Abstract
Breast cancer cells are known to overexpress Glut5, a sugar transporter responsible for the transfer of fructose across the cell membrane. Since Glut5 transporter is not significantly expressed in normal breast cells, fructose uptake can potentially be used to differentiate between normal and cancerous cells. Fructose was labeled with two fluorophores at the C-1 position: 7-nitro-1,2,3-benzadiazole (NBD) and Cy5.5. The labeling site was chosen on the basis of the presence and substrate specificity of the key proteins involved in the first steps of fructose metabolism. Using fluorescence microscopy, the uptake of the probes was studied in three breast cancer cell lines: MCF 7, MDA-MB-435, and MDA-MB-231. Both fluorescent fructose derivatives showed a very good uptake in all tested cell lines. The level of uptake was comparable to that of the corresponding glucose analogs, 2-NBDG and Cy5.5-DG. Significant uptake of 1-NBDF derivative was not observed in cells lacking Glut5 transporter, while the uptake of the 1-Cy5.5-DF derivative was independent of the presence of a fructose-specific transporter. While 1-NBDF showed Glut5-specific accumulation, the coupling of a large fluorophore such as Cy5.5 likely introduces big structural and electronic changes, leading to a fructose derivative that does not accurately describe the uptake of fructose in cells.
INTRODUCTION
With an estimated 211 240 diagnosed cases and 40 870 deaths in 2005, breast cancer represents the second leading cause of cancer deaths and one of the most frequently diagnosed malignancies among U.S. women (1). When it is detected early, breast cancer patients have a survival rate of 98% (1). Despite its limitations (2, 3), screening mammography is still the most widely used method for early detection. Mammography relies on anatomical differences between normal and cancerous tissues, and as such does not provide specific information about the molecular processes and changes at the cellular level. Modalities that offer functional imaging (4), such as positron emission tomography (PET1), single photon emission computed tomography (SPECT), and magnetic resonance imaging (MRI), are increasingly gaining importance in breast cancer detection (5–8). Detection using probes that differentiate between cancerous and normal breast cells at a molecular level provides specific information that can potentially be utilized for earlier detection, therapy response assessment, and overall better management of patients, ultimately leading to a better survival rate.
The most common, best-studied example of imaging molecular processes at a cellular level is the use of the glucose analog, 2-[18F] fluoro-2-deoxy glucose (FDG) with PET (9). Most cancers overexpress Glut1, a high-affinity glucose transporter, and therefore have an increased capacity for glucose uptake when compared to normal cells (10, 11). Once inside the cell, FDG undergoes the first step of metabolism, phosphorylation by hexokinase. Phosphorylated FDG cannot be further metabolized because it is not a substrate for aldolase, an enzyme involved in the second step of metabolism, nor can it leave the cell due to the charged phosphate. The accumulation of FDG thus describes specific molecular processes: transport by Glut1 and phosphorylation with hexokinase.
Glut transporters are transmembrane proteins responsible for transporting sugars such as glucose, fructose, and galactose through the cell membrane. To date, 13 members of the Glut family have been identified, all having a similar sequence but different tissue expression and substrate specificity (11, 12). To satisfy high-energy needs associated with increased proliferation, cancers commonly overexpress one or more Glut transporters. Breast cancer cells are found to have high levels of Glut5, a fructose-specific transporter, not significantly expressed in normal breast cells (13). Fructose can be transported by another member of the Glut family, Glut2, a low-affinity transporter that can also transport glucose. Glut2 is not found to be differentially expressed in normal and breast tumor cells (13) and as such does not likely represent a relevant target for detecting molecular changes in breast cancer cells. A recent study (14) reported 85% of the 33 tested breast cancer tissues expressing Glut 5. The same study done on 215 different tumor samples has found that, besides the ubiquitous glucose transporter, Glut1, the most commonly expressed transporters in cancer tissues are Glut2 and Glut5, both fructose transporters, suggesting the importance of fructose metabolism not only in breast but also in other cancers.
Molecules tagged with fluorescent labels have found a broad application in imaging as a cheaper and safer alternative to radioactive probes (15). Although poor light penetration through tissues makes their utilization in humans difficult, they are successfully applied in preclinical, small animal research (15–18). For the purpose of developing more specific imaging probes for breast cancer, we have synthesized fructose-based optical probes using two fluorophores as labels: 7-nitro-1,2,3-benzoxadiazole, NBD, and cyanine dye, Cy5.5. NBD fluorophore has been used to label glucose at two positions, C-2 and C-6. Fluorescent glucose analog, 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-d-glucose (2-NBDG) was developed for the viability assessment of E. coli (19). It has already been shown that it is transported through the cell membrane using the same mechanism as glucose, and that it is a substrate for hexokinase (19–21). Although transported by the same mechanism, 6-NBDG has a fluorophore at the phosphorylation site and is, therefore, not a substrate for hexokinase (22). Cy5.5 dye has better spectral characteristics than NBD, emitting light in the NIR region where there is minimal spectral interference from biological tissues (23). In preclinical settings, Cy5.5-labeled tracers have found broad application in tumor detection and drug treatment assessment (24–27).
In order to choose the labeling position that would allow transport and phosphorylation by the same proteins used by fructose, we looked into substrate specificity of the Glut5 transporter and kinases that can phosphorylate fructose. Fructose can be phosphorylated by two enzymes: hexokinase (HK) and fructokinase (ketohexokinase, KHK). Hexokinase phosphorylates fructose at the C-6 position, while fructokinase phosphorylates it at the C-1 position (Scheme 1). Ideally, we would like to label fructose in such a way to keep both C-1 and C-6 positions available for whichever of the two enzymes might be involved in phosphorylation. However, studies on substrate specificity of Glut5 show bulky groups being tolerated only at C-1 and C-6 positions (28, 29). Furthermore, for fructokinase, C-2, C-3, and C-4 seem to be important for binding and enzyme activity, while groups at C-6 are well-tolerated (30, 31). Similarly, hexokinase allows modification of structure at the C-1 position, the site furthest away from the site of phosphorylation, while hydroxyls at C-3 and C-4 are needed for the productive binding to the enzyme and the subsequent turnover (32). Because derivatization at either the C-1 or C-6 position allows phosphorylation by only one of the kinases but prevents enzyme activity of the other, we first wanted to determine the metabolic pathway that fructose takes once inside the cell by looking into the presence and the levels of the two kinases in the breast cancer cell lines. On the basis of the results of the Western analysis and considering substrate specificity of proteins involved in the first steps of fructose metabolism, we chose to label fructose in the C-1 position. Fructose has been labeled at the C-1 position with 18F and evaluated as a PET imaging agent for fibrosarcoma (33). Possibly due to the use of fibrosarcoma as a tumor model, for which levels of Glut5 have not been determined, 1-deoxy-1-[18F]fluoro-d-fructose did not show good imaging potential.
