||
| Injection site reaction | 1 (20) | 4 (67) | 5 (83) | 3 (100) | 13 (65) |
| Renal toxicity | 0 | 0 | 0 | 1 (33) | 1 (5) |
| Inflammation | 0 | 0 | 1 (17) | 3 (100) | 4 (20) |
Table 5.
Summary of adverse events of special interest by treatment.
| Special interest category preferred term | Placebo (N = 5) | 3 mg/kg Drisapersen (N = 6) | 6 mg/kg Drisapersen (N = 6) | 9 mg/kg Drisapersen (N = 3) |
|---|---|---|---|---|
| Injection site reaction, any event, n (%) | 1 (20) | 4 (67) | 5 (83) | 3 (100) |
| Injection site discoloration | 1 (20) | 3 (50) | 5 (83) | 3 (100) |
| Injection site induration | 1 (20) | 3 (50) | 4 (67) | 2 (67) |
| Injection site erythema | 0 | 3 (50) | 1 (17) | 2 (67) |
| Injection site hematoma | 0 | 1 (17) | 2 (33) | 1 (33) |
| Injection site inflammation | 0 | 0 | 1 (17) | 3 (100) |
| Injection site edema | 0 | 0 | 0 | 2 (67) |
| Injection site pruritus | 0 | 2 (33) | 0 | 0 |
| Injection site pain | 0 | 1 (17) | 0 | 0 |
| Injection site warmth | 0 | 0 | 1 (17) | 0 |
| Renal Toxicity, any event, n (%) | 0 | 0 | 0 | 1 (33) |
| Alpha 1 microglobulin increaseda | 0 | 0 | 0 | 1 (33) |
| Inflammation, any event, n (%) | 0 | 0 | 1 (17) | 3 (100) |
| Pyrexia | 0 | 0 | 1 (17) | 3 (100) |
| C reactive protein increasedb | 0 | 0 | 0 | 1 (33) |
| Immunology test abnormalc | 0 | 0 | 0 | 1 (33) |
Laboratory reference range: less than 12.0 mg/L.
Laboratory reference range: 0–3.0 mg/L.
Increase in complement split products.
All study subjects had elevated ALT and AST values at baseline, and there were no effects seen during the treatment period. Boys with DMD typically have elevations in ALT and AST due to leakage from muscle (versus hepatic source) due to disease [4]. Direct bilirubin, total bilirubin, gamma glutamyl transferase (GGT), and glutamate dehydrogenase (GLDH) values in each drisapersen treated cohort remained within the established reference range throughout the study. No urinary protein increases were seen post dose in the 3 and 6 mg/kg groups, but the placebo and, of greater magnitude, the 9 mg/kg treated groups had increases in random urine protein values at Day 8 compared with pre-dose values (placebo: Day 1, 169.8 ± 89.4 mg/L; Day 8, 185.4 ± 123.6 mg/L; 9 mg/kg: Day 1, 86.5 ± 51.6 mg/L; Day 8, 205.0 ± 99.6 mg/L). These values declined by the follow-up visit. Serum cystatin C values remained within normal limits. Elevations in mean urine α1-microglobulin values were seen in all drisapersen treated groups over the course of the study up to Day 8. These changes appeared dose dependent and reversible (Table 6). A slight decrease in mean platelet measurements was observed immediately following dosing (Day 2) in all groups; however all values stayed within the normal reference range and returned to pre-dose levels by Day 8. All aPTT values remained within the established reference range for all treatment groups except the 9 mg/kg drisapersen treated group, which had 3 h post-dose values higher than the upper limit of normal (35.0 ± 2.8 s; reference range: 22–34 s). All of the aPTT values for the 9 mg/kg group returned to within normal limits by 6 h post-dose. Other hematology and biochemistry values were unremarkable.
Table 6.
