Abstract
Background
Arterial (AC) and central venous catheterization (CVC) are common in intensive care units (ICUs). Few data describe which patients receive these devices and whether variability in practice exists.
Methods
We conducted an observational cohort study of adult ICU admissions during 2001–2008 using Project IMPACT to determine whether AC and CVC use is consistent across United States ICUs. We examined trends over time and patients more (mechanically ventilated or on vasopressors) or less (predicted risk of hospital mortality ≤2%) likely to receive either catheter.
Results
Our cohort included 334,123 patients across 122 hospitals and 168 ICUs. Unadjusted AC usage rates remained constant (36.9% (2001) versus 36.4% (2008); P = 0.212) while CVC use increased (from 33.4% (2001) to 43.8% (2008), P < 0.001 comparing 2001 and 2008); adjusted AC usage rates were constant from 2004 (35.2%) to 2008 (36.4%, P = 0.43 for trend). Surgical ICUs used both catheters most often (unadjusted rates, ACs: 56.0% of patients vs. 22.4% in medical and 32.6% in combined units, P < 0.001; CVCs: 46.9% vs. 32.5% and 36.4%, P < 0.001). There was wide variability in AC use across ICUs in patients receiving mechanical ventilation (median (interquartile range): 49.2% (29.9%, 72.3%); adjusted Median Odds-Ratio (AMOR) 2.56), vasopressors (51.7% (30.8%,76.2%); AMOR 2.64), and with predicted mortality ≤2% (31.7% (19.5, 49.3%); AMOR 1.94). There was less variability in CVC use (mechanical ventilation: 63.4% (54.9%, 72.9%), AMOR 1.69; vasopressors: 71.4% (59.5%, 85.7%), AMOR 1.93; predicted mortality ≤2%: 18.7% (11.9%, 27.3%), AMOR 1.90).
Conclusions
Both ACs and CVCs are common in ICU patients. There is more variation in use of ACs than CVCs.
Introduction
Intravascular catheterization is a common procedure in critically ill patients. Arterial catheters (AC) are placed to facilitate frequent blood sampling and to closely monitor blood pressure.1–4 Central venous catheters (CVCs) are used for many reasons including facilitating administration of certain medications, augmenting hemodynamic monitoring including determination of central venous oxygenation, and providing venous access when peripheral access is limited.5–8
The risk-benefit calculus of these catheters may not justify their widespread use. At the American Thoracic Society International Conference (May, 2013) the Choosing Wisely campaign9 for Critical Care Medicine highlighted the recommendation to use intravascular catheters only if specifically indicated (unpublished data: Robert Fowler, M.D., M.Sc.; presented at the American Thoracic Society International Conference [Philadelphia, Pennsylvania] in a session entitled “‘Choosing Wisely’ (©ABIM Foundation Symposium): Top Ways to Reduce Low Value Care in Pulmonary and Critical Care Medicine”, May 20, 2013). Most importantly, these catheters can result in significant complications.10–19 Second, both the supplies and the labor associated with the placement and maintenance of these devices are financially costly. Finally, there is controversy over whether their use is associated with a clinically relevant benefit for any group of patients.20 A first step in analyzing the potential impact of these catheters is to understand whether there is variability in their use.
Small studies and surveys have reported widely varying rates of AC and CVC use in the intensive care unit (ICU) setting. Rates of AC use have been estimated to range from one-third to nearly all patients in certain ICU patient subgroups.15,21,22 Reported rates of CVC usage range from 13% to 91% for ICU patients.15,23,24 Most of these data on catheter epidemiology come from studies that are focused on investigating complications associated with their use rather than the decision to use them at all; a detailed understanding of in which ICUs and patient subgroups clinicians use these catheters, therefore, is lacking.
We hypothesized that there is wide variation across ICUs in the use of ACs and CVCs in the care of critically ill patients. To test this hypothesis, we evaluated the variability in AC and CVC usage over time, across patient subgroups, and, finally, across individual ICUs for selected homogeneous subpopulations to characterize use of intravascular catheters.
Materials and Methods
We performed an observational cohort study of adult patients (≥18 yr of age) admitted from 2001 through 2008 to ICUs in the United States participating in the Project IMPACT database (Cerner Corporation, Kansas City, MO).25 Project IMPACT was not created as a research database. Rather, Project IMPACT provided regular performance audits and feedback to participating ICUs. Participation in the database was voluntary and hospitals and ICUs paid for the service. Data were collected at each institution by on-site data collectors who were certified by Project IMPACT to assure standardization and uniformity in data definitions and entry. Hospitals participating in Project IMPACT tended to be larger and more urban than the general population hospitals, but were diverse in size and location. Data were either from consecutive admissions to each ICU or a random sample of admissions. Sites using the latter method collected information on 50% or 75% of all patients; the percentage was determined quarterly before data collection commenced. Only the initial ICU admission for a given hospital stay was included.
Data on patient demographics (age, gender, race) and health problems including severity of illness as described by the mortality probability model predicted hospital mortality at ICU admission (MPM0-III),26 preference for cardiopulmonary resuscitation at ICU admission, acute diagnostic category, location prior to ICU arrival, patient type (medical, emergent surgical, or elective surgical), number of comorbidities, year of ICU admission, and number of organs failing during the ICU admission were available. Explicit definitions for organ failures were provided by Project IMPACT (appendix 127). Data on interventions included the use of invasive mechanical ventilation (MV) and vasopressor administration by continuous intravenous infusion at any point during the ICU stay. Information on patient outcomes included ICU and hospital lengths of stay and hospital mortality. ICUs and hospitals were characterized according to ICU specialty, number of operable ICU beds, ICU model (based on degree of critical care consultation mandated and/or available), community setting (urban, suburban, rural), academic affiliation, and number of licensed hospital beds.
Appendix 1 - Table 5.
Project IMPACT Definitions for Organ Failures
| Organ System | Definition of Failure |
|---|---|
|
| |
| Cardiovascular | Any of the following for >1 h despite adequate fluid resuscitation:
|
|
| |
| Hyperlactatemia | Both of:
|
|
| |
| Respiratory | Noncardiogenic pulmonary edema |
| and | |
Either of:
|
|
|
| |
| Renal | Patient not on chronic dialysis |
| and | |
Either of:
|
|
|
| |
| Hematologic | Any of:
|
|
| |
| Hepatic | Serum total bilirubin >2 mg/dl (must be acute, not chronic) |
|
| |
| Neurologic | All of:
|
CNS = central nervous system; FiO2 = fraction of inspired oxygen; GCS = Glasgow coma score; MAP = mean arterial pressure; PaO2 = partial pressure of oxygen in the arterial blood; PEEP = positive end-expiratory pressure; PT = prothrombin time; PTT = partial thromboplastin time; SBP = systolic blood pressure.
Statistical Analysis
The primary outcome of interest was use of an AC (including catheters placed in the radial, femoral, brachial, dorsalis pedis, or axillary arteries) or CVC (including catheters placed in the subclavian, femoral, brachial, internal or external jugular veins) during the ICU stay. This definition included catheters inserted prior to admission to ICU if they remained in place for some portion of the ICU stay. We also examined the data stratified by whether catheters were inserted prior to or during the ICU stay. Patient characteristics and ICU/hospital characteristics associated with AC and CVC use were analyzed using Chi-squared and analysis of variance as appropriate. The exact dates of catheter use—specifically in relation to dates of other interventions (e.g., vasopressor use)—were not available.