Scheme 1. Transport and Phosphorylation of Fructosea.
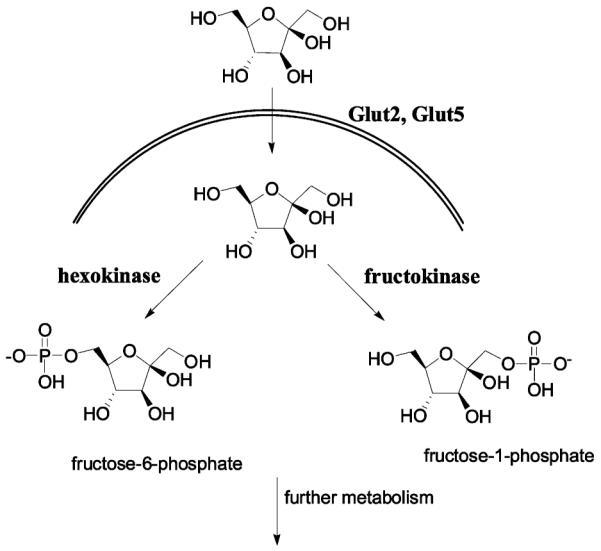
a Fructose is transported by Glut2 and Glut65 transporters across the cell membrane. While Glut2 can transport both glucose and fructose, Glut5 is a fructose-specific transporter. Fructose can be phosphorylated by two enzymes: hexokinase that phopsphorylates fructose in C-6 position and fructokinase (ketohexokinase) that phosphorylates fructose in C-6 position.
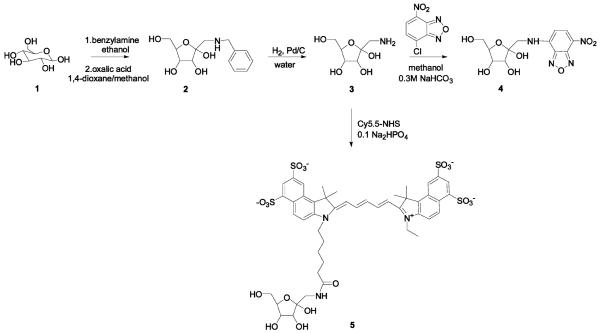
Here, we wish to present the syntheses of two fluorescent fructose derivatives, 1-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-amino]-1-deoxy-d-fructose (1-NBDF) and 1-Cy5.5-1-deoxy-d-fructose (1-Cy5.5-DF), and evaluation of their possible application as breast cancer imaging probes.
MATERIALS AND METHODS
General
Ketohexokinase (fructokinase) rabbit polyclonal antibody was purchased from Abnova corporation (Taipei, Taiwan). Hexokinase I rabbit polyclonal antibody was purchased from Abgent (San Diego, CA). Hexokinase II rabbit polyclonal antibody was purchased from Chemicon International, Inc. (Temecula, CA). Peroxidase conjugated goat anti-mouse IgG and goat anti-rabbit IgG were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). Enhanced chemiluminescence (ECL) reagents and Cy5.5 N-hydroxysuccinimide ester (Cy5.5-NHS) were purchased from Amersham Biosciences (Piscataway, NJ). 2-NBDG was obtained from Molecular Probes (Eugene, OR). 2-Cy5.5-2-deoxy-d-glucose (Cy5.5-DG) was synthesized using the procedure reported earlier (34). All other chemicals were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO) and were used without further purification. Glass-bottomed tissue culture dishes were acquired from MatTek Corporation (Ashland, MA). Purification and analysis of the products was performed using the Dionex Summit high-performance liquid chromatography (HPLC) system (Dionex Corporation, Sunnyvale, CA), equipped with a 340U four-channel UV–vis absorbance detector. For the analysis of the products, reverse-phase HPLC column Dionex Acclaim120 (C18, 4.6 mm × 250 mm) was used. Reverse-phase semipreparative HPLC column Zorbax SB (C18, 9.4 mm × 250 mm) was used for purification and isolation of products. The mobile phase was 0.1% trifluoroacetic acid (TFA) in water and 0.1% TFA in acetonitrile (CH3CN). The flow was 1 mL/min with gradient starting at 5% CH3CN and ending at 80% CH3CN at 42 min. UV wavelengths used for detection of NBD-labeled fructose were 218 and 475 nm. For Cy5.5-labeled fructose, detection wavelengths were 218, 280, and 590 nm. Electron spray ionization (ESI) mass spectrometry was done by Vincent Coates Foundation Mass Spectrometry Laboratory, Stanford University. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) was performed by the Protein and Nucleic Acid Biotechnology Facility, Stanford University. All NMR spectra were obtained on a Varian XL-400 (Varian, Palo Alto, CA) at 400 MHz 1H (100 MHz 13C) .
Cell Lines
Human breast cancer cell lines MCF-7, MDA-MB-435, MDA-MB-231 and human hepatocellular carcinoma cells, HepG2, were obtained from American Type Culture Collection (Manassas, VA). MCF-7 cells were grown in minimum essential medium (Eagle) (MEM, Invitrogen, Carlsbad, CA) supplemented with 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 1.5 g/L sodium bicarbonate, 0.01 mg/mL bovine insulin, 10% fetal bovine serum (FBS), and 1% penicillin–streptomycin. MDA-MB-435 cells were cultured in Leibovitz’s L-15 medium (Invitrogen, Carlsbad, CA) supplemented with 0.01 mg/mL insulin, 10% FBS, and 1% penicillin–streptomycin. MDA-MB-231 cells were cultured in Dulbecco’s modified Eagle high glucose medium (DMEM, Invitrogen, Carlsbad, CA) supplemented with 10% FBS and 1% penicillin–streptomycin. HepG2 cells were grown in MEM supplemented with 10% FBS and 1% penicillin-streptomycin.
