Abstract
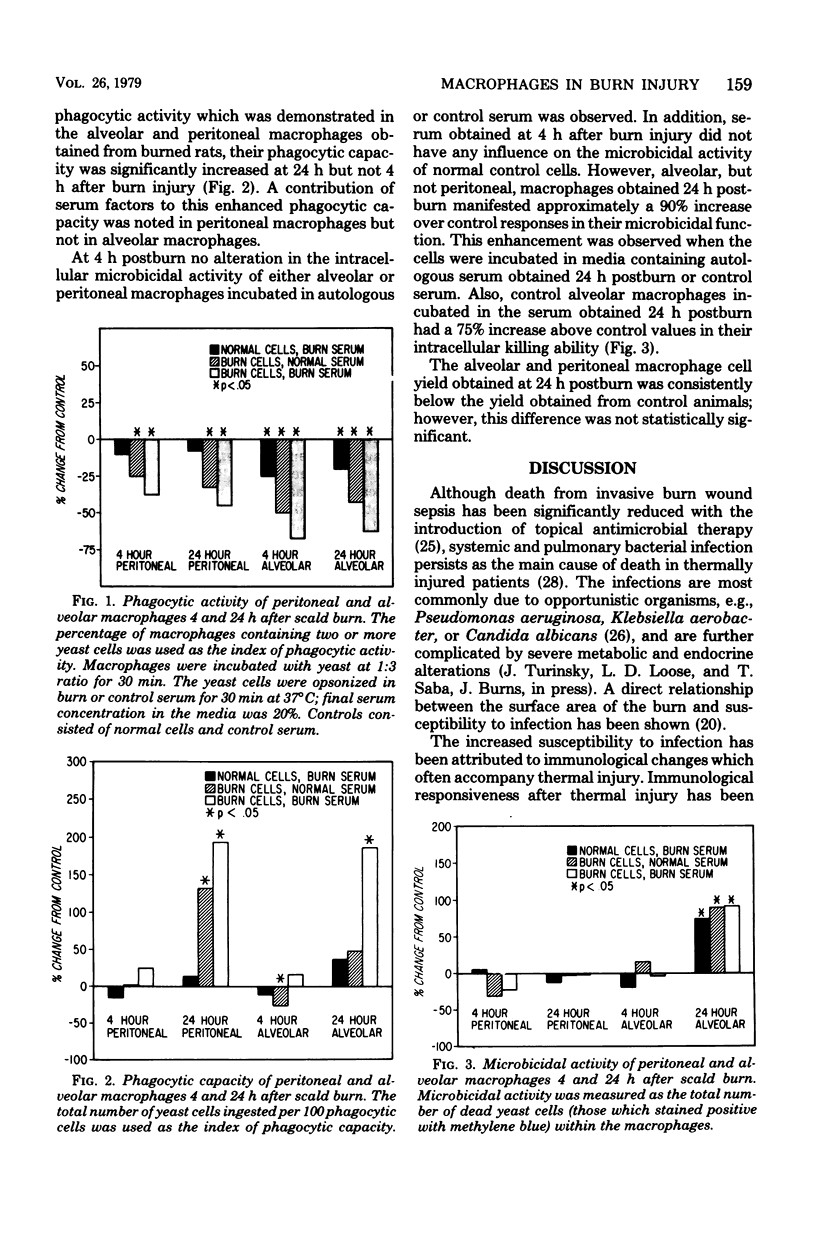
The phagocytic and microbicidal activities of alveolar and peritoneal macrophages were evaluated 4 and 24 h after a full-thickness scald burn of 26 to 28% body surface area in anesthetized rats. The contribution of serum factors to the macrophage functions was studied concurrently. The phagocytic activity of alveolar macrophages obtained 4 or 24 postburn was reduced approximately 65% below control values when they were incubated in media containing autologous serum and approximately 45% (below controls) when they were incubated in media containing normal (control) serum. A similar, although not as marked, decrease in the phagocytic activity of peritoneal macrophages was also demonstrated. Serum obtained from rats 4 or 24 h postburn had a significant suppressive effect on the phagocytic activity of control alveolar, but not peritoneal, macrophages. The intracellular microbicidal activity of peritoneal macrophages obtained at the postburn intervals and incubated in media containing either autologous or control serum was unaltered from control values. However, alveolar macrophages obtained 24 h, but not 4 h, postburn had a significant (approximately 80%) increase, above control values, in their microbicidal activity. Serum obtained 24 h, but not 4 h, postburn stimulated control alveolar macrophage killing ability. These data indicate that thermal injury induces a defect in the phagocytic activity rather than the microbicidal activity of macrophages. This phagocytic alteration is mediated, in part, by serum.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. W., Dionigi R., Meakins J. L. Periodic variation in the antibacterial function of human neutrophils and its relationship to sepsis. Ann Surg. 1971 Feb;173(2):206–213. [PMC free article] [PubMed] [Google Scholar]
- Alexander J. W. Effect of thermal injury upon the early resistance to infection. J Surg Res. 1968 Mar;8(3):128–137. doi: 10.1016/0022-4804(68)90074-7. [DOI] [PubMed] [Google Scholar]
- Alexander J. W., Wixson D. Neutrophil dysfunction and sepsis in burn injury. Surg Gynecol Obstet. 1970 Mar;130(3):431–438. [PubMed] [Google Scholar]
- Bjornson A. B., Altemeier W. A., Bjornson H. S. Changes in humoral components of host defense following burn trauma. Ann Surg. 1977 Jul;186(1):88–96. doi: 10.1097/00000658-197707000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J. F., Quinby W. C., Bondoc C. C., Cosimi A. B., Russell P. S., Szyfelbein S. K. Immunosuppression and temporary skin transplantation in the treatment of massive third degree burns. Ann Surg. 1975 Sep;182(3):183–197. doi: 10.1097/00000658-197509000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole P., Brostoff J. Intracellular killing of Listeria monocytogenes by activated macrophages (Mackaness system) is due to antibiotic. Nature. 1975 Aug 7;256(5517):515–517. doi: 10.1038/256515a0. [DOI] [PubMed] [Google Scholar]
- Daniels J. C., Sakai H., Ritzmann S. E. Lymphoid response of the burn patient. South Med J. 1975 Jul;68(7):865–870. [PubMed] [Google Scholar]
- Dhennin Ch, Pinon G., Greco J. M. Alterations of complement system following thermal injury: use in estimation of vital prognosis. J Trauma. 1978 Feb;18(2):129–133. doi: 10.1097/00005373-197802000-00010. [DOI] [PubMed] [Google Scholar]