We calculated the absolute (unadjusted) rate of catheter use in each study year; statistical differences over time in the adjusted odds of use were assessed using univariable linear regression (with modeling using one or two trend lines determined by visual inspection of the data for the presence of a point at which the rate of change over time may have become notably different). We then examined the adjusted odds of receiving a catheter over time using multivariate multilevel mixed effects logistic modeling including independent variables: year, age, gender, race, comorbidities, MPM0-III, resuscitation status on ICU admission, organ failures, use of MV, use of vasopressors, use of other indwelling catheters, acute diagnostic group, location prior to ICU arrival, patient type, whether the facility had an academic affiliation, and ICU specialty. Multivariate multilevel mixed effects modeling allows for evaluation of associations of individual patient- and ICU/hospital-level variables with a given outcome after accounting for the clustering of individual patients in specific ICUs. For the purposes of the multivariate multilevel mixed effects logistic modeling, patients were clustered by ICU rather than by hospital. Sensitivity analyses were conducted to test the robustness of the results of the model in which: (1) the independent variables of organ failures and chronic illnesses were categorized differently and (2) AC or CVC use was excluded as an independent variable in the model of the other catheter.
We first compared the frequency of AC and CVC use across patients grouped by ICU specialty and location prior to the ICU using Chi-squared tests. We summarized the variability by individual ICU in the use of ACs and CVCs using median, interquartile ranges (IQR) and full ranges as well as adjusted median odds-ratios (AMOR). AMORs have been promoted to describe practice pattern variation between hospitals and are preferred to intraclass correlation coefficients when reporting multilevel modeling of binary outcomes.28–30 The AMOR quantitatively describes the variability between clusters and can be easily calculated from the cluster variance.28 An AMOR of 1.5 indicates that for two patients who are otherwise identical except that one was admitted to a “high catheter using ICU” and the other was admitted to a “low catheter using ICU”, the odds of having had a catheter is 1.5-fold higher in the “high catheter using ICU”. By definition, the AMOR is ≥1. We quantified the predictive power of three variable sets—(1) patient factors, (2) ICU/hospital factors, and (3) being clustered in individual ICUs—on catheter use using the unitless quotient of the Akaike information criterion of a model excluding the variable set of interest to the full model as described by Harrell; a number closer to one indicates a larger relative impact of the excluded variable set on catheter use prediction.31 We examined three specific subgroups of patients; first, patients where we expected catheter use to be high: (1) patients requiring MV and (2) patients requiring vasopressors during their ICU stay and, second, patients with low expected use: (3) predicted hospital mortality (using MPM0-III) on ICU arrival of ≤2%.32 For the analyses of each subgroup of patients, we only included ICUs with ≥20 patients in the given subgroup.
Results were considered statistically significant if P < 0.05. No adjustments were made to this significance level for multiple models. Database management and statistical analyses were performed using Excel (Microsoft, Redmond, WA) and Stata 11.0 (StataCorp LP, College Station, TX). Institutional Review Board approval was obtained from Beth Israel Medical Center (New York, New York, #200-10).
Results
Our cohort included 334,123 ICU patients across 122 hospitals and 168 ICUs (with 16.7% of ICUs reporting data on all admissions and 83.3% on a random sample of patients). Most of the ICUs were mixed medical-surgical units (52.9%) with an even breakdown of surgical ICUs (SICUs, 24.4%) and medical ICUs (MICUs, 22.6%) (table 1). A majority of hospitals were in urban environments (54.5%) and were nonacademic (79.2%). The mean age of patients in the cohort was 60.6 ±18.0 yr with a mean MPM0-III predicted hospital mortality of 13.7% ± 16.5%. Overall hospital mortality was 13.0% with a median ICU length of stay of 2 (IQR 1,4) days and a median hospital length of stay of 7 (IQR 3,12) days.
Characteristics of patients who received ACs and CVCs
In our cohort, 47.8% had neither an AC nor CVC, 14.3% of patients had only an AC, 16.2% had only a CVC, and 21.7% had both. Surgical patients were more likely than medical patients to receive either catheter (table 2). Patients at the extremes of age (<50 or 85+ yr) were less likely to receive either an AC or a CVC. The use of ACs was highest in patients at the extremes of illness severity (MPM0-III predicted hospital mortality ≤2% or >20%) while CVC use increased steadily with illness severity. Patients received ACs and CVCs more often with more organ failures, receipt of MV, or receipt of vasopressor medications. Patients with both ACs and CVCs had longer ICU lengths of stay (median (IQR): 2 (1,5) vs. 2 (1,3) days for AC; 3 (2,7) vs. 1 (1,2) days for CVC), longer hospital lengths of stay (8 (5,15) vs. 6 (3,10) days for AC; 10 (6,19) vs. 5 (3,6) days for CVC), and higher hospital mortality (15.4% vs. 11.6% for AC; 21.4% vs. 7.9% for CVC) than those without. All comparisons were significant at P < 0.001.
Trends in catheter use
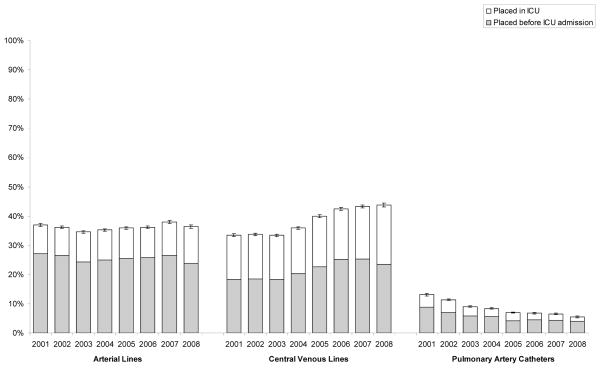
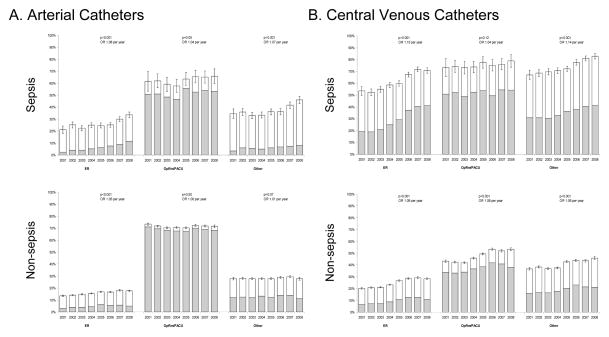
Absolute rates of AC use remained fairly constant from 2001 to 2008 (minimum rate in 2003 of 34.6% of patients to a maximum in 2007 of 38.0%, P = 0.21 comparing 2001 and 2008) while CVC use increased from 33.4% in 2001 to 43.8% in 2008 (P < 0.001 comparing 2001 and 2008) (fig. 1). Over this same time period, the use of pulmonary artery catheters (PACs) steadily declined (from 13.1% in 2001 to 5.5% in 2008, P < 0.001). The changes in use of CVCs were due to changes in the number of catheters placed in both the pre-ICU and ICU setting. Increased use of ACs and CVCs was greater in septic patients admitted from nonoperating room/postanesthesia care unit (OpRm/PACU) locations than for nonseptic patients admitted from non-OpRm/PACU (appendix 2).
Figure 1.
Trends in Catheter Utilization, 2001–2008.
Trends evaluated using logistic regression revealed odds-ratio (OR) 1.01 per year (P < 0.001) for arterial catheter use, OR 1.08 per year (P < 0.001) for central venous catheter use, and OR 0.87 per year (P < 0.001) for pulmonary artery catheter use.
ICU = intensive care unit
Appendix 2 - Figure 4.
Trends in AC and CVC (2001–2008) stratified by sepsis diagnosis and location prior to ICU arrival.
(A) arterial catheters, (B) central venous catheters
ER = emergency room; ICU = intensive care unit; OpRm/PACU = operating room/postanesthesia care unit; OR = odds-ratio calculated by logistic regression.