Ketohexokinase and Hexokinase Western Analysis
Cells were washed with ice-cold phosphate buffered saline (PBS) once and lysed in 1× passive lysis buffer (Promega, Madison, WI) for 10 min on ice. After centrifugation at 13 500 rpm for 15 min at 4 °C, protein content was determined using Bradford assay (Biorad, Hercules, CA). 75 μg of total protein were resolved using the 4–12% NU-PAGE gradient gel (Invitrogen, Carlsbad, CA) and electroblotted onto nitrocellulose membrane (Schleicher & Schuell, Keene, NH). The membrane was first incubated with blocking buffer (5% nonfat dry milk in Tris-buffered saline containing 0.01% Tween 20 (TBST)) for 1 h, and then overnight at 4 °C with an appropriate primary antibody diluted in blocking buffer according to manufacturer’s recommendations. The membrane was washed three times with TBST and incubated with appropriate secondary antibodies: peroxidase-conjugated anti-mouse IgG (1:3000 dilution in the blocking buffer) or peroxidase-conjugated anti-rabbit IgG (1:3000 dilution in the blocking buffer) for 1 h. As a loading control, the membrane was incubated with the monoclonal anti-human α-tubulin antibody (1:5000 dilution in the blocking buffer). Enhanced chemiluminescent method was used for visualization of protein bands.
The extraction of mouse liver was performed according to a protocol approved by the Stanford University Administrative Panels on Laboratory Animal Care (A-PLAC). Normal mouse liver was harvested and immediately frozen in dry ice. SDS lysis buffer (50 mM Tris pH 7.4, 2% SDS) containing Complete Mini protease inhibitor (Roche, Palo Alto, CA) was used to lyse the liver. After freezing at −80 °C and thawing at 37 °C three times, liver lysate was centrifuged, and supernatant used for protein detection as described above.
Chemical Syntheses
1-(Benzylamino)-1-deoxy-d-fructose (2)
1-(Benzylamino)-1-deoxy-d-fructose was synthesized following the published procedure (35). Our synthesis starts with d-glucose instead of the d-mannose used in the paper. Briefly, d-glucose (1.8 g, 10 mmol, 1 equiv) was reacted with benzylamine (1.1 mL, 10 mmol, 1 equiv) in boiling absolute ethanol for 5 min. After cooling, the solid was collected and used in the next step without further purification. N-Benzyl-β-glucopyranosylamine (1 g, 3.7 mmol, 1 equiv) was heated at 80 °C in the 10:3 mixture of 1,4-dioxane and methanol, in the presence of 0.5 g (5.5 mmol, 1.5 equiv) of oxalic acid for 10 min. The product was collected and recrystallized from isopropanol. 1H NMR (400 MHz, D2O): 7.28 (5H, m), 4.10 (2H, s), 3.77 (2H, m), 3.66 (1H, dd, J = 3.35 Hz, J = 9.76 Hz), 3.53-3.46 (2H, m), 3.08 (2H, s). 13C NMR (100 MHz, D2O): 165.55, 130.29, 130.084, 129.88, 129.37, 95.53, 69.69, 69.34, 68.97, 63.97, 52.13, 51.72. ESI+ 270.1. The calculated molecular weight is 269.1 g/mol.
1-Amino-1-deoxy-d-fructose Oxalate Salt (3)
1-(Benzylamino)-1-deoxy-d-fructose oxalate salt (1 g) was dissolved in 30 mL water. 1 g of Pd/C (10 wt %) was added and the mixture hydrogenated overnight. The catalyst was filtered off and the solvent removed in vacuo. 1H NMR (400 MHz, D2O): 3.74 (m, 2H), 3.62 (m, 1H), 3.48 (m, 2H), 2.97 (m, 2H). 13C NMR (100 MHz, D2O): 95.45, 69.62, 69.38, 68.97, 63.91, 45.16. ESI+ 179.9. The calculated molecular weight is 179.1 g/mol.
1-NBDF
10 mg amino fructose was dissolved in 200 μL 0.3 M NaHCO3 and added to the solution of 10 mg 4-chloro-7-nitrobenzofurazan (NBD chloride) in 400 μL methanol. The mixture was stirred at room temperature for 20 h. Solvents were removed in vacuo, water (400 μL) was added, and the precipitate removed by centrifugation. The filtrate was injected onto the HPLC column. Two major isomers are observed with retention times of 11.5 min and 12.05 min. Both peaks show the same molecular ion in negative ESI 341. Isomers were combined for subsequent uptake studies.
1-Cy5.5-DF
1 mg Cy5.5-NHS ester was dissolved in 300 μL sodium phosphate buffer (pH 8.5-9). To this solution was added a solution of ~0.5 mg of 1-amino-1-deoxy-d-fructose in 100 μL phosphate buffer. The mixture was kept at room temperature for 4 h and then directly injected onto HPLC. The 1-Cy5.5-DF fraction was eluted from the column at 15.8 min. MALDI-MS analysis of the collected fraction shows a molecular ion peak of 1077.82. The calculated Mw is found to be 1078.24 g/mol.
Cell Uptake Studies
1 × 105 MCF-7, MDA-MB-435, MDA-MB-231, and HepG2 cells were plated in 35 mm MatTek glass-bottomed culture dishes 1 day ahead of the imaging study. Cells were washed once with PBS right before incubation with 10 μM probes at 37 °C or 4 °C for 15 min. For the staining of nuclei, after the incubation with 1-Cy5.5-DF, MDA-MB-231 cells were incubated with a solution of Sytox Green (Molecular Probe, Eugene, OR) in 95% ethanol for 15 min. To assess the effect of d-fructose and d-glucose on the uptake of fluorescent-labeled fructose, 1 × 105 MDA-MB-435 cells were coincubated with 10 μM labeled probe and 50 mM d-fructose or 50 mM d-glucose for 15 min at 37 °C. After the incubation, cells were washed three times with cold PBS and imaged using a microscope.