Summary of mean and median urine α-1-microglobulin values over time.
| Lab Test | Treatment | N | Visit | n | Mean (SD) | Median (Range) |
|---|---|---|---|---|---|---|
| Urine alpha 1 microglobulin (mg/L) | Placebo | 5 | Screening | 3 | 5.70 (0.000) | 5.70 (5.7–5.7) |
| Day 1 | 5 | 6.14 (0.984) | 5.70 (5.7–7.9) | |||
| Day 8 | 5 | 5.70 (0) | 5.70 (5.7–5.7) | |||
| Follow-up | 5 | 7.76 (3.871) | 5.70 (5.7–14.6) | |||
| 3mg/kg Drisapersen | 6 | Screening | 3 | 5.70 (0.000) | 5.70 (5.7–5.7) | |
| Day 1 | 6 | 5.70 (0.000) | 5.70 (5.7–5.7) | |||
| Day 8 | 6 | 7.27 (2.563) | 5.70 (5.7–11.7) | |||
| Follow-up | 6 | 5.70 (0) | 5.70 (5.7–5.7) | |||
| 6mg/kg Drisapersen | 6 | Screening | 0 | No data | No data | |
| Day 1 | 6 | 5.78 (0.204) | 5.70 (5.7–6.2) | |||
| Day 8 | 6 | 16.83 (11.767) | 14.05 (5.7–31.0) | |||
| Follow-up | 6 | 6.82 (2.735) | 5.70 (5.7–12.4) | |||
| 9mg/kg Drisapersen | 3 | Screening | 0 | No data | No data | |
| Day 1 | 2 | 5.70 (0.000) | 5.70 (5.7–5.7) | |||
| Day 8 | 3 | 31.03 (12.974) | 25.20 (22.0–45.9) | |||
| Follow-up | 3 | 5.70 (0) | 5.70 (5.7–5.7) |
Laboratory Reference range for urine alpha 1 microglobulin: less than 12.0 mg/L.
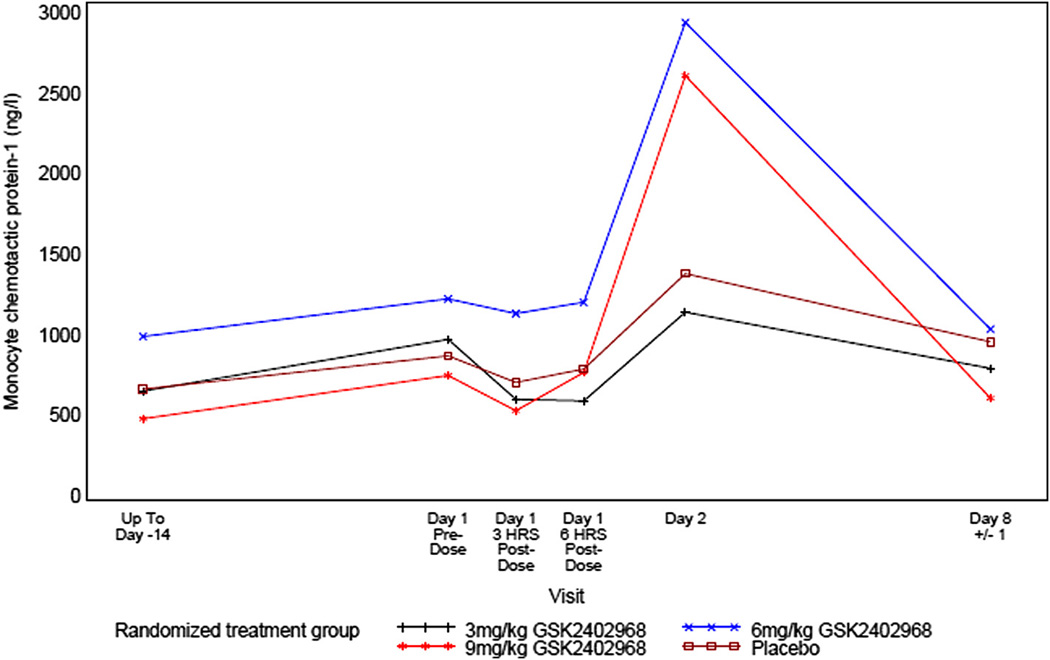
Several inflammatory biomarkers were measured in the study, including: high sensitivity C-reactive protein (hsCRP); monocyte chemotactic protein-1 (MCP-1); interleukin-6 (IL-6); tumor necrosis factor-α (TNF-α); complement split factors C3a, Bb, and SC5b-9; fibrinogen; and haptoglobin. Across treatment groups, there were fluctuations in complement split factors C3a, and SC5b-9 and in TNF-α but no clear trends were observed. In the 3 and 6 mg/kg drisapersen treated groups, reversible changes were observed post-dose for complement split factor Bb, hsCRP, fibrinogen, haptoglobin, IL-6 and MCP-1. These returned to baseline between 6 h and 1 week post-dose suggesting a low local inflammatory response to administration of product. In the 9 mg/kg drisapersen treated group, 1 subject had values of hsCRP >2 × ULN and 2 × baseline value. In addition, all 3 subjects treated with 9 mg/kg drisapersen had fever and elevations of other inflammatory biomarkers (predominantly hsCRP, MCP-1 and IL-6) (Figs. 2–4). Although transient increases were also seen for these parameters in the 6 mg/kg group, these were generally less than for the 9 mg/kg group. The highest temperature recorded by the investigator in the 9 mg/kg group was 37.9 °C; however temperatures up to 39.0 °C were reported to the investigators by the subject’s families (measured outside of study procedures). These reportedly occurred in 2 subjects approximately 20 h and 30 h after dosing and resolved without sequelae. All other vital signs were within normal ranges for all groups.
Fig. 2.
Mean profile plot of high sensitivity C-reactive protein (hsCRP).
Fig. 4.