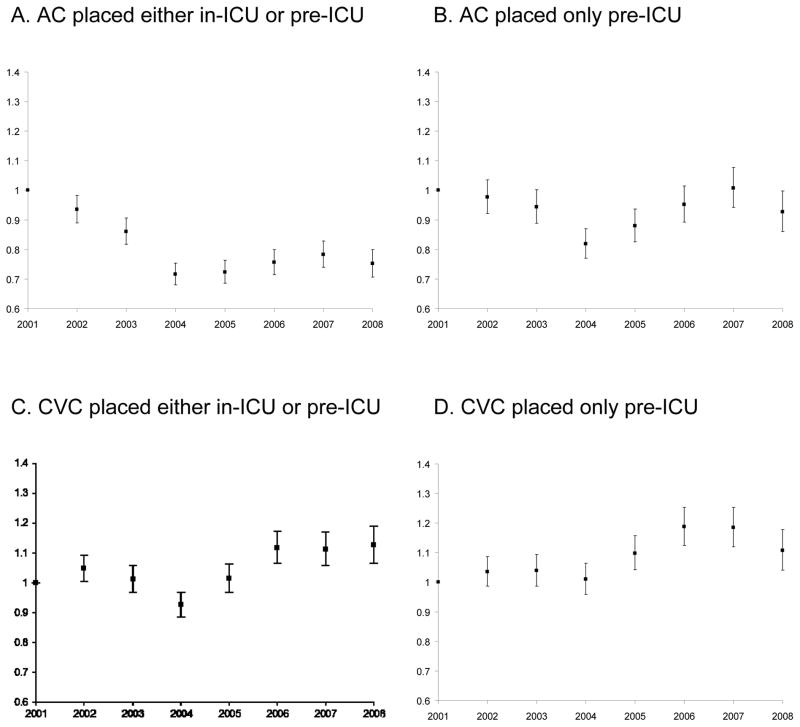
Patient-, ICU-, and hospital-level characteristics of the cohort changed over time (appendix 3). After multivariate adjustment (table 3, appendix 4), the odds of AC use decreased with time between 2001 and 2004 (regression coefficient for linear trend in odds −0.93, P = 0.02), but remained constant from 2004 to 2008 (P = 0.43). There was no significant trend in AC placement before ICU arrival (P = 0.79) (appendix 4). In contrast, after multivariate adjustment, the odds of receiving a CVC did not change over time (P = 0.07) (appendix 4). These results were robust to sensitivity analyses conducted by the construction of alternative multivariate models (data not shown).
Appendix 3 - Table 6.
Characteristics of Cohort over Time, 2001–2008
| 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | p-value** | |
|---|---|---|---|---|---|---|---|---|---|
| Age (years), mean±sd | 61.6±17.5 | 61.4±17.8 | 61.4±17.8 | 60.7±18.0 | 60.1±18.1 | 59.8±18.1 | 59.6±18.2 | 59.5±18.1 | <0.001 |
| Gender, % | <0.001 | ||||||||
| male | 53.8 | 54.5 | 54.4 | 55.3 | 55.5 | 56.3 | 56.1 | 55.4 | |
| female | 46.2 | 45.5 | 45.6 | 44.7 | 44.5 | 43.7 | 43.9 | 44.6 | |
| Race, % | <0.001 | ||||||||
| white | 79.7 | 81.5 | 83.0 | 80.5 | 78.7 | 79.1 | 79.0 | 79.6 | |
| other | 15.8 | 13.8 | 11.5 | 12.5 | 14.0 | 14.2 | 13.7 | 15.2 | |
| black | 4.6 | 4.7 | 5.5 | 7.0 | 7.3 | 6.7 | 7.3 | 5.1 | |
| Comorbidities, mean ± SD | 0.3±0.6 | 0.2±0.5 | 0.3±0.5 | 0.3±0.5 | 0.3±0.5 | 0.3±0.6 | 0.3±0.5 | 0.3±0.5 | <0.001 |
| MPM0-III predicted hospital mortality (%)*, mean ± SD | 13.2±16.1 | 13.6±16.6 | 13.9±17.0 | 13.6±16.5 | 13.6±16.3 | 13.8±16.5 | 13.8±16.4 | 14.4±16.6 | <0.001 |
| Acute Diagnostic Group, % | <0.001 | ||||||||
| trauma | 5.1 | 5.7 | 5.5 | 7.0 | 8.2 | 8.6 | 9.5 | 10.2 | |
| respiratory/thoracic | 21.1 | 21.7 | 21.6 | 19.5 | 19.8 | 18.2 | 17.8 | 18.9 | |
| cardiovascular/vascular | 33.9 | 33.2 | 33.1 | 33.2 | 29.2 | 31.0 | 29.0 | 25.2 | |
| sepsis† | 4.8 | 5.5 | 6.0 | 6.6 | 7.3 | 7.9 | 9.1 | 10.5 | |
| neurologic (nontraumatic) | 13.2 | 13.0 | 13.2 | 13.8 | 15.1 | 15.5 | 16.3 | 17.1 | |
| metabolic/renal | 8.7 | 8.5 | 8.6 | 8.2 | 8.2 | 7.8 | 7.6 | 7.5 | |
| gastrointestinal | 13.1 | 12.4 | 12.2 | 11.7 | 12.1 | 10.9 | 10.8 | 10.6 | |
| DNR on ICU Admission, % | 0.0 | 0.0 | 0.3 | 0.6 | 0.4 | 0.4 | 0.4 | 0.4 | <0.001 |
| Organs failing, mean±sd | 0.0±0.0 | 0.0±0.0 | 0.2±0.6 | 0.5±0.9 | 0.5±1.0 | 0.5±1.0 | 0.6±1.1 | 0.7±1.1 | <0.001 |
| Mechanical ventilation, % | 35.1 | 35.8 | 33.9 | 34.9 | 37.3 | 37.2 | 39.5 | 41.0 | <0.001 |
| Vasopressors§, % | 20.3 | 20.5 | 19.9 | 19.3 | 19.5 | 20.2 | 21.1 | 21.7 | <0.001 |
| Arterial Catheter, % | <0.001 | ||||||||
| none | 63.1 | 63.8 | 65.4 | 64.8 | 64.1 | 63.8 | 62.0 | 63.6 | |
| in-place on ICU arrival | 27.2 | 26.5 | 24.3 | 25.0 | 25.5 | 25.7 | 26.4 | 23.8 | |
| placed while in ICU | 9.8 | 9.7 | 10.3 | 10.3 | 10.4 | 10.5 | 11.6 | 12.6 | |
| Central Venous Catheter, % | <0.001 | ||||||||
| none | 66.6 | 66.3 | 66.6 | 64.1 | 60.1 | 57.5 | 56.7 | 56.2 | |
| in-place on ICU arrival | 18.3 | 18.4 | 18.3 | 20.3 | 22.7 | 25.2 | 25.3 | 23.5 | |
| placed while in ICU | 15.1 | 15.3 | 15.1 | 15.6 | 17.2 | 17.3 | 18.0 | 20.3 | |
| Location prior to ICU, % | <0.001 | ||||||||
| ward | 9.7 | 9.4 | 9.9 | 8.4 | 8.5 | 7.7 | 7.4 | 7.4 | |
| emergency room | 42.3 | 43.4 | 43.9 | 43.6 | 43.8 | 45.0 | 44.0 | 47.0 | |
| OpRm/PACU | 32.7 | 31.5 | 28.7 | 29.1 | 29.3 | 28.2 | 29.6 | 27.0 | |
| stepdown/telemetry unit | 5.0 | 5.6 | 5.9 | 5.2 | 5.0 | 4.8 | 4.5 | 4.8 | |
| other | 10.4 | 10.1 | 11.5 | 13.7 | 13.5 | 14.3 | 14.6 | 13.8 | |
| Patient type, % | <0.001 | ||||||||
| medical | 62.9 | 64.6 | 67.2 | 66.5 | 66.5 | 67.6 | 65.2 | 68.8 | |
| elective surgical | 25.9 | 24.4 | 22.8 | 22.6 | 21.1 | 20.6 | 21.5 | 18.9 | |
| emergent surgical | 11.2 | 11.0 | 10.0 | 10.9 | 12.4 | 11.8 | 13.2 | 12.4 | |
| ICU type‖, % | <0.001 | ||||||||
| SICU | 16.6 | 14.1 | 10.7 | 19.4 | 24.7 | 26.4 | 33.3 | 31.7 | |
| MICU | 13.2 | 10.9 | 10.9 | 15.1 | 16.9 | 19.8 | 20.3 | 20.3 | |
| combined | 70.3 | 75.0 | 78.4 | 65.6 | 58.5 | 53.7 | 46.4 | 47.9 | |
| Academic hospital, % | 13.1 | 11.6 | 11.5 | 19.7 | 27.1 | 28.8 | 35.7 | 34.9 | <0.001 |
DNR = do-not-resuscitate order; ICU = intensive care unit; MICU = medical ICU; OpRm/PACU = operating room/post-anesthesia care unit; SICU = surgical ICU.