The uptake of 1-NBDF in MCF7 cells was also followed by flow cytometry. Cells (0.1 million) were incubated for 1 h with four different concentrations of 1-NBDF: 10 μM, 7 μM, 5 μM, and 3 μM. After washing two times with 0.5 mL cold PBS, the cells were suspended in 200 μL PBS and analyzed on FACS-Calibur (Becton Dickinson, Franklin Lakes, NJ) using FL-2 channel. The samples were done in triplicate.
Fluorescence Microscopy Studies
Cells were imaged using an Axiovert 200M fluorescence microscope (Carl Zeiss Micro-Imaging, Inc., Thornwood, NY). To image 1-NBDF in cells and Sytox Green stained nuclei, the eGFP filter set (excitation 450/490 nm, emission 515/565 nm) was used, while for Cy5.5-labeled fructose, the fluorescent signal was recorded using the Cy5 filter set (exciter 640/30 nm; emitter 700/50 nm). An AttoArc HBO 100 W microscopic illuminator was used as a light source. Images were recorded using a thermoelectrically cooled charged-coupled device (CCD) (Micromax, model RTE/CCD-576, Princeton Instruments, Inc., Trenton, NJ) and analyzed using MetaMorph software version 6.2r4 (Molecular Devices Corporation, Downingtown, PA).
RESULTS AND DISCUSSION
Fructose can be metabolized via two pathways, one that involves phosphorylation at the C-6 position by hexokinase and the other that starts with phosphorylation at C-1 by fructokinase. Since substrate specificity studies of the proteins involved in transport and phosphorylation point to substitutions at C-1 and C-6 positions as the only ones permissible, the fluorophore labeling site had to be chosen on the basis of the presence and levels of the two kinases that can phosphorylate fructose. That is why we first investigated the expression of hexokinase and fructokinase in the breast cancer cell lines. There are four hexokinase isoforms in mammalian cells. Of those four, two isoforms, hexokinase I and II, are found overexpressed in many cancer cells, hexokinase II being recognized as the key isoform associated with high glycolytic rates (36). Among the three breast cancer cell lines studied, MCF7 cell line shows the highest levels of both hexokinase I and II (Figure 1), whereas the other two cell lines, MDA-MB-435 and MDA-MB-231, show considerably lower expression. While Western analysis shows the presence of fructokinase in the liver, the main organ of fructose metabolism, no fructokinase is detected in any of the breast cancer cell lines tested (Figure 1). This suggests that, because only hexokinase is detected in these breast cancer cells, fructose, once inside the cell, is phosphorylated in the C-6 position. In view of these results, and considering Glut5 and hexokinase substrate specificity, only fructose labeled in the C-1 position should be considered a possible imaging probe–it is expected to be transported by the Glut 5 transporter and trapped in the cells by hexokinase.
Figure 1.
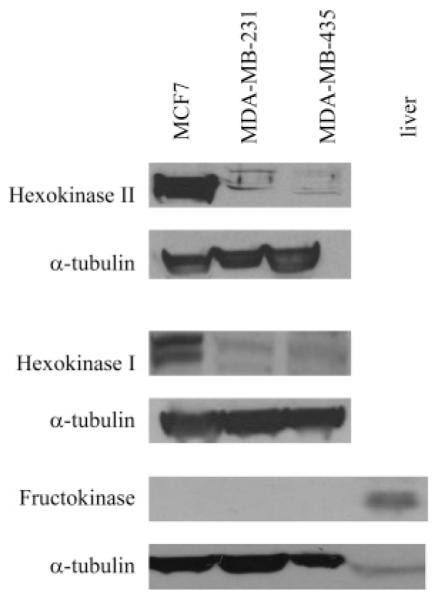
Western blot analysis of hexokinase and fructokinase levels in breast cancer cells and liver. The highest levels of both hexokinase I and II are found in MCF7 cells. Fructokinase was not observed in any of the breast cancer cell lines tested.
Two fluorescent labels were conjugated to fructose in the C-1 position: NBD and Cy5.5. The syntheses of the fluorescent fructose derivatives are shown in Scheme 2. Both syntheses required 1-amino-1-deoxy-d-fructose (3), which was prepared by hydrogenation of the Amadori rearrangement product (2). 1-NBDF was synthesized by reacting 1-amino-1-deoxy-d-fructose with NBD chloride under basic conditions. The coupling reaction is not very efficient and has a typical yield of only 5%. Two peaks were observed in the HPLC chromatogram, corresponding to different isomers of 1-NBDF. For uptake studies, these isomers were combined, since the Glut5 transporter seems to be accepting all of the four possible fructose isomers, α/β fructopyranose and R/β fructofuranose (28). 1-Cy5.5-DF was synthesized by coupling 1-amino-1-deoxy-d-fructose with Cy5.5-NHS ester. Only one broad peak was observed in the HPLC chromatogram, suggesting closely running 1-Cy5.5-DF isomers.
Scheme 2. Synthesis of 1-NBDF and 1-Cy5.5-Fa.
a Note that fructose exists as a mixture of pyranose and furanose forms but is represented here in the β-furanose form for simplicity.
NBD-chloride is nonfluorescent, but when coupled to the amine group of amino fructose, the NBD group shows fluorescence with the excitation maximum at 472 nm and the emission maximum at 538 nm (Figure 2a). For 1-Cy5.5-DF, the maximum excitation wavelength was found to be 675 nm, while the maximum emission wavelength was at 695 nm (Figure 4b). Cy5.5 dye shows the same spectral characteristics as its fructose conjugate (data not shown). Since the NBD group absorbs and emits light in the region where the interference from endogenous fluorophores is expected, it is important to determine the degree of intrinsic fluorescence of the cells. The extent of autofluorescence of different cell lines was investigated by imaging cells, using a fluorescent microscope and appropriate filter sets. Autofluorescence was detected only when using eGFP filter set (λex 450/490 nm, λem 515/565 nm) (Figure 3a–d), while no significant autofluorescence was observed in the NIR region (data not shown). All imaged cell lines showed autofluorescence of very low intensity. Since the observed autofluorescence was limited to the cell membrane and was of relatively small intensity, it is not expected to present a problem in studying the uptake of the 1-NBDF in cell culture.