- Dressler D. P., Skornik W. A. Alveolar macrophage in the burned rat. J Trauma. 1974 Dec;14(12):1036–1041. doi: 10.1097/00005373-197412000-00006. [DOI] [PubMed] [Google Scholar]
- Dressler D. P., Skornik W. A. Pulmonary bacterial susceptibility in the burned rat. Ann Surg. 1974 Aug;180(2):221–227. doi: 10.1097/00000658-197408000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman A. S., Rudloff H. B., McNamee R., Loose L. D., DiLuzio N. R. Deficiency of plasma humoral recognition factor activity following burn injury. J Reticuloendothel Soc. 1974 Mar;15(3):193–198. [PubMed] [Google Scholar]
- Grogan J. B. Altered neutrophil phagocytic function in burn patients. J Trauma. 1976 Sep;16(9):734–738. doi: 10.1097/00005373-197609000-00009. [DOI] [PubMed] [Google Scholar]
- Grogan J. B., Miller R. C. Impaired function of polymorphonuclear leukocytes in patients with burns and other trauma. Surg Gynecol Obstet. 1973 Nov;137(5):784–788. [PubMed] [Google Scholar]
- Grogan J. B. Suppressed in vitro chemotaxis of burn neutrophils. J Trauma. 1976 Dec;16(12):985–988. doi: 10.1097/00005373-197612000-00008. [DOI] [PubMed] [Google Scholar]
- Harmon J. W., Skornik W. A., McDonald J., Dressler D. P. Pulmonary bacterial defense. Effect of the burn wound on transfer of alveolar macrophage activation in rats by parabiosis. Am J Surg. 1976 Apr;131(4):447–451. doi: 10.1016/0002-9610(76)90155-0. [DOI] [PubMed] [Google Scholar]
- Heck E. L., Browne L., Curreri P. W., Baxter C. R. Evaluation of leukocyte function in burned individuals by in vitro oxygen consumption. J Trauma. 1975 Jun;15(6):486–489. doi: 10.1097/00005373-197506000-00005. [DOI] [PubMed] [Google Scholar]
- Howes R. M., Allen R. C., Su C. T., Hoopes J. E. Altered polymorphonuclear leukocyte bioenergetics in patients with thermal injury. Surg Forum. 1976;27(62):558–560. [PubMed] [Google Scholar]
- Leguit P., Jr, Meinesz A., Zeijlemaker W. P., Schellekens P. T., Eijsvoogel V. P. Immunological studies in burn patients. I. Lymphocyte transformation in vitro. Int Arch Allergy Appl Immunol. 1973;44(1):101–121. doi: 10.1159/000230921. [DOI] [PubMed] [Google Scholar]
- Lindberg R. B., Moncrief J. A., Mason A. D., Jr Control of experimental and clinical burn wounds sepsis by topical application of sulfamylon compounds. Ann N Y Acad Sci. 1968 Aug 14;150(3):950–960. doi: 10.1111/j.1749-6632.1968.tb14747.x. [DOI] [PubMed] [Google Scholar]
- Loose L. D., Silkworth J. B., Simpson D. W. Influence of cadmium on the phagocytic and microbicidal activity of murine peritoneal macrophages, pulmonary alveolar macrophages, and polymorphonuclear neutrophils. Infect Immun. 1978 Nov;22(2):378–381. doi: 10.1128/iai.22.2.378-381.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markley K., Smallman E. T. Effect of thermal trauma on numbers and function of T and B cells from mouse spleen. Int Arch Allergy Appl Immunol. 1977;54(3):238–246. doi: 10.1159/000231832. [DOI] [PubMed] [Google Scholar]
- Markley K., Smallman E., Evans G. Effect of E. coli and other bacteria on mortality of burned mice. J Trauma. 1968 Nov;8(6):1052–1064. doi: 10.1097/00005373-196811000-00007. [DOI] [PubMed] [Google Scholar]
- McEuen D. D., Gerber G. C., Blair P., Eurenius K. Granulocyte function and Pseudomonas burn wound infection. Infect Immun. 1976 Aug;14(2):399–402. doi: 10.1128/iai.14.2.399-402.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncrief J. A. The status of topical antibacterial therapy in the treatment of burns. Surgery. 1968 May;63(5):862–867. [PubMed] [Google Scholar]