MPM0-III = the mortality probability model at ICU admission.
By definition for Project IMPACT, ‘sepsis’ means that a “patient is septic with or without significantly low blood pressure” and he/she “may or may not have positive blood cultures upon ICU admission.”
Vasopressors include infusions of dopamine, epinephrine, norepineprhine, phenylephrine, and/or vasopressin.
MICU and combined units can include coronary care.
p-values calculated using Chi-square or ANOVA as appropriate.
Appendix 4 - Figure 5.
Adjusted odds of catheter use by ICU admission year, 2001–2008.
(A) AC placed either in-ICU or pre-ICU, (B) AC placed only pre-ICU, (C) CVC placed either in-ICU or pre-ICU (D) CVC placed only pre-ICU
AC = arterial catheter; CVC = central venous catheter; ICU = intensive care unit.
Variability in catheter use
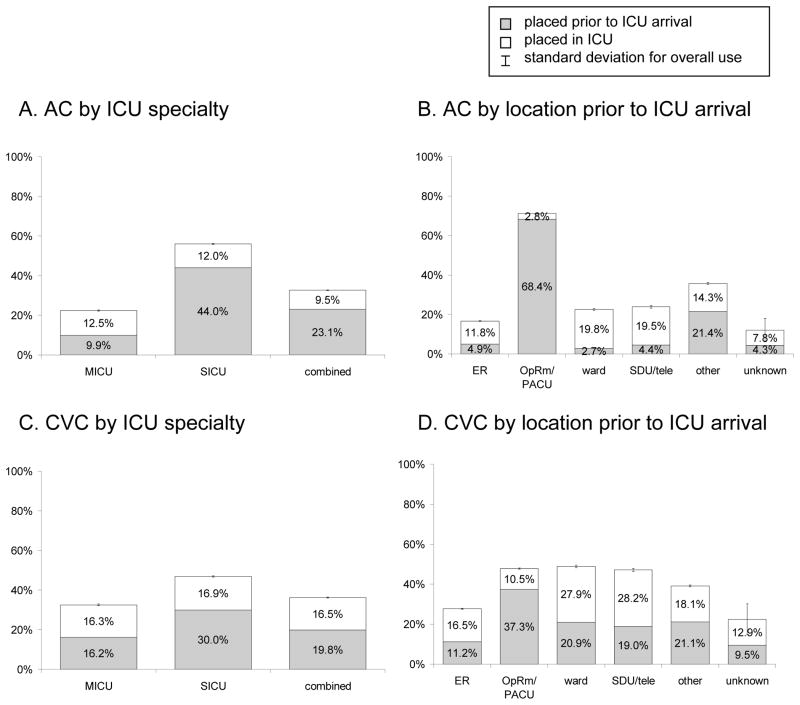
AC use varied by type of ICU (fig. 2, table 3). Compared to SICU patients (56.0% of whom received an AC), patients admitted to either a MICU (22.4%, adjusted OR (95% confidence interval, CI): 0.52 (0.37,0.73)) or a combined unit (32.6%, adjusted OR (CI): 0.63 (0.48,0.84)) were less likely to receive an AC (fig. 2A). The timing of placement of ACs also varied based on type of ICU. The majority of ACs used in SICU and combined unit patients were placed prior to arrival in the ICU whereas in the MICU population, more were placed in the ICU itself. The high frequency of ACs placed prior to ICU admission in SICUs was driven by the admission of patients from the OpRm/PACU who were nearly twice as likely to receive an AC (71.2%) compared with patients coming to the ICU from other locations (≤35.7%, P < 0.001, fig. 2B).
Figure 2.
Catheter use by ICU specialty and location prior to ICU arrival.
(A) AC by ICU specialty, (B) AC by location prior to ICU arrival, (C) CVC by ICU speciality, (D) CVC by location prior to ICU arrival.
AC = arterial catheter; combined = medical and surgical ICU; CVC = central venous catheter; ER = emergency room; ICU = intensive care unit; MICU = medical ICU; OpRm/PACU = operating room/postanesthesia care unit; SDU/tele = step-down unit/telemetry unit; SICU = surgical ICU.
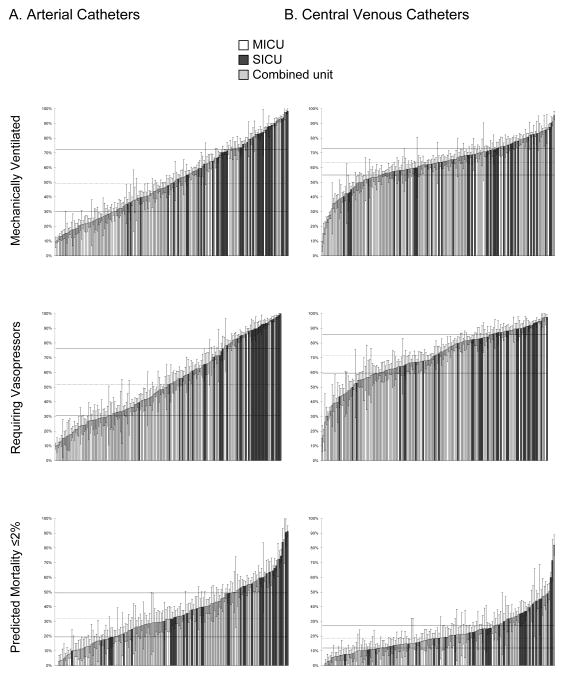
While the majority of variability was explained by patient characteristics (table 4), there was substantial variability in AC usage across individual ICUs (AMOR 2.04; table 3). For patients who received MV, median AC use across units was 49.2% with an IQR of 29.2% to 72.3% (AMOR 2.56; fig. 3, appendix 5). Similarly, there was wide variability in the rates of AC usage for patients requiring vasopressors during their ICU stay (median (IQR): 51.7% (30.8%, 76.2%); AMOR 2.64) and for low risk patients (those with predicted mortality of ≤2%: 31.7% (19.5, 49.3%); AMOR 1.94). SICUs used ACs more commonly across both high-risk (MV and vasopressor) and low-risk (MPM0-III predicted hospital mortality ≤2%) patients and account for a majority of the 10 highest usage units in each subgroup.
Figure 3.
Variation in catheter use across individual ICUs.