Figure 2.
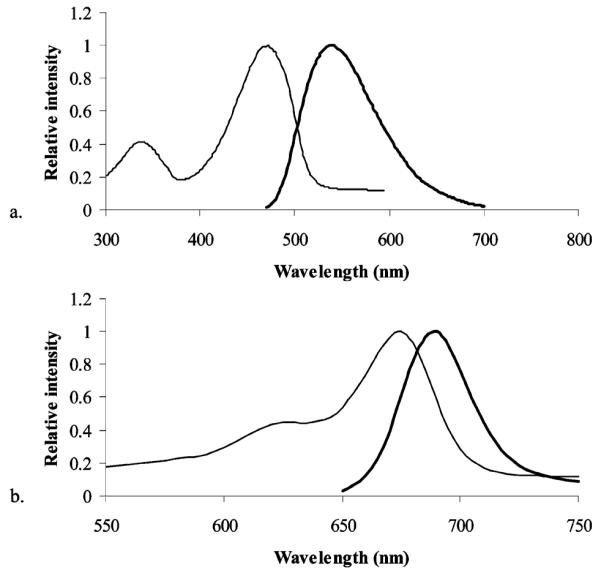
Absorbance (thin line) and fluorescence (thick line) spectra for 1-NBDF (a) and 1-Cy5.5-fructose (b).
Figure 4.
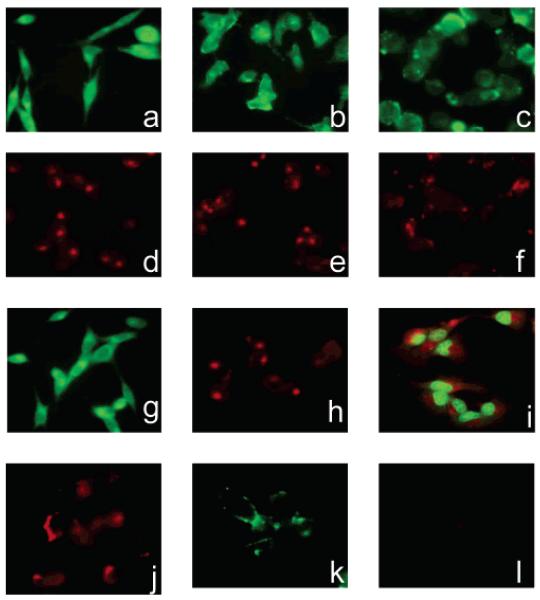
Uptake of fluorescent fructose derivatives in MDA-MB-435 (a,d), MDA-MB-231 (b,e), and MCF-7 cells (c,f). MDA-MB-435 cells incubated with 2-NBDG (g) showed comparable fluorescence intensities to cells incubated with 1-NBDF. Fluorescence of MDA-MB-435 cells incubated with Cy5.5-DG (h) was similar to the fluorescence of cells incubated with 1-Cy5.5-F. Incubation of MDA-MB-231 and MCF7 cells with Cy5.5-DG and NBDG resulted in similar fluorescence intensities as the ones observed after uptake of fluorescent fructose derivatives (data not shown). 1-Cy5.5-DF showed cytoplasmic rather than nuclear accumulation in MDA-MB-435 cells (i). Incubation with Cy5.5-NHS ester results in the staining of MDA-MB-435 cells (j). Incubation of MDA-MB-435 cells with C-1 fructose derivatives at low temperature results in minimal uptake of the probes (k,l).
Figure 3.
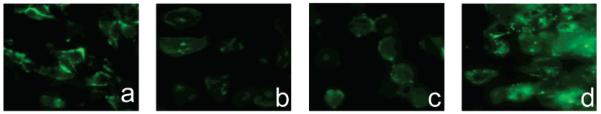
Autofluorescence of MDA-MB-435 (a), MDA-MB-231 (b), MCF7(c), and HepG2 cells (d). Cells were imaged using eGFP filter set, λex 450/490 nm, λem 515/565 nm. All cells showed autofluorescence of low intensity, mostly localized on the cell membrane.
In order to investigate the tumor targeting ability of the two fluorescent fructose derivatives in cell culture, breast cancer cell lines (MDA-MB-435, MDA-MB-231, and MCF7) were incubated with the probes at 37 °C and imaged using a fluorescent microscope. As a positive control, cells were incubated with the corresponding fluorescent glucose analogs, 2-NBDG and Cy5.5-DG. Good uptake in cancer cells for those two glucose analogs has been previously established (34, 37). Both fructose derivatives were taken up by the breast cancer cells (Figure 4a–f). The level of uptake, as judged by the fluorescence intensity, was comparable to the uptake of the positive probes, 2-NBDG and Cy5.5-DG (Figure 4 g,h). The images suggest a difference in accumulation of the two fructose derivatives. While incubation with 1-NBDF resulted in uniform cell fluorescence, incubation with 1-Cy5.5-DF led to more localized accumulation. In order to investigate localization of 1-Cy5.5-DF, after incubation with the probe, cell nuclei were stained with Sytox Green. The overlay of the images showed cytoplasmic localization of 1-Cy5.5-DF clearly separated from the nucleus (Figure 4i). For the purpose of comparison, cells were also incubated with the Cy5.5-NHS ester. As mentioned earlier, the NBD chloride is nonfluorescent, and as such, its uptake cannot be investigated using the fluorescent microscope. Incubation of cells with the Cy5.5 dye resulted in a cell-associated fluorescence signal (Figure 4j). Although the fluorescence was mostly localized on the cell surface, the uptake of the dye was also observed. Staining of the cell membrane with the Cy5.5 dye alone could be explained by the likely reaction of amine-reactive Cy5.5-NHS ester with many amino groups present on the cell surface. The observed uptake of the dye was likely due to the subsequent endocytosis. The nature of the transport mechanism was investigated by incubating cells with the probes at 4 °C. At low temperature, the uptake of the probes was not observed, indicating an energy-dependent transport (Figure 4k,l).