Mean profile plot of interleukin-6 (IL-6).
No clinically significant ECG abnormalities were observed at any time throughout the study for any treatment group.
4. Discussion
This study was the first double-blind placebo-controlled study to administer drisapersen in non-ambulant DMD subjects. This study was also the first time doses higher than 6 mg/kg of drisapersen have been administered in the DMD population. The study showed that single doses of 3 and 6 mg/kg were generally well tolerated, but that the 9 mg/kg dose was limited by acute (though self-resolving) inflammatory responses. Additionally, the 9 mg/kg dose did not offer any apparent increase in exposure as compared to the 6 mg/kg dose.
Pharmacokinetic analysis demonstrated less than proportional increase in exposure (in terms of AUC) over the dose range of 3–9 mg/kg, though post hoc analysis showed that proportionality was more feasible over the 3–6 mg/kg range than the 3–9 mg/kg range. The reason for the observed non-proportionality cannot be concluded from this study. It may be related to absorption saturation at the higher dose, since the slope of Cmax with dose is lower than that of AUC, indicating that it might be related to absorption rather than distribution or elimination. The later Tmax as observed after 9 mg/kg (6 h compared to 3 h for the 3 and 6 mg/kg doses) seems to be in line with this hypothesis.
The pharmacokinetics of drisapersen after a single subcutaneous dose of 6 mg/kg were similar between non-ambulant subjects in the current study and ambulant subjects in the Goemans et al. [10] study. Since a dose of 3 mg/kg was not tested in the previous study [10], the results of this dose could not be compared. However, the data of the 2 and 4 mg/kg doses that were tested in the Goemans et al. study [10] indicated that the lower dose also resulted in similar PK between the ambulant and non-ambulant population. Although in the current study t1/2 could not be calculated, this comparable PK between the two studies indicates that the PK at steady state after multiple dosing at doses up to 6 mg/kg in nonambulant subjects may also be similar to that observed in ambulant subjects.
Since antisense oligonucleotides like drisapersen are known to distribute into adipose tissue, and non-ambulant boys with DMD generally have a high percentage body fat, the relation between body fat and exposure was explored. Plotting Cmax and AUC(0–24) versus the percentage body fat showed that these PK parameters appeared constant over the range of body fat percentages tested. It should be noted that data from younger boys, who are likely to have lower body fat percentages, should be included in order to make a definitive conclusion, but from the data collected within this study, no relationship between percentage body fat and PK of drisapersen have been observed.