(A) arterial catheters, (B) central venous catheters
Error bars = 95% confidence interval for each ICUs utilization rate; dotted line = median of all unit rates; solid lines = interquartile range of all unit rates.
ICU = intensive care unit; MICU = medical ICU; SICU = surgical ICU.
Appendix 5 - Table 7.
Multilevel Mixed Effects Models for the Use of Arterial and Central Venous Catheters in High- and Low-Risk Patient Subgroups
| Arterial Catheters | Central Venous Catheters | |||||
|---|---|---|---|---|---|---|
| MV | Vasopressors | MPM0-III≤2% | MV | Vasopressors | MPM0-III≤2% | |
| Age (per 5 yr) | 1.00 (1.00,1.00) | 1.00 (0.99,1.00) | 1.03 (1.03,1.03) | 1.00 (1.00,1.00) | 0.99 (0.99,1.00) | 1.00 (1.00,1.00) |
| Female | 0.86 (0.83,0.89) | 0.87 (0.83,0.91) | 0.93 (0.87,0.98) | 1.28 (1.24,1.33) | 1.27 (1.22,1.33) | 1.29 (1.22,1.37) |
| Race | ||||||
| white | 1 | 1 | 1 | 1 | 1 | 1 |
| other | 0.93 (0.89,0.98) | 0.88 (0.81,0.94) | 0.89 (0.81,0.97) | 1.09 (1.03,1.14) | 1.03 (0.96,1.11) | 1.19 (1.09,1.30) |
| black | 0.98 (0.92,1.05) | 0.90 (0.81,1.01) | 0.99 (0.88,1.12) | 0.92 (0.86,0.98) | 1.03 (0.92,1.15) | 0.83 (0.74,0.94) |
| 1+ Comorbidities | 0.84 (0.80,0.87) | 0.79 (0.75,0.83) | 0.98 (0.90,1.07) | 1.23 (1.18,1.27) | 1.11 (1.05,1.16) | 1.39 (1.28,1.51) |
| MPM0-III predicted hospital mortality (per 10% increase)* | 1.00 (0.99,1.00) | 0.99 (0.99,0.99) | 1.00 (1.00,1.00) | 0.99 (0.99,1.00) | ||
| Acute Diagnostic Group | ||||||
| trauma | 1 | 1 | 1 | 1 | 1 | 1 |
| respiratory/thoracic | 0.48 (0.45,0.51) | 0.34 (0.29,0.38) | 0.72 (0.61,0.83) | 0.71 (0.67,0.75) | 0.77 (0.67,0.88) | 0.71 (0.61,0.83) |
| cardiovascular/vascular | 0.87 (0.81,0.94) | 0.52 (0.46,0.60) | 1.51 (1.30,1.75) | 0.82 (0.77,0.88) | 0.58 (0.51,0.66) | 1.03 (0.89,1.20) |
| sepsis† | 0.64 (0.59,0.70) | 0.43 (0.38,0.49) | 0.38 (0.29,0.50) | 1.69 (1.55,1.85) | 1.35 (1.18,1.55) | 4.74 (3.78,5.95) |
| neurologic (nontraumatic) | 1.02 (0.96,1.09) | 0.69 (0.60,0.80) | 1.29 (1.12,1.50) | 0.57 (0.54,0.61) | 0.48 (0.42,0.55) | 0.62 (0.54,0.73) |
| metabolic/renal | 0.45 (0.41,0.49) | 0.33 (0.28,0.38) | 0.40 (0.34,0.48) | 0.58 (0.53,0.62) | 0.82 (0.70,0.96) | 1.50 (1.28,1.75) |
| gastrointestinal | 0.68 (0.63,0.74) | 0.39 (0.33,0.45) | 0.52 (0.44,0.61) | 1.85 (1.72,1.99) | 1.52 (1.31,1.77) | 2.90 (2.49,3.39) |
| DNR on ICU Admission | 0.92 (0.63,1.32) | 0.75 (0.52,1.10) | 6.58 (1.10,39.17) | 0.96 (0.68,1.36) | 1.05 (0.77,1.45) | 0.23 (0.06,0.89) |
| Organs failing (per 1 organ increase) | 1.22 (1.20,1.24) | 1.18 (1.16,1.21) | 1.21 (1.09,1.34) | 1.54 (1.51,1.58) | 1.36 (1.32,1.39) | 1.92 (1.74,2.12) |
| Mechanical ventilation | 4.39 (4.14,4.66) | 4.26 (3.70,4.91) | 2.84 (2.68,3.00) | 4.12 (3.62,4.69) | ||
| Vasopressors§ | 2.40 (2.30,2.50) | 2.40 (2.03,2.82) | 2.69 (2.58,2.80) | 1.98 (1.72,2.27) | ||
| Arterial Catheter | ||||||
| none | 1 | 1 | 1 | |||
| in-place on ICU arrival | 3.44 (3.28,3.60) | 2.36 (2.18,2.55) | 2.61 (2.43,2.82) | |||
| placed while in ICU | 4.78 (4.56,5.01) | 3.55 (3.31,3.81) | 4.72 (4.05,5.49) | |||
| Central Venous Catheter | ||||||
| none | 1 | 1 | 1 | |||
| in-place on ICU arrival | 3.62 (3.46,3.78) | 2.57 (2.41,2.75) | 2.90 (2.68,3.13) | |||
| placed while in ICU | 4.67 (4.47,4.87) | 3.28 (3.08,3.50) | 3.27 (2.85,3.75) | |||
| Location prior to ICU | ||||||
| ward | 1 | 1 | 1 | 1 | 1 | 1 |
| emergency room | 0.93 (0.88,0.99) | 1.00 (0.93,1.08) | 1.87 (1.52,2.29) | 0.46 (0.43,0.49) | 0.58 (0.54,0.62) | 0.30 (0.26,0.35) |
| OpRm/PACU | 2.67 (2.45,2.90) | 4.68 (4.16,5.27) | 10.11 (8.55,11.96) | 0.54 (0.49,0.59) | 0.61 (0.53,0.69) | 0.67 (0.58,0.77) |
| stepdown/telemetry unit | 1.00 (0.92,1.09) | 0.97 (0.88,1.07) | 1.98 (1.46,2.67) | 0.89 (0.82,0.97) | 0.96 (0.86,1.06) | 1.04 (0.81,1.34) |
| other | 1.25 (1.16,1.34) | 1.42 (1.31,1.55) | 3.83 (3.14,4.65) | 0.87 (0.80,0.93) | 0.88 (0.80,0.96) | 0.57 (0.49,0.68) |
| Patient type | ||||||
| medical | 1 | 1 | 1 | 1 | 1 | 1 |
| elective surgical | 2.82 (2.60,3.05) | 2.93 (2.62,3.29) | 2.41 (2.05,2.84) | 0.99 (0.92,1.08) | 0.88 (0.78,0.99) | 1.16 (1.00,1.36) |
| emergent surgical | 2.37 (2.20,2.55) | 1.71 (1.54,1.91) | 1.55 (1.30,1.86) | 1.28 (1.19,1.38) | 1.37 (1.21,1.54) | 1.01 (0.85,1.19) |
| ICU type‖ | ||||||
| SICU | 1 | 1 | 1 | 1 | 1 | 1 |
| MICU | 0.46 (0.30,0.71) | 0.43 (0.27,0.68) | 0.60 (0.41,0.88) | 0.98 (0.75,1.27) | 0.93 (0.67,1.30) | 0.85 (0.59,1.24) |
| combined | 0.48 (0.33,0.71) | 0.41 (0.27,0.61) | 0.79 (0.58,1.07) | 1.02 (0.81,1.28) | 0.97 (0.73,1.31) | 0.86 (0.64,1.16) |
| Academic hospital | 1.97 (1.31,2.96) | 2.32 (1.51,3.57) | 1.74 (1.26,2.40) | 1.03 (0.81,1.30) | 1.36 (1.00,1.84) | 1.34 (0.98,1.84) |
| ICU admission year | ||||||
| 2001 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2002 | 0.88 (0.81,0.95) | 0.89 (0.80,0.98) | 1.02 (0.90,1.16) | 1.07 (1.00,1.15) | 1.04 (0.95,1.14) | 1.05 (0.93,1.19) |
| 2003 | 0.80 (0.74,0.86) | 0.72 (0.65,0.80) | 0.95 (0.84,1.08) | 0.96 (0.90,1.03) | 0.98 (0.89,1.09) | 1.14 (1.00,1.30) |
| 2004 | 0.61 (0.56,0.66) | 0.60 (0.53,0.67) | 0.83 (0.72,0.94) | 0.88 (0.81,0.94) | 0.85 (0.77,0.95) | 1.05 (0.92,1.20) |
| 2005 | 0.61 (0.56,0.66) | 0.61 (0.54,0.69) | 0.82 (0.71,0.94) | 0.98 (0.91,1.06) | 1.02 (0.92,1.14) | 1.16 (1.01,1.33) |
| 2006 | 0.64 (0.58,0.69) | 0.62 (0.55,0.70) | 0.94 (0.81,1.08) | 1.05 (0.97,1.14) | 1.18 (1.05,1.32) | 1.22 (1.06,1.41) |
| 2007 | 0.63 (0.58,0.69) | 0.69 (0.61,0.78) | 1.05 (0.91,1.21) | 1.07 (0.98,1.16) | 1.34 (1.19,1.51) | 1.09 (0.94,1.26) |
| 2008 | 0.62 (0.56,0.68) | 0.82 (0.71,0.93) | 0.88 (0.75,1.03) | 1.11 (1.02,1.21) | 1.38 (1.21,1.58) | 1.15 (0.97,1.35) |
| Adjusted MOR | 2.56 (2.31,2.87) | 2.64 (2.37,2.99) | 1.94 (1.78,2.13) | 1.69 (1.59,1.81) | 1.93 (1.78,2.11) | 1.90 (1.75,2.09) |
DNR = do-not-resuscitate order; ICU = intensive care unit; MICU = medical ICU; MOR = median odds-ratio; OpRm/PACU = operating room/postanesthesia care unit; SICU = surgical ICU.
MPM0-III is the mortality probability model at ICU admission.
By definition for Project IMPACT, ‘sepsis’ means that a “patient is septic with or without significantly low blood pressure” and he/she “may or may not have positive blood cultures upon ICU admission.”
Vasopressors include infusions of dopamine, epinephrine, norepineprhine, phenylephrine, and/or vasopressin.
MICU and combined units can include coronary care.