The uptake of 1-NBDF in MCF 7 cells was further analyzed and quantified using flow cytometry. The amount of probe accumulated clearly correlated with the concentration of the probes that the cells were exposed to (data not shown).
Seeking to explore whether fluorescent fructose probes are specific for the Glut5-expressing cells, we studied their uptake in hepatocarcinoma cells, HepG2, a cell line expressing glucose transporters Glut1–3, while no significant levels of Glut5 transporter are detected (38, 39). The uptake of fructose analogues is not expected in these cells due to the lack of transporter. No significant uptake of 1-NBDF was observed, while glucose analog, 2-NBDG, transported by glucose transporters, showed accumulation in HepG2 cells (Figure 5). These results imply that the uptake of 1-NBDF depends on the presence of the Glut5 transporter and can thus be used to distinguish between the cells expressing and the cells lacking this transporter. In contrast, 1-Cy5.5-DF derivative accumulated in HepG2 cells, showing that its uptake was not associated with the presence of the Glut 5 transporter.
Figure 5.
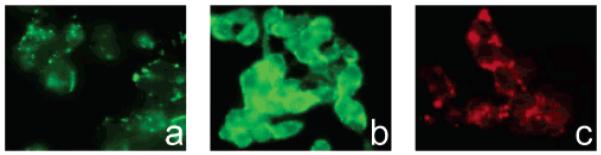
Uptake of 1-NBDF, 2-NBDG, and 1-Cy5.5-DF in HepG2 cells. HepG2 cells lacking Glut5 transporter showed fluorescence of low intensity after incubation with 1-NBDF (a), indicating the absence of the probe transport. 2-NBDG and 1-Cy5.5-F, transported through a different mechanism, were readily taken up by HepG2 cells (b,c).
To further examine the mechanism of transport, MDA-MB-435 cells were coincubated with fluorescent fructose derivatives and unlabeled d-fructose. As judged by the decrease in fluorescence intensity (Figure 6), the uptake of 1-NBDF seems to be diminished by coincubation with d-fructose. The addition of d-glucose has a similar effect, suggesting the inhibition of uptake through the Glut2 transporter, which can transfer both fructose and glucose across the cell membrane. On the other hand, the uptake of 1-Cy5.5-DF was not affected by the addition of either d-fructose or d-glucose, implying that 1-Cy5.5-DF does not use Glut transporters to enter the cell. However, the investigation of the uptake inhibition by flow cytometry failed to show decreased uptake in the cells coincubated with various concentrations of fructose and glucose. The observed decrease in cell fluorescence (Figure 6) could be due to the qualitative rather than quantitative changes that unlabeled fructose and glucose introduce. Other studies report uptake of d-[U-14C]-fructose in cells coincubated with excess cold fructose suggesting that transport is not blocked by cold substrate (13, 40). For the purpose of blocking the transport, the most suitable agents would be substances that bind to the transporter and prevent their function. Cytochalasin B is known to prevent glucose transport by binding to glucose transporters (41), but to the best of our knowledge, a molecule preventing the function of Glut5 has not been reported. Fused-ring glyco-1,3-oxazolidin-2-thiones and glyco-1,3-oxazolidin-2-ones have been reported to inhibit the Glut5 transporter, but their specificity and mechanism of action has not been fully evaluated, which limits their current use (42).
Figure 6.
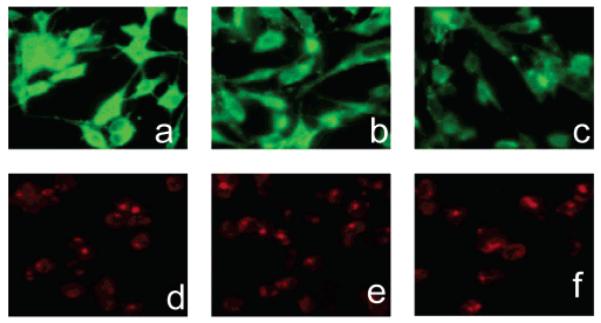
The effect of coincubation with d-fructose and d-glucose on the uptake of the probes in MDA-MB-435 cells. Addition of 50 mM d-fructose (b), as well as addition of 50 mM d-glucose (c) to the incubation medium containing 10 μm 1-NBDF, resulted in decreased fluorescence compared to the cells that have been incubated with 1-NBDF only (a). No effect on the uptake of Cy 5.5-labeled fructose was observed (d,e,f). Analysis of the inhibition by flow cytometry did not show blocking of the transport, indicating that the observed decrease in fluorescence in the cells coincubated with unlabeled fructose and glucose cannot be explained by the inhibitory effect of the sugars on the uptake of 1-NBDF, but rather by the qualitative effect that high concentration of the sugars might have on the fructose transport.
Although this study suggests uptake of 1-NBDF through Glut5, it remains to be determined whether 1-NBDF gets phosphorylated by hexokinase and at what relative rate compared to glucose. It will also be important for future studies to determine if there is efflux of the probes out of cells, since Glut5 is a bidirectional transporter. On the basis of its behavior in cell culture, it would be interesting to explore the possibility of using 1-NBDF for in vivo studies in small animals. As discussed earlier, 1-NBDF does not have optimal spectral characteristics, but using instruments with multispectral imaging techniques might minimize interference caused by autofluorescence.
In summary, two fluorescent fructose derivatives were synthesized and evaluated in cell culture for their Glut5-detecting ability in breast cancer. Both derivatives show good uptake in breast cancer cells tested. Importantly, we found that the uptake of 1-NBDF appears to correspond with the uptake of fructose in cells. The NBD group is small in size and does not seem to perturb the structure of the fructose to the point at which the labeled analogue is no longer recognized by the Glut5 transporter. In contrast, the coupling of fructose with a very bulky fluorophore such as Cy5.5 results in a derivative that shows nonspecific uptake in breast cancer cells, and as such cannot likely be used in breast cancer imaging.
ACKNOWLEDGMENT
We would like to thank the NCI ICMIC P50 CA114747 (SSG) for funding support.
Footnotes
Abbreviations: NIR, near-infrared; NBD, 7-nitro-1,2,3-benzoxadiazole; Cy5.5-DG, Cy5.5-d-glucosamine; Cy5.5-NHS, Cy5.5 N-hydroxysuccinimide ester; MRI, magnetic resonance imaging; PET, positron emission tomography; [18F]FDG, 2-deoxy-2-[18F]fluoro-d-glucose; HPLC, high-performance liquid chromatography; eGFP, enhanced green fluorescent protein.