- Ninnemann J. L., Fisher J. C., Wachtel T. L. Thermal injury-associated immunosuppression: occurrence and in vitro blocking effect of post recovery serum. J Immunol. 1979 May;122(5):1736–1741. [PubMed] [Google Scholar]
- Pavlovskis O. R., Pollack M., Callahan L. T., 3rd, Iglewski B. H. Passive protection by antitoxin in experimental Pseudomonas aeruginosa burn infections. Infect Immun. 1977 Dec;18(3):596–602. doi: 10.1128/iai.18.3.596-602.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt B. A., Jr, DiVincenti F. C., Mason A. D., Jr, Foley F. D., Flemma R. J. The occurrence and significance of pneumonia and other pulmonary complications in burned patients: comparison of conventional and topical treatments. J Trauma. 1970 Jul;10(7):519–531. doi: 10.1097/00005373-197007000-00001. [DOI] [PubMed] [Google Scholar]
- Rapaport F. T., Bachvaroff R. J. Kinetics of humoral responsiveness in severe thermal injury. Ann Surg. 1976 Jul;184(1):51–59. doi: 10.1097/00000658-197607000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saelinger C. B., Snell K., Holder I. A. Experimental studies on the pathogenesis of infections due to Pseudomonas aeruginosa: direct evidence for toxin production during Pseudomonas infection of burned skin tissues. J Infect Dis. 1977 Oct;136(4):555–561. doi: 10.1093/infdis/136.4.555. [DOI] [PubMed] [Google Scholar]
- Schmid L., Brune K. Assessment of phagocytic and antimicrobial activity of human granulocytes. Infect Immun. 1974 Nov;10(5):1120–1126. doi: 10.1128/iai.10.5.1120-1126.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenenberger G. A., Burkhardt F., Kalberer F., Müller W., Städtler K., Vogt P., Allgöwer M. Experimental evidence for a significant impairment of host defense for gram-negative organisms by a specific cutaneous toxin produced by severe burn injuries. Surg Gynecol Obstet. 1975 Oct;141(4):555–561. [PubMed] [Google Scholar]
- Silverstein P., Dressler D. P. Effect of current therapy on burn mortality. Ann Surg. 1970 Jan;171(1):124–129. doi: 10.1097/00000658-197001000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Goldman A. S. Selective effects of thermal injury on mouse peritoneal macrophages. Infect Immun. 1972 Jun;5(6):938–941. doi: 10.1128/iai.5.6.938-941.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERDER E., ROSENTHAL S. M. Role of infection in the delayed deaths of mice following extensive burn injury. Proc Soc Exp Biol Med. 1961 Nov;108:501–505. doi: 10.3181/00379727-108-26978. [DOI] [PubMed] [Google Scholar]
- Warden G. D., Mason A. D., Jr, Pruitt B. A., Jr Evaluation of leukocyte chemotaxis in vitro in thermally injured patients. J Clin Invest. 1974 Oct;54(4):1001–1004. doi: 10.1172/JCI107815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg D. S., Fishman M., Veit B. C. Functional heterogeneity among peritoneal macrophages. 1. Effector cell activity of macrophages against syngeneic and xenogeneic tumor cells. Cell Immunol. 1978 Jun;38(1):94–104. doi: 10.1016/0008-8749(78)90035-7. [DOI] [PubMed] [Google Scholar]
- Wood G. W., Volenec F. J., Mani M. M., Humphrey L. J. Dynamics of T-lymphocyte subpopulations and T-lymphocyte function following thermal injury. Clin Exp Immunol. 1978 Feb;31(2):291–297. [PMC free article] [PubMed] [Google Scholar]