CVCs were also more common in the SICU population (46.9% of patients as compared with 32.5% (MICU) and 36.3% (combined), P < 0.001, fig. 2C). After multivariate adjustment this difference was not statistically significant (table 3). Nearly two-thirds of CVCs were placed prior to ICU arrival for patients in the SICU, while for MICU and combined unit patients, the placement of catheters was more evenly divided between pre-ICU and in-ICU locations (fig. 2C and D). Compared with ACs, for the entire cohort and in each patient subgroup analyzed, there was less variability in CVC use amongst individual ICUs (fig. 3, table 3, and appendix 5).
Discussion
The use of intravascular catheters in the United States varies significantly across individual ICUs, with greater variability associated with the use of ACs than with CVCs. Remarkably, the median odds of receiving an AC was more than twice as high if the same patient received mechanical ventilation or vasopressors in a “high catheter using ICU” as opposed to a “low catheter using ICU”. These findings suggest that practice patterns—rather than patient factors—often determine whether or not a patient undergoes this procedure. This practice pattern variation persisted even among the patients with the lowest predicted mortality (AMOR 1.94).
Patients in surgical units receive both ACs and CVCs more frequently than patients admitted to medical units and the timing of insertion of these catheters is different—patients in surgical units more commonly have catheters placed prior to ICU arrival whereas patients in medical units are more likely to have their ACs/CVCs placed once in the ICU. These findings are consistent with the trends seen in past studies on PAC use.33–35 While specific differences in patient casemix may justify this difference in use, it may also be a consequence of clinician experience and comfort. In our cohort, a significant proportion of patients admitted to the SICU arrive with an AC (44.0%) and/or a CVC (30.0%) already in place. There are studies that report on the discordance of noninvasive blood pressure and intra-arterial measurements intraoperatively;36,37 however, the practice patterns and clinical implications of intravascular catheter use in the operating room setting are unknown. Regardless, healthcare providers in surgical ICUs might be either more familiar with managing patients using such devices and/or slow to remove catheters others have deemed necessary. Given this disparate use, potential future studies of the impact of these devices on patient outcomes should be stratified by unit type and generalization to other unit types may not be appropriate.
Our data reveal a wider variation in the rates of use for AC catheters across individual units than for CVCs, even within fairly homogeneous subgroups of patients. While some variation can likely be attributed to unmeasured differences in patient casemix, it is more likely this variability stems from differences in practice patterns and culture within each unit. We know that these devices are not without risk; the complication of bloodstream infections is associated with an increased mortality, cost, and length of stay.38–40 Moreover, whether the routine use of these catheters confers quantifiable meaningful benefits to the care of critically ill patients is not known; thus, the optimal rate of use of these catheters is unclear. However, with this degree of disparity, all providers cannot be operating optimally;32,41,42 some clinicians likely use these catheters more often than is necessary while others may not use them frequently enough.
The lack of change in adjusted CVC use and the transient (early) nature of the decrease in adjusted AC use over time stands in contrast to the steady reduction in PAC use over the past two decades.34,43 This lack of persistent decline in adjusted AC/CVC use over time may be due to a recent focus on two very important paradigms of thinking in critical care. The first is a heavy reliance on standardized/protocolized care.44,45 To this end, bundles of care (e.g., from the Surviving Sepsis Campaign) have been promoted which call for early and frequent use of ACs and CVCs, although those components of the bundles have not been separately assessed.46–48 Similarly, recent guidelines to care for cardiogenic shock following myocardial infarction have encouraged intravascular catheterization.49,50 Coincident with this focus on protocols of care—which may push clinicians to use catheters more than they might otherwise—has been a move to eliminate the use of “unnecessary” intravascular devices. The Centers for Disease Control’s checklist to prevent central line associated bloodstream infections recommends, firstly, that clinicians should “perform daily audits to assess whether each central line is still needed.”51 These two competing movements—on the one hand to utilize catheters more quickly and more often in certain situations and, on the other, to seriously contemplate their prompt removal—may have offset one another and led to a fairly constant rate of AC and CVC use in U.S. ICUs.
This study is limited by the fact that we did not have information about why an AC or CVC was placed. Specifically, the indication (e.g., frequent phlebotomy, blood pressure monitoring) as well as the thought process by the clinician (e.g., “we should place an AC because all patients requiring vasopressors should have one”) were not known. This dearth of information rendered further study of specific findings (e.g., the relatively higher use of both ACs and CVCs in the extreme age groups) infeasible. Moreover, many of the ACs/CVCs were placed prior to ICU arrival in patients coming to the ICU from the OpRm/PACU and we did not have information about their OpRm/PACU course of events. While this information would not have changed our findings, it might have provided us with a more comprehensive explanation for the variation observed. Additionally, we were unable to confirm the exact timing of placement of the catheters beyond the basic information of whether they were placed prior to or in the ICU. Again, this information would help to further understand usage. Also, we did not have information about protocols/guidelines available or reimbursement schemes at each ICU nor information about the individual physician whose decision it was to insert an AC or a CVC; the ability to adjust for this information may have improved our understanding of residual variability. Finally, Project IMPACT is a database which consists of patients in ICUs which paid for the service; while diverse, these ICUs are not a completely representative sample of U.S. ICUs.