LITERATURE CITED
- (1).American Cancer Society: Fact Sheets: Breast Cancer. American Cancer Society; Atlanta: 2005. [Google Scholar]
- (2).Buist DS, Porter PL, Lehman C, Taplin SH, White E. Factors contributing to mammography failure in women aged 40–49 years. J. Natl. Cancer Inst. 2004;96:1432–1440. doi: 10.1093/jnci/djh269. [DOI] [PubMed] [Google Scholar]
- (3).Berlin L. The missed breast cancer redux: time for educating the public about the limitations of mammography? AJR Am. J. Roentgenol. 2001;176:1131–1134. doi: 10.2214/ajr.176.5.1761131. [DOI] [PubMed] [Google Scholar]
- (4).Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Gen. DeV. 2003;17(5):545–580. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- (5).Koomen M, Pissano ED, Kuzmiak C, Pavic D, McLelland R. Future directions in breast imaging. J. Clin. Oncol. 2005;23(8):1674–1677. doi: 10.1200/JCO.2005.11.039. [DOI] [PubMed] [Google Scholar]
- (6).Lehman CD, Schnall MD. Imaging in breast cancer: magnetic resonance imaging. Breast Cancer Res. 2005;7:215–219. doi: 10.1186/bcr1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Quon A, Gambhir SS. FDG-PET and beyond: molecular breast cancer imaging. J. Clin. Oncol. 2005;23(8):1664–1673. doi: 10.1200/JCO.2005.11.024. [DOI] [PubMed] [Google Scholar]
- (8).Benard F, Turcotte E. Imaging in breast cancer: single-photon emission computed tomography and positron emission tomography. Breast Cancer Res. 2005;7:153–162. doi: 10.1186/bcr1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat. ReV. Cancer. 2002;2(9):683–693. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- (10).Younes M, Lechago LV, Somoano JR, Mosharaf M, Lechago J. Wide expression of the human erythrocyte glucose transporter Glut1 in human cancers. Cancer Res. 1996;56:1164–1167. [PubMed] [Google Scholar]
- (11).Medina RA, Owen GI. Glucose transporters: expression, regulation and cancer. Biol. Res. 2002;35:9–26. doi: 10.4067/s0716-97602002000100004. [DOI] [PubMed] [Google Scholar]
- (12).Joost HG, Thorens B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members. Mol. Membr. Biol. 2001;18(4):247–256. doi: 10.1080/09687680110090456. [DOI] [PubMed] [Google Scholar]
- (13).Zamora-León P, Golde DW, Concha II, Rivas CI, Delgado-López F, Baselga J, Nualart F, Vera JC. Expression of the fructose transporter Glut5 in human breast cancer. Proc. Natl. Acad. Sci. U.S.A. 1996;93:1847–1852. doi: 10.1073/pnas.93.5.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Godoy A, Ulloa V, Rodriguez F, Reinicke K, Yanez A, De Los Angeles, Garcia M, Medina R, Carrasco M, Barberis S, Castro T, Martinez F, Koch H, Vera JC, Poblete MT, Figureoa CD, Peruzzo B, Perez F, Nualart F. Differential subcellular distribution of glucose transporters Glut1–6, and Glut9 in human cancer: ultrastructural localization of Glut1 and Glut5 in breast tumor tissues. J. Cell. Physiol. 2006;207:614–627. doi: 10.1002/jcp.20606. [DOI] [PubMed] [Google Scholar]
- (15).Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nat. Med. 2003;2(2):123–31. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- (16).Kim S, Lim YT, Soltesz EG, De Grand AM, Lee J, Nakayama A, Parker JA, Mihaljevic T, Laurence RG, Dor DM, Cohn LH, Bawendi MG, Frangioni JV. Near infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat. Biotechnol. 2004;22:93–97. doi: 10.1038/nbt920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Graves EE, Weissleder R, Ntziachristos V. Fluorescence molecular imaging of small animal tumor model. Curr. Mol. Med. 2004;4:419–430. doi: 10.2174/1566524043360555. [DOI] [PubMed] [Google Scholar]
- (18).Choy G, Choyke P, Libutti SK. Current advances in molecular imaging: non-invasive in vivo bioluminescent and fluorescent optical imaging in cancer research optical imaging in cancer research. Mol. Imaging. 2003;2:303–312. doi: 10.1162/15353500200303142. [DOI] [PubMed] [Google Scholar]
- (19).Yoshioka K, Takahashi H, Homma T, Saito M, Oh K-B, Nemoto Y, Matsuoka H. A Novel fluorescent derivative of glucose applicable to the assessment of glucose uptake activity of Escherichia coli. Biochim. Biophys. Acta. 1996;1289:5–9. doi: 10.1016/0304-4165(95)00153-0. [DOI] [PubMed] [Google Scholar]
- (20).Yoshioka K, Oh K-B, Saito M, Nemoto Y, Matsuoka H. Evaluation of 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-amino]-2-deoxy-D-glucose, a new fluorescent derivative of glucose, for viability assessment of yeast Candida albicans. Appl. Microbiol. Biotechnol. 1996;46:400–404. doi: 10.1007/BF00166236. [DOI] [PubMed] [Google Scholar]
- (21).Yoshioka K, Saito M, Oh K-B, Nemoto Y, Matsuoka H, Natsume M, Abe H. Intracellular fate of 2-NBDG, a fluorescent probe for glucose uptake activity in Escherichia coli cells. Biosci. Biotechnol. Biochem. 1996;60:1899–1901. doi: 10.1271/bbb.60.1899. [DOI] [PubMed] [Google Scholar]
- (22).Speizer L, Haugland R, Kutchai H. Assymetric transport of a fluorescent glucose analogue by human erythrocytes. Biochim. Biophys. Acta. 1985;815:75–84. doi: 10.1016/0005-2736(85)90476-6. [DOI] [PubMed] [Google Scholar]