Single doses of drisapersen at 3 mg/kg and 6 mg/kg did not result in significant safety or tolerability concerns in the non-ambulant DMD population. Adverse events and laboratory abnormalities were similar to those reported to date in the previous open-label drisapersen DMD study [10].
Drisapersen at the 9 mg/kg dose showed tolerability issues (pyrexia) in the treated subjects. As dose proportionality in PK was not demonstrated in this study, it is unknown why these occurred at this dose level. Although the reason for lower tolerability of the drisapersen 9 mg/kg dose is not known, it is possible that the larger volume of administration, the greater local drug exposure, and/or the need for more than a single injection could result in an increased risk of local inflammatory reactions. This could then be complicated by inflammatory biomarker release with subsequent pyrexia.
Injection site reactions were also seen across the drisapersen and placebo groups, though with a higher incidence in the active treatment group. This finding is consistent with what has previously been observed in predominantly ambulant boys [10].
Antisense oligonucleotides such as drisapersen have known preclinical class effects. These fall into four main categories: inflammation, thrombocytopenia, accumulation in the kidneys and liver, and increases in aPTT. With the exception of pyrexia reported in one subject who received drisapersen 6 mg/kg, subjects receiving drisapersen at doses of 3 mg/kg and 6 mg/kg did not experience any AEs related to class effects. In contrast, all subjects receiving 9 mg/kg drisapersen experienced AEs suggestive of inflammation together with increases in hsCRP, MCP-1 and IL-6. Although transient increases were also seen for these parameters in the 6 mg/ kg group, these were generally less than for the 9 mg/kg group. These effects were time-limited and self-resolving, without any further clinical sequelae. A repeat dose study in ambulant subjects treated at 6 mg/kg/week did not show evidence of acute or persistent systemic inflammatory responses [10].
Elevations in mean urine α1-microglobulin values were seen in all groups up to Day 8. These changes appeared dose dependant and reversible. Increases in α1-microglobulin were also seen in the repeat dose study of predominantly ambulant boys [10], and possibly represent an adaptive process within renal tubules (which may absorb oligonucleotides).
It was concluded that the study objectives had been met following analysis of cohort 3a (9 mg/kg) and the decision was made not to proceed to cohort 3b (9 mg/kg) or cohort 4 (12 mg/kg). The study was considered to have met the objectives of the study because: (1) doses of 3 mg/kg and 6 mg/kg were generally well tolerated; (2) there were less than proportional increases in exposure from 6 to 9 mg/ kg, suggesting that investigation of higher doses administered subcutaneously would not likely lead toincreased systemic exposure; and (3) tolerability at the 9 mg/kg dose was limited by acute (though self-resolving) inflammatory reactions.
Therefore, the maximum tolerated dose of 6 mg/kg of drisapersen was identified for further characterization in multiple dose studies in the non-ambulant DMD population. This finding is consistent with those from the ambulant DMD population, where the dose of 6 mg/kg/ week is being studied in double-blind, placebo-controlled, 24–48 week studies of the efficacy and safety of drisapersen.
Fig. 3.
Mean profile plot of monocyte chemotactic protein-1 (MCP-1).
Acknowledgements
This study was funded by GlaxoSmithKline. The authors wish to acknowledge Gail Arthur and Kandice Roush at Nationwide Children’s Hospital, as well as Denis de Castro MD (Institut de Myologie) and Valerie Doppler MD for their invaluable contributions to the study. The authors would also like to thank Katie Rolfe (GlaxoSmithKline) for critical review and Tommy Thompson (GlaxoSmithKline) for providing medical writing support.