While invasive interventions will likely always have a place in the care of the critically ill, there are numerous examples in healthcare of movement away from the more invasive alternative when a less invasive option becomes available (e.g., the evolution of surgeries from open to laparascopic; cardiac valvular interventions from surgical to endovascular; diagnostic testing for pulmonary embolism from angiography to computed tomography scanning). Our data demonstrate that there has been no recent change in the incidence of AC and/or CVC placement in the ICU setting, but that use is disproportionately driven by care in surgical units with certain individual units being higher users. As technology evolves to allow for potential replacement of these invasive interventions and/or new studies reveal information about their impact on clinically meaningful outcomes, it will be imperative to target efforts to standardize use.
Supplementary Material
Acknowledgments
Funding support: Dr. Wunsch – K08AG038477 from the National Institute on Aging (Bethesda, Maryland); Dr. Scales — New Investigator Award from the Canadian Institutes for Health Research (Ottawa, Ontario, Canada) and a Fellowship in Translational Health Research from the Physicians’ Services Incorporated Foundation (Toronto, Ontario, Canada).
Footnotes
This work was performed at Beth Israel Medical Center in the Division of Pulmonary, Critical Care, and Sleep Medicine within the Department of Medicine.
The authors declare no competing interests.
References
- 1.Manios E, Vemmos K, Tsivgoulis G, Barlas G, Koroboki E, Eleni K, Spengos K, Zakopoulos N. Comparison of noninvasive oscillometric and intra-arterial blood pressure measurements in hyperacute stroke. Blood Press Monit. 2007;12:149–56. doi: 10.1097/MBP.0b013e3280b083e2. [DOI] [PubMed] [Google Scholar]
- 2.Bur A, Herkner H, Vlcek M, Woisetschläger C, Derhaschnig U, Delle Karth G, Laggner AN, Hirschl MM. Factors influencing the accuracy of oscillometric blood pressure measurement in critically ill patients. Crit Care Med. 2003;31:793–9. doi: 10.1097/01.CCM.0000053650.12025.1A. [DOI] [PubMed] [Google Scholar]
- 3.Bur A, Hirschl MM, Herkner H, Oschatz E, Kofler J, Woisetschläger C, Laggner AN. Accuracy of oscillometric blood pressure measurement according to the relation between cuff size and upper-arm circumference in critically ill patients. Crit Care Med. 2000;28:371–6. doi: 10.1097/00003246-200002000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Lakhal K, Macq C, Ehrmann S, Boulain T, Capdevila X. Noninvasive monitoring of blood pressure in the critically ill: Reliability according to the cuff site (arm, thigh, or ankle) Crit Care Med. 2012;40:1207–13. doi: 10.1097/CCM.0b013e31823dae42. [DOI] [PubMed] [Google Scholar]
- 5.Kahn JM, Kress JP, Hall JB. Skin necrosis after extravasation of low-dose vasopressin administered for septic shock. Crit Care Med. 2002;30:1899–901. doi: 10.1097/00003246-200208000-00038. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JR, Johnston GW. Development of cutaneous gangrene during continuous peripheral infusion of vasopressin. Br Med J (Clin Res Ed) 1983;287:1657–8. doi: 10.1136/bmj.287.6406.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wormser GP, Kornblee LV, Gottfried EB. Cutaneous necrosis following peripheral intravenous vasopressin therapy. Cutis. 1982;29:249–52. [PubMed] [Google Scholar]
- 8.Zingg W, Sandoz L, Inan C, Cartier V, Clergue F, Pittet D, Walder B. Hospital-wide survey of the use of central venous catheters. J Hosp Infect. 2011;77:304–8. doi: 10.1016/j.jhin.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Bloche MG. Beyond the “R word”? Medicine’s new frugality. N Engl J Med. 2012;366:1951–3. doi: 10.1056/NEJMp1203521. [DOI] [PubMed] [Google Scholar]
- 10.Esteve F, Pujol M, Pérez XL, Ariza J, Gudiol F, Limón E, Verdaguer R, Mañez R. Bacteremia related with arterial catheter in critically ill patients. J Infect. 2011;63:139–43. doi: 10.1016/j.jinf.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 11.Lucet JC, Bouadma L, Zahar JR, Schwebel C, Geffroy A, Pease S, Herault MC, Haouache H, Adrie C, Thuong M, Français A, Garrouste-Orgeas M, Timsit JF. Infectious risk associated with arterial catheters compared with central venous catheters. Crit Care Med. 2010;38:1030–5. doi: 10.1097/CCM.0b013e3181d4502e. [DOI] [PubMed] [Google Scholar]
- 12.Gowardman JR, Lipman J, Rickard CM. Assessment of peripheral arterial catheters as a source of sepsis in the critically ill: A narrative review. J Hosp Infect. 2010;75:12–8. doi: 10.1016/j.jhin.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Koh DB, Gowardman JR, Rickard CM, Robertson IK, Brown A. Prospective study of peripheral arterial catheter infection and comparison with concurrently sited central venous catheters. Crit Care Med. 2008;36:397–402. doi: 10.1097/CCM.0b013e318161f74b. [DOI] [PubMed] [Google Scholar]
- 14.Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: A systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81:1159–71. doi: 10.4065/81.9.1159. [DOI] [PubMed] [Google Scholar]
- 15.Traoré O, Liotier J, Souweine B. Prospective study of arterial and central venous catheter colonization and of arterial- and central venous catheter-related bacteremia in intensive care units. Crit Care Med. 2005;33:1276–80. doi: 10.1097/01.ccm.0000166350.90812.d4. [DOI] [PubMed] [Google Scholar]
- 16.Wilson TJ, Stetler WR, Fletcher JJ. Comparison of catheter-related large vein thrombosis in centrally inserted versus peripherally inserted central venous lines in the neurological intensive care unit. Clin Neurol Neurosurg. 2013;115:879–82. doi: 10.1016/j.clineuro.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 17.Gaertner WB, Santilli SM, Reil TD. Radial artery pseudoaneurysm in the intensive care unit. Ann Vasc Surg. 2010;24:554.e13–6. doi: 10.1016/j.avsg.2009.07.039. [DOI] [PubMed] [Google Scholar]
- 18.Nazeri A, Sohawon S, Papadopoulou B, Georgala A, Dernier Y, Noordally SO. A late complication of percutaneous radial artery cannulation. Acta Clin Belg. 2011;66:223–5. doi: 10.2143/ACB.66.3.2062552. [DOI] [PubMed] [Google Scholar]
- 19.Valentine RJ, Modrall JG, Clagett GP. Hand ischemia after radial artery cannulation. J Am Coll Surg. 2005;201:18–22. doi: 10.1016/j.jamcollsurg.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Garland A, Connors AF. Indwelling arterial catheters in the intensive care unit: Necessary and beneficial, or a harmful crutch? Am J Respir Crit Care Med. 2010;182:133–4. doi: 10.1164/rccm.201003-0410ED. [DOI] [PubMed] [Google Scholar]
- 21.Angus DC, Shorr AF, White A, Dremsizov TT, Schmitz RJ, Kelley MA Committtee on Manpower for Pulmonary and Critical Care Societies. Critical care delivery in the United States: Distribution of services and compliance with Leapfrog recommendations. Crit Care Med. 2006;34:1016–24. doi: 10.1097/01.CCM.0000206105.05626.15. [DOI] [PubMed] [Google Scholar]