- (23).Richards-Kortum R, Sevick-Muraca E. Quantitative optical spectroscopy for tissue diagnostics. Annu. ReV. Phys. Chem. 1996;47:555–606. doi: 10.1146/annurev.physchem.47.1.555. [DOI] [PubMed] [Google Scholar]
- (24).Ke S, Wen X, Gurfinkel M, Charnsangavej C, Wallace S, Sevick-Muraca EM, Li C. Near-infrared optical imaging of epidermal growth factor receptor in breast cancer xenografts. Cancer Res. 2003;63:7870–7875. [PubMed] [Google Scholar]
- (25).Veiseh O, Sun C, Gunn J, Kohler N, Gabikian P, Lee D, Bhattarai N, Ellenbogen R, Sze R, Hallahan A, Olson J, Zhang M. Optical and MRI multifunctional nanoprobe for targeting gliomas. Nano Lett. 2005;5:1003–1008. doi: 10.1021/nl0502569. [DOI] [PubMed] [Google Scholar]
- (26).Pham W, Choi Y, Weissleder R, Tung C-H. Developing a peptide-based near-infrared molecular probe for protease sensing. Bioconjugate Chem. 2004;15:1403–1407. doi: 10.1021/bc049924s. [DOI] [PubMed] [Google Scholar]
- (27).Ntziachristos V, Schellenberger EA, Ripoll J, Yessayan D, Graves E, Bogdanov A, Jr., Josephson L, Weissleder R. Visualization of antitumor treatment by means of fluorescence molecular tomography with annexin V-Cy 5.5 conjugate. Proc. Natl. Acad. Sci. U.S.A. 2004;101:12294–12299. doi: 10.1073/pnas.0401137101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Tatibuet A, Yang J, Morrin C, Holman GD. Synthesis and evaluation of fructose analogs as inhibitors of the D-fructose transporter Glut5. Bioorg. Med. Chem. 2000;8:1825–1833. doi: 10.1016/s0968-0896(00)00108-5. [DOI] [PubMed] [Google Scholar]
- (29).Yang J, Dowden J, Tatibuet A, Hatanaka Y, Holman GD. Development of high-affinity ligands and photoaffinity labels for the D-fructose transporter. Biochem. J. 2002;367:533–539. doi: 10.1042/BJ20020843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Raushel FM, Cleland WW. The substrate and anomeric specificity of fructokinase. J. Biol. Chem. 1973;248:8174. [PubMed] [Google Scholar]
- (31).Raushel FM, Cleland WW. Bovine liver fructokinase: purification and kinetic properties. Biochemistry. 1977;16:2169–2175. doi: 10.1021/bi00629a020. [DOI] [PubMed] [Google Scholar]
- (32).Chenault HK, Mandes RF, Hornberger KR. Synthetic utility of yeast hexokinase. Substrate specificity, cofactor regeneration, and product isolation. J. Org. Chem. 1997;62:331–336. doi: 10.1021/jo961715g. [DOI] [PubMed] [Google Scholar]
- (33).Haradahira T, Tanaka A, Maeda M, Kanazawa Y, Ichiya Y-I, Masuda K. Radiosynthesis, rodent biodistribution, and metabolism of 1-deoxy-1-[18F]fluoro-D-fructose. Nucl. Med. Biol. 1995;22:719–725. doi: 10.1016/0969-8051(95)00018-s. [DOI] [PubMed] [Google Scholar]
- (34).Cheng Z, Levi J, Xiong Z, Gheysens O, Keren S, Chen X, Gambhir SS. Near-infrared fluorescent glucose analog and cyanin dye for tumor optical imaging in cell culture and in living mice. Bioconjugate Chem. 2006;17:662–669. doi: 10.1021/bc050345c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Ponpipom MM, Bugianesi RL, Shen TY. Synthesis of N-substituted D-mannosylamines and 1-(benzylamino)-1-deoxy-alditols for biological evaluation. Carbohydr. Res. 1980;82:135–140. [Google Scholar]
- (36).Aloj L, Caraco C, Jagoda E, Eckelman WC, Neumann RD. Glut-1 and hexokinase expression: relationship with 2-fluoro-2-deoxy-D-glucose uptake in A431 and T47D cells in culture. Cancer Res. 1999;59:4709–4714. [PubMed] [Google Scholar]
- (37).O’ Neil RG, Wu L, Mullani N. Uptake of a fluorescent deoxyglucose analog (2-NBDG) in tumor cells. Mol. Imaging Biol. 2005;7:388–392. doi: 10.1007/s11307-005-0011-6. [DOI] [PubMed] [Google Scholar]
- (38).Yamamoto T, Seino Y, Fukumoto H, Koh G, Yano H, Inagaki N, Yamada Y, Inoue K, Manabe T, Imura H. Over-expression of facilitative glucose transporter genes in human cancer. Biochem. Biophys. Res. Commun. 1990;170:223–230. doi: 10.1016/0006-291x(90)91263-r. [DOI] [PubMed] [Google Scholar]
- (39).Chan KK, Chan JYW, Chang KKW, Fung K-P. Inhibition of cell proliferation in human breast tumor cells by antisense oligonucleotides against facilitative glucose transporter 5. J. Cell. Biochem. 2004;93:1134–1142. doi: 10.1002/jcb.20270. [DOI] [PubMed] [Google Scholar]
- (40).Concha II, Velasquez FV, Martinez JM, Angulo C, Droppelmann A, Reyes AM, Slebe JC, Vera JC, Golde DW. Human erythrocytes express Glut5 and transport fructose. Blood. 1997;89:4190–4195. [PubMed] [Google Scholar]
- (41).Bell GI, Burant CF, Takeda J, Gould GW. Structure and function of mammalian facilitative sugar transporters. J. Biol. Chem. 1993;268:19161–19164. [PubMed] [Google Scholar]
- (42).Girniene J, Tatibuet A, Sackus A, Yang J, Holman GD, Rollin P. Inhibition of the D-fructose transporter protein Glut5 by fused-glyco-1,3-oxazolidin-2-thiones and -oxazolidin-2-ones. Carbohydr. Res. 2003;338:711–719. doi: 10.1016/s0008-6215(03)00007-7. [DOI] [PubMed] [Google Scholar]