Footnotes
Disclosures
John E. Kraus, Joanna Nakielny, Naashika Quarcoo, Lia Liefaard, and Tom Drury are GlaxoSmithKline employees and shareholders. Claire Wardell was a GlaxoSmithKline employee at the time the study was conducted and is a shareholder. Padraig Wright was a GlaxoSmithKline employee at the time the study was conducted and is currently employed with Takeda.
Allison Morgan, Susie Dorricott, and Giles Campion are Prosensa Therapeutics BV employees.
References
- 1.Emery AEH. Duchenne muscular dystrophy. 2nd ed. Oxford: Oxford University Press; 1993. [Google Scholar]
- 2.Hoffman EP, Fischbeck KH, Brown RH, et al. Characterization of dystrophin in muscle-biopsy specimens from patients with Duchenne’s or Becker’s muscular dystrophy. N Engl J Med. 1988;318:1363–1368. doi: 10.1056/NEJM198805263182104. [DOI] [PubMed] [Google Scholar]
- 3.Koenig M, Monaco AP, Kunkel LM. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988;53:219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- 4.Bushby K, Finkel R, Birnkrant DJ, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9:77–93. doi: 10.1016/S1474-4422(09)70271-6. [DOI] [PubMed] [Google Scholar]
- 5.Ishikawa Y, Miura T, Ishikawa Y, et al. Duchenne muscular dystrophy: survival by cardio-respiratory interventions. Neuromuscul Disord. 2011;21:47–51. doi: 10.1016/j.nmd.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Van Deutekom JC, van Ommen GJ. Advances in Duchenne muscular dystrophy gene therapy. Nat Rev Genet. 2003;4:774–784. doi: 10.1038/nrg1180. [DOI] [PubMed] [Google Scholar]
- 7.Helderman-van den Enden AT, Straathof CS, Aartsma-Rus A, et al. Becker muscular dystrophy patients with deletions around exon 51; a promising outlook for exon skipping therapy in Duchenne patients. Neuromuscul Disord. 2010;20:251–254. doi: 10.1016/j.nmd.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Aartsma-Rus A, Fokkema I, Verschuuren J, et al. Theoretic applicability of anti-sense-mediated exon skipping for duchenne muscular dystrophy mutations. Hum Mutat. 2009;30:293–299. doi: 10.1002/humu.20918. [DOI] [PubMed] [Google Scholar]
- 9.Van Deutekom JC, Janson AA, Ginjaar IB, et al. Local dystrophin restoration with antisense oligonucleotide PRO051. N Engl J Med. 2007;357:2677–2686. doi: 10.1056/NEJMoa073108. [DOI] [PubMed] [Google Scholar]
- 10.Goemans NM, Tulinius M, van den Akker JT, et al. Systemic administration of PRO051 in Duchenne’s muscular dystrophy. N Engl J Med. 2011;364:1513–1522. doi: 10.1056/NEJMoa1011367. [DOI] [PubMed] [Google Scholar]
- 11.DMD114044. A phase III, randomized, double blind, placebo-controlled clinical study to assess the efficacy and safety of GSK2402968 in subjects with Duchenne muscular dystrophy. ClinicalTrials.gov Identifier: NCT01254019
- 12.DMD114117. A phase II, double blind, exploratory, parallel-group, placebo-controlled clinical study to assess two dosing regimens of GSK2402968 for efficacy, safety, tolerability and pharmacokinetics in ambulant subjects with Duchenne muscular dystrophy. ClinicalTrials. gov Identifier: NCT01153932
- 13.DMD114349. An open-label extension study of the long-term safety, tolerability and efficacy of GSK2402968 in subjects with Duchenne muscular dystrophy. ClinicalTrials.gov Identifier: NCT01480245
- 14.DMD114876. An exploratory study to assess two doses of GSK2402968 in the treatment of ambulant boys with Duchenne muscular dystrophy (DMD) ClinicalTrials.gov Identifier: NCT01462292
- 15.DMD115501. An open-label extension study of the long-term safety, tolerability and efficacy of drisapersen (GSK2402968) in US subjects with Duchenne muscular dystrophy. ClinicalTrials.gov Identifier: NCT01803412