- 22.McIntyre LA, Hébert PC, Fergusson D, Cook DJ, Aziz A Canadian Critical Care Trials Group. A survey of Canadian intensivists’ resuscitation practices in early septic shock. Crit Care. 2007;11:R74. doi: 10.1186/cc5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyon SM, Benson NM, Cooke CR, Iwashyna TJ, Ratcliffe SJ, Kahn JM. The effect of insurance status on mortality and procedural use in critically ill patients. Am J Respir Crit Care Med. 2011;184:809–15. doi: 10.1164/rccm.201101-0089OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burgmann H, Hiesmayr JM, Savey A, Bauer P, Metnitz B, Metnitz PG. Impact of nosocomial infections on clinical outcome and resource consumption in critically ill patients. Intensive Care Med. 2010;36:1597–601. doi: 10.1007/s00134-010-1941-2. [DOI] [PubMed] [Google Scholar]
- 25.Cook S, Visscher W, Hobbs C, Williams R. Project IMPACT: Results from a pilot validity study of a new observational database. Crit Care Med. 2002;30:2765–70. doi: 10.1097/00003246-200212000-00024. [DOI] [PubMed] [Google Scholar]
- 26.Higgins T, Teres D, Copes W, Nathanson B, Stark M, Kramer A. Assessing contemporary intensive care unit outcome: An updated Mortality Probability Admission Model (MPM0-III) Crit Care Med. 2007;35:827–35. doi: 10.1097/01.CCM.0000257337.63529.9F. [DOI] [PubMed] [Google Scholar]
- 27.Project IMPACT Participation Manual, Project IMPACT. 2003. Data Collection, Therapies & Drugs; pp. 77–96. [Google Scholar]
- 28.Larsen K, Merlo J. Appropriate assessment of neighborhood effects on individual health: Integrating random and fixed effects in multilevel logistic regression. Am J Epidemiol. 2005;161:81–8. doi: 10.1093/aje/kwi017. [DOI] [PubMed] [Google Scholar]
- 29.Merlo J, Chaix B, Ohlsson H, Beckman A, Johnell K, Hjerpe P, Råstam L, Larsen K. A brief conceptual tutorial of multilevel analysis in social epidemiology: Using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006;60:290–7. doi: 10.1136/jech.2004.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wijeysundera DN, Austin PC, Beattie WS, Hux JE, Laupacis A. Variation in the practice of preoperative medical consultation for major elective noncardiac surgery: A population-based study. Anesthesiology. 2012;116:25–34. doi: 10.1097/ALN.0b013e31823cfc03. [DOI] [PubMed] [Google Scholar]
- 31.Harrell F. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer; 2001. [Google Scholar]
- 32.Chen LM, Render M, Sales A, Kennedy EH, Wiitala W, Hofer TP. Intensive care unit admitting patterns in the veterans affairs health care system. Arch Intern Med. 2012;172:1220–6. doi: 10.1001/archinternmed.2012.2606. [DOI] [PubMed] [Google Scholar]
- 33.Rapoport J, Teres D, Steingrub J, Higgins T, McGee W, Lemeshow S. Patient characteristics and ICU organizational factors that influence frequency of pulmonary artery catheterization. JAMA. 2000;283:2559–67. doi: 10.1001/jama.283.19.2559. [DOI] [PubMed] [Google Scholar]
- 34.Koo KK, Sun JC, Zhou Q, Guyatt G, Cook DJ, Walter SD, Meade MO. Pulmonary artery catheters: Evolving rates and reasons for use. Crit Care Med. 2011;39:1613–8. doi: 10.1097/CCM.0b013e318218a045. [DOI] [PubMed] [Google Scholar]
- 35.Wiener RS, Welch HG. Trends in the use of the pulmonary artery catheter in the United States, 1993–2004. JAMA. 2007;298:423–9. doi: 10.1001/jama.298.4.423. [DOI] [PubMed] [Google Scholar]
- 36.Collins VJ, Magora F. Sphygmomanometry: The indirect measurement of blood pressure. A review with recommendations for the operating room. Anesth Analg. 1963;42:443–52. [PubMed] [Google Scholar]
- 37.Tao G, Chen Y, Wen C, Bi M. Statistical analysis of blood pressure measurement errors by oscillometry during surgical operations. Blood Press Monit. 2011;16:285–90. doi: 10.1097/MBP.0b013e32834dc5bc. [DOI] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention (CDC) Vital signs: Central line-associated blood stream infections--United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011;60:243–8. [PubMed] [Google Scholar]
- 39.Laupland KB, Lee H, Gregson DB, Manns BJ. Cost of intensive care unit-acquired bloodstream infections. J Hosp Infect. 2006;63:124–32. doi: 10.1016/j.jhin.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 40.Garrouste-Orgeas M, Timsit JF, Tafflet M, Misset B, Zahar JR, Soufir L, Lazard T, Jamali S, Mourvillier B, Cohen Y, De Lassence A, Azoulay E, Cheval C, Descorps-Declere A, Adrie C, Costa de Beauregard MA, Carlet J OUTCOMEREA Study Group. Excess risk of death from intensive care unit-acquired nosocomial bloodstream infections: A reappraisal. Clin Infect Dis. 2006;42:1118–26. doi: 10.1086/500318. [DOI] [PubMed] [Google Scholar]
- 41.Seymour CW, Iwashyna TJ, Ehlenbach WJ, Wunsch H, Cooke CR. Hospital-level variation in the use of intensive care. Health Serv Res. 2012;47:2060–80. doi: 10.1111/j.1475-6773.2012.01402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gershengorn HB, Iwashyna TJ, Cooke CR, Scales DC, Kahn JM, Wunsch H. Variation in use of intensive care for adults with diabetic ketoacidosis*. Crit Care Med. 2012;40:2009–15. doi: 10.1097/CCM.0b013e31824e9eae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiener RS, Welch HG. Trends in the use of the pulmonary artery catheter in the United States, 1993–2004. JAMA. 2007;298:423–9. doi: 10.1001/jama.298.4.423. [DOI] [PubMed] [Google Scholar]
- 44.Wall RJ, Dittus RS, Ely EW. Protocol-driven care in the intensive care unit: A tool for quality. Crit Care. 2001;5:283–5. doi: 10.1186/cc1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morris AH. Treatment algorithms and protocolized care. Curr Opin Crit Care. 2003;9:236–40. doi: 10.1097/00075198-200306000-00012. [DOI] [PubMed] [Google Scholar]
- 46.Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche J, Keh D, Marshall JC, Parker MM, Ramsay G, Zimmerman JL, Vincent JL, Levy MM Surviving Sepsis Campaign Management Guidelines Committee. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858–73. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- 47.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL for the International Surviving Sepsis Campaign Guidelines Committee. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 48.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R Surviving Sepsis Campaign Guideline Committee including the Pediatric Subrgoup. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 49.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC, Alpert JS, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK American College of Cardiology, American Heart Association Task Force on Practice Guidelines, Canadian Cardiovascular Society. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction) Circulation. 2004;110:e82–292. [PubMed] [Google Scholar]
- 50.Kushner FG, Hand M, Smith SC, King SB, Anderson JL, Antman EM, Bailey SR, Bates ER, Blankenship JC, Casey DE, Green LA, Hochman JS, Jacobs AK, Krumholz HM, Morrison DA, Ornato JP, Pearle DL, Peterson ED, Sloan MA, Whitlow PL, Williams DO. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205–41. doi: 10.1016/j.jacc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 51.Centers for Disease Control. Checklist for Prevention of Central Line Associated Bloodstream Infections. 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.